Abstract
The association between developmental trajectories of language-related white matter fiber pathways from 6 to 24 months of age and individual differences in language production at 24 months of age was investigated. The splenium of the corpus callosum, a fiber pathway projecting through the posterior hub of the default mode network to occipital visual areas, was examined as well as pathways implicated in language function in the mature brain, including the arcuate fasciculi, uncinate fasciculi, and inferior longitudinal fasciculi. The hypothesis that the development of neural circuitry supporting domain-general orienting skills would relate to later language performance was tested in a large sample of typically developing infants. The present study included 77 infants with diffusion weighted MRI scans at 6, 12 and 24 months and language assessment at 24 months. The rate of change in splenium development varied significantly as a function of language production, such that children with greater change in fractional anisotropy (FA) from 6 to 24 months produced more words at 24 months. Contrary to findings from older children and adults, significant associations between language production and FA in the arcuate, uncinate, or left inferior longitudinal fasciculi were not observed. The current study highlights the importance of tracing brain development trajectories from infancy to fully elucidate emerging brain-behavior associations while also emphasizing the role of the splenium as a key node in the structural network that supports the acquisition of spoken language.
Keywords: neurodevelopment, splenium, language, infancy, development, MRI
Acquiring spoken language is one of the major achievements of the first two years of life. Around the time of the first birthday, infants typically speak their first words. They are combining two words together by their second birthday and, by their third, are producing sentences. Yet behavioral and functional neuroimaging studies have suggested that infants develop a capacity to process speech and learn language long before they begin speaking (Dehaene-Lambertz & Baillet, 1998; Dehaene-Lambertz & Pena, 2001; Dehaene-Lambertz et al., 2006; Dehaene-Lambertz, Dehaene, & Hertz-Pannier, 2002; Guttorm, Leppanen, Richardson, & Lyytinen, 2001; Kuhl, 2004, 2010; Redcay, Haist, & Courchesne, 2008; Shultz, Vouloumanos, Bennett, & Pelphrey, 2014).
This early neural commitment culminates in an adult brain with a sophisticated language network including two cortical association areas, Wernicke’s region (Brodmann area 22) in the posterior superior temporal gyrus and Broca’s region (Brodmann areas 44 and 45) in the frontal lobe. These two regions are primarily connected by a dorsal white matter fiber tract, the arcuate fasciculus (Catani & Mesulam, 2008). Together this system is often referred to as the perisylvian language network and it is considered functionally important for speech production and speech processing (Breier, Hasan, Zhang, Men, & Papanicolaou, 2008; Hickok & Poeppel, 2007; Warren, Wise, & Warren, 2005). In more contemporary models of language neurobiology a complementary ventral pathway has been proposed to support the mapping of auditory speech sounds to meaning (Hickok & Poeppel, 2004, 2007; Saur et al., 2008). This ventral pathway courses laterally along temporal lobes connecting adjacent cortices and includes the uncinate fasciculus and the inferior and middle longitudinal fasciculi (Dick & Tremblay, 2012; Saur et al., 2008).
While informative, most studies of the neurobiology of language are based on the adult brain, which make implicit assumptions about developmentally invariant structure-function associations across time. These studies provide evidence for specialized cortical functions, but do not provide evidence of the process of cortical specialization. The anatomical substrates that underlie the acquisition of language are currently poorly understood.
Structural and diffusion tensor imaging of typically developing infants have thus far largely been limited by imaging data collected at a single time point, thus restricting the inferences that can be made about brain development. In a structural magnetic resonance imaging study, Ortiz-Mantilla and colleagues observed that infants with larger right amygdala at 6 months had lower expressive and receptive language scores at 2, 3, and 4 years of age (Ortiz-Mantilla, Choe, Flax, Grant, & Benasich, 2010). The amygdalae are a pair of limbic-system substructures located deep in the temporal lobes with putative primary functions including emotional reactions, memory, and decision making (Phelps, 2006). The uncinate fasciculi, bi-lateral fiber tracts from the ventral pathway of language neurobiology, connects the amygdalae and other limbic system structures to the orbitofrontal cortex (Colnat-Coulbois et al., 2010; Kier, Staib, Davis, & Bronen, 2004). Elison and colleagues (Elison, Wolff, et al., 2013) reported that individual differences in the right uncinate fasciculus at 6 months predicted individual differences in joint attention behaviors at 9 months. Joint attention is consistently associated with receptive and expressive language abilities during toddlerhood (Mundy et al., 2007). Additional diffusion tensor imaging studies of preterm infants have found that microstructure in the left superior temporal gyrus at term equivalent age is related to language scores at 2 years of age (Aeby et al., 2013), and that microstructure in the centrum semiovale and subventricular zone soon after birth was related to standardized language scores at 18-24 months of age (Pogribna et al., 2014).
In the last decade, as more research has shown that the classic perisylvian model of language in the brain may be anatomically under-specified and overly exclusive (Poeppel & Hickok, 2004), researchers have begun to explore brain regions outside of the perisylvian language network that may be involved in language processing and production. One such region that may be uniquely important early in development is the posterior hub of the default mode network (Damaraju et al., 2014; Gao et al., 2009, 2013; Pruett et al., 2015). This densely connected region is well positioned to function as an information processing way station early in development, situated to some degree at the intersection of ventral and dorsal attention streams. The functions of this hetero-modal region which includes the retro-splenial cortex, posterior cingulate cortex, and precuneus are varied in the adult brain (Margulies et al., 2009; Pearson, Heilbronner, Barack, Hayden, & Platt, 2011; Vann, Aggleton, & Maguire, 2009). Yet there is compelling evidence to suggest that this region may support rapid visual orienting during infancy (Elison, Paterson, et al., 2013), research that augments previous findings suggesting that cellular firing rates in the posterior cingulate cortex mediate attentional engagement (Hayden, Smith, & Platt, 2009).
The splenium of the corpus callosum, the most posterior sector of the corpus callosum, contains fibers that course through the posterior hub of the default mode network and project to primary visual cortices among additional cortical targets (Putnam, Steven, Doron, Riggall, & Gazzaniga, 2010). Anchored by this pattern of connectivity, recent research suggests the splenium plays an important role in orienting to salient information during infancy and adulthood (Elison, Paterson, et al., 2013; Niogi et al., 2010). Visual orienting has been shown to facilitate spoken word acquisition, namely label mapping, and hence the splenium may be an important neurobiological region for emerging language production during infancy through its role in visual orienting (Keehn, Müller, & Townsend, 2013; Koegel, Shirotova, & Koegel, 2009; Vouloumanos & Curtin, 2014).
Further support for the role of the splenium in language comes from studies of adults with dyslexia (Vandermosten, Poelmans, Sunaert, Ghesquière, & Wouters, 2013) and studies of school-aged children born prematurely, that show associations between language skills and structural properties of the splenium (Feldman, Lee, Yeatman, & Yeom, 2012; Northam et al., 2012). Despite these recent advances the direct association between language development and the splenium during infancy has yet to be tested.
The current study aims to begin to fill this research gap by examining diffusion tensor imaging brain scans at three narrowly-defined age points, 6, 12 and 24 months, and standardized language production at 24 months. The aim of this study was to delineate the association between longitudinal trajectories of white matter growth and individual differences in language production at 2 years of age. White matter fiber pathways characterized as integral to language function in the mature brain, the arcuate fasciculi, the uncinate fasciculi, and the inferior longitudinal fasciculi were examined. To investigate the contribution of visual-spatial coordination in the development of language, the contribution of the splenium of the corpus callosum was also examined. The primary hypothesis of the current study is that development of neural circuitry supporting domain-general orienting skills, as measured by longitudinal development of the splenium, relates to later language. If this hypothesis is correct and white matter fiber tract growth of the splenium is related to later language ability, this would provide evidence that domain-general orienting skills may be core building blocks for early associative language learning in infants. This finding would also suggest that the systems supporting language functions are dynamic over time, and while the splenium may play a unique role early on in supporting language production, later in life the splenium may no longer support language production and this task may be handled by other specialized domain-specific systems (e.g., the perisylvian network).
Methods
Participants
This study included a subset of data from the Infant Brain Imaging Study (IBIS), an ongoing longitudinal study of infants at high and low familial-risk for autism spectrum disorder (ASD). IBIS, the parent network, is funded as an Autism Center for Excellence by the National Institutes of Health. The parent network includes four clinical sites: University of North Carolina, Chapel Hill; University of Washington, Seattle; The Children’s Hospital of Philadelphia; and Washington University, St. Louis. Data coordination was managed by the Montreal Neurological Institute (MNI) at McGill University and neuroimaging data processing was performed at the University of North Carolina and the Scientific Computing and Imaging Institute at the University of Utah. Infants and their families were enrolled and assessed at 6-months, with follow-up assessments at 12 and 24 months of age. Parents provided written informed consent prior to participating in this study. Procedures for this study were approved by the Institutional Review Boards at each clinical data collection site.
The present study included all infants from the low familial risk sample that contributed up to three MRI scans at 6, 12 and 24 months and the MacArthur-Bates Communicative Development Inventory (Fenson et al., 2007) at 24 months (n= 77; 45 male, 32 female). These infants did not meet criteria for ASD at 24 months of age and had no first-degree relatives with ASD. Further exclusionary criteria included: significant medical conditions known to affect brain development, sensory impairment, low birth weight (< 2,200 g) or prematurity (<36 weeks gestation), perinatal brain injury secondary to birth complications or exposure to specific medication or neurotoxins during gestation, non-English speaking immediate family, contraindication for MRI, adoption, and first degree relative with idiopathic intellectual disability, psychosis, schizophrenia, or bipolar disorder.
Procedures
Diffusion tensor imaging brain scans and behavioral assessments were completed at 6-, 12-, and 24-month time points. Table 1 includes information on the Mullen Scales of Early Learning (Mullen, 1995) and child chronological age at each time point. Mullen Early Learning composite scores were within the normal range for all time points suggesting the current sample is representative of typically developing infants. Parent-reported number of words produced on the MacArthur-Bates Communicative Development Inventory (M-CDI) was used to quantify language production at 24 months. The M-CDI is a parent-report questionnaire measure intended for typically developing children between the ages of 8 and 30 months that has shown reasonable concurrent and predictive validity (Feldman et al., 2005). Although parents completed the M-CDI when the infants were 24 months, they completed the Words and Gesture version of the M-CDI. In the larger study the M-CDI was administered at 12, 18, and 24 months and the Words and Gesture version was consistently administered to allow for the comparison of raw scores across multiple time points. The current study focuses on one component of the M-CDI, number of words produced at 24 months. The Vineland Adaptive Behavior Scales-II motor subscale score (VABS-II) (Sparrow, Cicchetti, & Balla, 2005) was used to test specificity of hypothesized brain-behavior associations. The VABS-II is a standardized parent report measure used to assess adaptive skills across numerous domains in individuals of all ages.
Table 1.
Participant developmental characteristics.
| n | Chronological Age (months) |
Developmental Characteristic | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | Range | ||
|
|
||||||
| 6 Month Visit | ||||||
| MSEL ELC1 | 60 | 6.71 | 0.60 | 101.80 | 11.44 | 76-122 |
| 12 Month Visit | ||||||
| MSEL ELC | 63 | 12.73 | 0.72 | 108.06 | 10.15 | 88-132 |
| 24 Month Visit | ||||||
| MSEL ELC | 46 | 24.63 | 0.83 | 110.26 | 14.47 | 82-134 |
| MSEL Language2 | 77 | 52.36 | 9.94 | 20-73 | ||
| Vineland Motor3 | 75 | 101.6 | 8.60 | 82-129 | ||
| M-CDI Words4 | 77 | 243.35 | 97.65 | 0-396 | ||
Mullen Scales of Early Learning, Early Composite Standard Score
Mullen Scales of Early Learning, Expressive Language Subscale Standard Score
Vineland Adaptive Behavior Scales Motor Subscale Standard Score
MacArthur-Bates Communicative Development Inventory Number of Words Produced
Image Acquisition
Pediatric imaging was completed during natural sleep at each clinical site using identical 3-T Siemens TIM Trio scanners (Siemens Medical Solutions, Malvern, Pa.) equipped with 12-channel head coils. The diffusion tensor imaging sequence was acquired as an ep2d_diff pulse sequence with a field of view of 190 mm (6 and 12 months) or 209 mm (24 months), 75–81 transversal slices, a slice thickness of 2 mm isotropic, 2×2×2-mm3 voxel resolution, a TR of 12,800–13,300 ms, a TE of 102 ms, variable b values between 0 and 1,000 s/mm2, 25 gradient directions, and a scan time of 5–6 minutes. Traveling volunteers and a standardized phantom were used to establish intra- and inter-site reliability (Gouttard, Styner, Prastawa, Piven, & Gerig, 2008).
Image Preprocessing
Data from diffusion-weighted imaging were processed for appropriate quality via DTIprep (Liu et al., 2010; Oguz et al., 2014), which automatically detects artifacts, corrects for motion and eddy current deformations, excludes diffusion weighted images with artifacts, and generates a full report. Expert raters manually removed additional images presenting with residual artifacts. Data sets with fewer than 18 (72%) gradient diffusion-weighted images after this quality procedure were excluded from further processing owing to a low signal-to-noise ratio and potential biases for fractional anisotropy assessment (Farzinfar et al., 2013).
DTI Processing Pipeline
Group analysis of the data from diffusion-weighted imaging, processed by means of diffusion tensor estimates, employed a sophisticated processing pipeline designed to process data obtained from neonates and infants (Goodlett, Fletcher, Gilmore, & Gerig, 2009; Verde et al., 2013). This processing overcomes the major challenge in implementing tract-oriented statistics in large study groups, which is acquiring consistent spatial parametrization within and between groups from longitudinal imaging data (Geng et al., 2012; Sadeghi et al., 2013). This includes a computational anatomy approach for nonlinear co-registration of the diffusion tensor imaging data to a study-specific template reference coordinate frame, a process to parameterize fiber tracts to functions of length, and the mapping of individual tract geometries into common coordinates. Fiber tracts of interest are tracked in the study-specific template as discussed below. All corresponding processing tools are publicly available as part of the UNC-Utah NA-MIC DTI fiber tract analysis framework (www.nitrc.org/projects/namicdtifiber). Diffusion-tensor imaging data for all subjects (ages 6 to 24 months) were mapped into a common atlas space by using the preceding procedure.
Fiber Tractography
3D Slicer (www.slicer.org) was used to perform deterministic tractography. Seed label maps were drawn in the common atlas according to anatomically informed, previously described tractography methods (Catani & Thiebaut de Schotten, 2008; Mori, Wakana, Van Zijl, & Nagae-Poetscher, 2005). These fiber tracts were defined in mean atlas space which includes all individual data sets across all time points. This approach has been successfully used to extract cross-sectional and longitudinal fiber tract data such as that in the current manuscript from infants starting at the neonate stage within the first year of life in over 2000 subjects (Geng et al., 2012; Sadeghi et al., 2013; Wolff et al., 2012). Label maps were created for each fiber tract and coarse cleaning of the resulting fiber tracts was completed in 3D Slicer using interactive 3D regions of interest (ROIs). Fiber tracts generated in 3D Slicer were processed to remove spurious, incomplete or anatomically incorrect fibers via FiberViewerLight.
Fiber profiles of fractional anisotropy (FA) diffusivity values were computed and averaged across each fiber tract. Fractional anisotropy is an index measuring the degree of anisotropy of local diffusivity, ranging from 0 for isotropic diffusion, to 1 for strongly directional diffusivity in highly structured axonal bundles (Basser & Pierpaoli, 1996). Fiber profiles of axial diffusivity (AD), which indexes diffusion along the principal fiber direction (1st eigenvalue), and radial diffusivity (RD), which indexes diffusion that is orthogonal to the major fiber direction (average of the 2nd and 3rd eigenvalues) were also produced. Mean diffusivity (MD), a measure of the average of the three eigenvalues, was included for completeness. Fractional anisotropy was chosen as the primary means of defining fiber profiles because it models the association between the three eigenvalues and is thought to correspond to organizational properties that reflect development (Beaulieu, 2009; Geng et al., 2012; Hermoye et al., 2006; Hüppi & Dubois, 2006). As a follow up RD and AD are examined independently, with the specific hypothesis that RD yields information about restriction of water movement due to myelin content and axon density, both of which are meaningful developmentally.
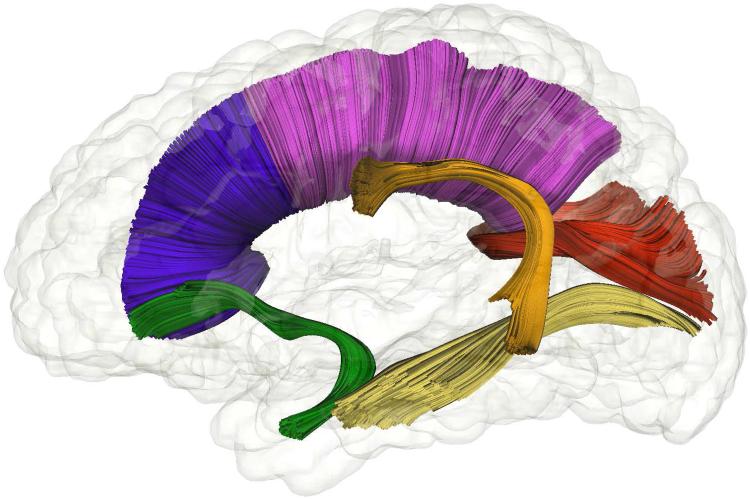
The current study focused on the longitudinal change in FA for the arcuate fasciculus, inferior longitudinal fasciculus, and uncinate fasciculus, as well as three segments of the corpus callosum (see Figure 1). These fiber tracts were selected based on previous research showing associations to various aspects of language (Dick & Tremblay, 2012) and feasibility of generating the fiber tract in the infant brain using label map tractography methods.
Figure 1.
White matter fiber bundles from tractography of DTI data. Blue, Anterior portion of the corpus callosum; magenta, corpus callosum body; red, splenium; green, uncinate fasciculus; yellow, inferior longitudinal fasciculus; orange, arcuate fasciculus.
Corpus Callosum
The corpus callosum is the main commissural fiber in the human brain connecting the left and right cerebral hemispheres in a mostly homologous pattern. For the purposes of the current study the corpus callosum was divided into three main segments: anterior portion, body, and splenium based on Witelson (Witelson, 1989), and following the work of Elison and colleagues (Elison, Paterson, et al., 2013). The splenium is the most posterior segment of the corpus callosum; in the current atlas the majority of its fibers project to the occipital lobes. The body of the corpus callosum includes fibers projecting to premotor and supplementary motor areas, the primary motor cortex, primary sensory cortex, and the parietal lobes. The anterior portion of the corpus callosum contains fibers connecting the frontal lobes (Hasan et al., 2009; Hofer & Frahm, 2006). The tapetum is not included in the segmentation of the corpus callosum in the current study.
Arcuate Fasciculus
The arcuate fasciculus is a bi-lateral fiber tract composed of three divisions (Catani, Jones, & ffytche, 2005). The largest of these divisions, the long direct segment, runs medially and originates in Wernicke’s territory (Brodmann area 22) in the posterior superior temporal gyrus, from there it travels posteriorly and superiorly before arcing anteriorly near the level of the corpus callosum and continuing medially toward Broca’s territory (Brodmann area 44 and 45) in the frontal lobe where it terminates. The long direct segment was defined bilaterally in the atlas space. The two smaller divisions were not segmented due the volume of these regions.
Uncinate Fasciculus
The uncinate fasciculus is a hook-shaped fiber bundle connecting several limbic system structures in the temporal lobe, such as the amygdala and hippocampus, to frontal lobe areas such as the orbitofrontal cortex (Dick & Tremblay, 2012).
Inferior Longitudinal Fasciculus
The inferior longitudinal fasciculus (ILF) is long fiber bundle running along the lateral walls of the lateral ventricles, connecting the temporal lobe to the occipital lobe. The anterior portion of the ILF is thought to join the uncinate fasciculus (Ashtari, 2012).
Statistical Analysis
The association between longitudinal trajectories of mean fractional anisotropy values for each fiber tract and dimensional language production were evaluated using mixed models for repeated measures design. The mixed-model framework accommodates different patterns of missing data and unbalanced design. Among the 77 infants in the current study successful scans were obtained for 60 infants at 6 months, 63 infants at 12 months, and 45 infants at 24 months, with 31 infants contributing scans at all three time points, an additional 30 infants contributing scans at 2 time points, and 16 infants contributing scans at 1 time point.
Preliminary analyses were conducted to determine if male and female participants differed on our dimensional language measure, The MacArthur-Bates Communicative Development Inventory number of words produced at 24 months. Males produced on average 225.87 (SD=99.45) words and females produced 272.21 (SD=87.86), t= 2.38, p = .02. Given this difference, participant sex was included in all following models.
Separate linear mixed models were fit for each fiber tract with fractional anisotropy (FA) development, language production, and FA development-by-language interaction as fixed effects. Sex, maternal education, and data collection site were entered as covariates in all following models. The primary hypotheses examining the trajectory of FA development and language production were tested in the interaction of FA development and language production for each tract. An adaptive step-down Bonferroni multiple comparison correction was applied to the results from all FA models (Hochberg & Benjamini, 1990). All analyses were done using SAS statistics software, version 9.3.
Results
According to parent-report at 24 months, toddlers in this study produced an average of 243.35 words (SD = 97.65, range 0-396). Shapiro-Wilk tests of normality suggest that 24 month word production approximated a normal distribution (W = .97, p > .05). Across time points infants scored broadly within normal ranges on cognitive function measured using the Mullen Scales of Early Learning (Table 1). Participant demographic information can be found in Table 2.
Table 2.
Participant demographics.
| Variable | Value |
|---|---|
| Males (%) | 58.7% |
| % Caucasian | 85.7% |
| % Hispanic | 4% |
| Average maternal age, years | 32.85 (SD= 4.20) |
| Average paternal age, years | 34.37 (SD = 5.43) |
| % Mothers with college degree or higher |
84.4% |
| % Fathers with college degree or higher |
75.3% |
| % Household income under 75K |
41.6% |
Diffusion tensor imaging was used to calculate mean fractional anisotropy (FA) along each fiber tract (see Figure 1 for an illustration of the fiber tracts). FA is an index of fiber bundle organization and tends to increase with age during early development. In the current study all white matter fiber tracts increased in FA as infants developed (Table S1). Next, the key hypothesis that longitudinal development of FA varied as a function of 24 month language production in infants was tested using longitudinal mixed modeling. All models included an interaction term to examine whether 6-24 month FA change in a given fiber tract varied as a function of language production (e.g., number of words spoken at 24 months). Significant interactions were reported between language production and development of the splenium, corpus callosum body, anterior corpus callosum, and right inferior longitudinal fasciculus over time, indicating that the developmental trajectory of FA from six to 24 months varied as a function of language production at 24 months (Table 3). In all three of these white matter fiber tracts the children with highest language production at 24 months had a steeper increase in FA development. Only the splenium survived an adaptive false discovery rate procedure (Table 3) (Hochberg & Benjamini, 1990).
Table 3.
Results of longitudinal mixed models testing the association between fractional anisotropy development and language outcome.
| Fiber tract | FA Change | Language at 24 months1 |
FA Change *Language |
Adaptive FDR |
|||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | p | |
|
|
|||||||
| Splenium | 6.76 | .012 | 2.45 | .127 | 9.58 | .004 | .029* |
| CC Body | 21.38 | .000 | 1.98 | .169 | 6.45 | .016 | .098 |
| Anterior CC | 43.95 | .000 | 1.95 | .172 | 4.40 | .044 | .264 |
| Arcuate, left | 55.13 | .000 | 0.06 | .807 | 0.33 | .571 | .902 |
| Arcuate, right | 66.25 | .000 | 0.15 | .701 | 0.34 | .562 | .902 |
| ILF, left | 23.86 | .000 | 0.00 | .976 | 0.02 | .902 | .902 |
| ILF, right | 19.65 | .000 | 2.26 | .143 | 6.78 | .014 | .084 |
| Uncinate, left | 39.96 | .000 | 0.57 | .456 | 0.05 | .830 | .902 |
| Uncinate, right | 29.37 | .000 | 0.01 | .928 | 1.47 | .234 | .902 |
Language measure by the MacArthur-Bates Communicative Development Inventory, Number of words produced at 24 months
Significant interaction that survived adaptive false-discovery rate procedure (Hochberg & Benjamini, 1990).
Note: FA, Fractional anisotropy; CC, Corpus Callosum; ILF, Inferior longitudinal fasciculus
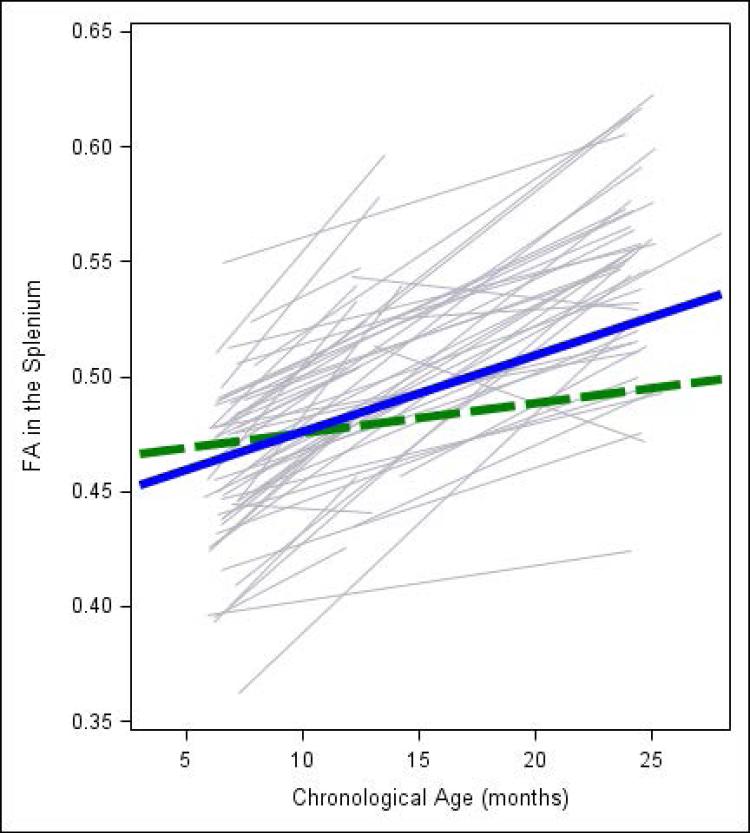
Figure 2 displays raw FA trajectory data for each participant. Bold lines are for illustration only and represent FA development at one standard deviation above (blue solid line) and below the mean (green dotted line) of the MacArthur-Bates Communicative Development Inventory, Number of words produced at 24 months. As depicted in Figure 2, the infants with high and low language production had similar FA in the splenium at 6 months, but by 24 months the high language production children generally had higher FA when compared to children with lower language production. We did not find significant associations between FA change across time and language production in the bi-lateral arcuate fasciculus, left inferior longitudinal fasciculus, or bi-lateral uncinate fasciculus.
Figure 2.
Individual trajectories of fractional anisotropy development in the splenium of the corpus callosum from 6 to 24 months. Bold lines are for illustration only and represent FA development at one standard deviation above (blue solid line) and below the mean (green dotted line) of the MacArthur-Bates Communicative Development Inventory, Number of words produced at 24 months.
Planned follow up linear mixed models of radial diffusivity yielded a trend between language production and the development of radial diffusivity for the splenium, F(59,31) = 3.71, p = .06 (see Table S2 for results from all other fiber tracts). Models of axial diffusivity did not yield significant associations between change in axial diffusivity and language production (Table S3). Results from models of MD did not provide additional insights as results are highly correlated with findings in AD due to interdependence of the two diffusion measures.
Next, cross-sectional analyses were conducted to determine if FA in the splenium at individual time points predicted language production at 24 months. Partial correlations controlling for age at MRI, sex, maternal education, and data collection site yielded non-significant correlations between language production and FA in the splenium at 6 (r = −.115, p = .41) or 12 months (r = −.018, p = .89). The association between FA in the splenium and language production at 24 months was statistically significant (r = .351, p = .03). These results, considered in the context of those from the longitudinal models, suggest that children with higher word production begin to show accelerated FA development sometime between the 12 and 24 month time point. Table S4 in the Supplementary Information contains cross-sectional analyses for the corpus callosum body, anterior corpus callosum, bilateral uncinate, arcuate, and inferior longitudinal fasciculi.
To test for convergent validity MSEL expressive language subscale t-scores at 24 months were used as an alternative language production variable. A linear mixed model was fit with FA splenium development, language production, and FA development-by-language interaction as fixed effects. The model yielded a significant interaction term indicating that that longitudinal development of FA varied as a function of Mullen expressive language, F(59,31) = 4.97, p < .05. As a final step, a control analysis was performed to examine whether FA change in the splenium varied as a function of motor development at 24 months, as measured by the Vineland Adaptive Behavior Scales-II. The model failed to yield a significant interaction, p > .31, suggesting that change in splenium FA between 6 and 24 months varied as a function of words spoken at 24 months, but not individual differences in motor development at 24 months. Lastly, it is worthwhile to acknowledge that infant brain imaging data is often highly variable. However, in the current data the removal of possible outlying FA trajectories (n= 10) did not affect the main findings of this study.
Discussion
The human brain undergoes dramatic change during the first years of life, yet few studies have examined longitudinal trajectories of brain development in young infants, and fewer yet have explored the association between longitudinal brain development and language ability. In the current study we provide evidence that the trajectory of 6 to 24 month white matter fiber tract development is related to language production at 24 months in typically developing infants. The strongest brain-behavior association was found in the splenium of the corpus callosum, a fiber tract that courses through the posterior hub of the default mode network and supports orienting to salient stimuli. Visual orienting has been shown to facilitate language acquisition through label mapping and consequently the splenium may support emerging language production through its role in visual orienting. Infants with superior language at 24 months displayed the greatest change over time in fractional anisotropy (FA) development from 6 to 24 months of age. Similar results were reported for radial diffusivity (RD), suggesting that differences in axon packing density and myelination may be driving the FA results, an interpretation consistent with previous work by Elison and colleagues and Wolff and colleagues (Elison, Paterson, et al., 2013; Wolff et al., 2015). Results did not support an association between early language production and classic perisylvian fiber tracts including the arcuate fasciculus, uncinate fasciculus, and inferior longitudinal fasciculus. This bolsters the position that the classic model of language-related neural circuitry cannot be downwardly extended to infants (Poeppel & Hickok, 2004). We also observed some evidence for structure-function specificity in that development of the splenium was not significantly associated with motor development, suggesting that this structure may facilitate the acquisition of spoken language but not all aspects of development. The findings presented here also bring attention to two matters often overlooked in neuroimaging research. One, understanding developmental processes requires longitudinal data (Karmiloff-Smith, 2012) and two, the association between cognitive function and brain structure is dynamic in nature (Johnson, 2000, 2011).
In regards to the first matter raised, previous studies in older children have highlighted the necessity to study trajectories of development by reporting longitudinal brain-behavior associations. Shaw and colleagues (Shaw et al., 2006) demonstrated that school-aged children with superior intelligence were characterized by a dynamic pattern of cortical development including a rapid increase in cortical thickness early in development, a later age of maximal cortical thickness, and more cortical thinning during late adolescence than children with high or normal intelligence. The significance of this pattern would not have been captured by cross-sectional research. Recently, Deoni and colleagues (2014) demonstrated that from infancy to five years children with above average cognitive ability showed initially slower, but prolonged myelin development, resulting in increased myelination when compared to children with lower cognitive ability. When compared to the current study, the Deoni study utilized a different imaging modality (mcDESPOT multicomponent relaxometry technique) with an older participant sample. However despite these differences the current results mirror the general findings of the youngest participants in that study. Specifically, the present results indicate that infants with superior language production display a period of prolonged, accelerated development through the second year of life, such that by two years of age children with superior language production displayed higher levels of splenium development.
Further support for the position that patterns of change may be more meaningful than structure at a given time point, comes from a DTI study of white matter development in infants at high familial risk for autism spectrum disorder by Wolff and colleagues (Wolff et al., 2012), who found a similar pattern of crossing trajectories over the first two years of life, wherein a steeper rate of FA development was associated with an absence of autistic symptoms, whereas a flatter FA trajectory was associated with later ASD diagnosis. Together, these studies (Deoni et al., 2014; Shaw et al., 2008; Wolff et al., 2012) point to dynamic phenomena that could not have been ascertained through cross-sectional design, demonstrating the necessity of longitudinal data to support inferences concerning developmental processes. In addition, this collection of studies highlights the developmental impact of individual differences in the timing and rate of early brain maturation in infancy and childhood. At the same time these studies have shown that associations between neural development and cognition are dynamic, and it remains unclear whether early patterns of timing and rate of brain maturation yield meaningful lifelong effects. Moving forward, prospective studies following children from infancy through late adolescence are needed to determine if the effects of early development of the splenium on later language production extend past childhood, or whether, and how, the splenium supports cortical specialization involving the perisylvian network. This last point underscores the second matter raised -- the association between cognitive function and brain structure is dynamic in nature.
The idea that the association between cognitive function and brain structure is dynamic stems from the theory of interactive specialization (Johnson, 2000, 2011) which posits in part that cognitive abilities develop through the initially widespread interaction of numerous brain regions, a process that through time and with experience results in cortical specialization (Redcay et al., 2008). The splenium of the corpus callosum may support language development in this way through its connections coursing through the posterior hub of the default mode and the dorsal and ventral visual attention networks (Putnam et al., 2010). For example, previous studies in infants and adults have reported associations between the splenium and visual orienting (Elison, Paterson, et al., 2013; Niogi et al., 2010), a foundational behavior that supports rapid, efficient processing of salient information, such as mapping object labels, which in turn supports language acquisition (Colombo, Shaddy, Richman, Maikranz, & Blaga, 2004; Frick, Colombo, & Saxon, 1999). Given that in the current study the majority of splenium projections terminated in the visual areas of the occipital lobe it is likely that our findings are a result of visual orienting supporting early language production. Future research might examine the possibility that targeted early intervention on the attention system can benefit later language production by rectifying early aberrant development of these key posterior inter-hemispheric connections (Pietrasanta, Restani, & Caleo, 2012).
One limitation of the current study is that the sample, although substantial, was largely homogenous in terms of racial background and socio-economic status, and as such may not be representative of the wider experiences of children in the United States. Future work should consider a more diverse sample of children in terms of racial and ethnic background as well as socio-economic status. Another potential limitation of our study is our use of the MacArthur-Bates Communicative Development Inventory (M-CDI), Words and Gestures in children that are 24 months of age, as this version is normed for infants 8-18 months. The M-CDI Words and Gestures was used in children 24 months of age to allow for longitudinal analyses of the M-CDI in a larger study of infants at high and low familial risk for autism spectrum disorder. While tests of normality indicated that scores of words produced at 24 months approximated a normal distribution, it is possible the distribution of scores in the range of our sample is truncated. While we found an association between infant brain development and language production at age 2, it is unknown whether more distal language function is similarly predicted by early trajectories. Future work might follow-up with children at school age to determine the relevance of early white matter development to language and related academic outcomes later in childhood.
Diffusion tensor imaging was used to approximate organizational properties of development by defining fractional anisotropy levels along targeted fiber tracts (Basser & Pierpaoli, 1996; Hermoye et al., 2006). However, fractional anisotropy is an imperfect index of white matter fiber structure that may reflect potential sources of error including crossing fibers and partial volume effects (Mori & Zhang, 2006). Recent studies show that fractional anisotropy may develop in a non-linear, asymptotic fashion, in which case a non-linear growth trajectory statistical approach would provide additional power (Geng et al., 2012; Sadeghi et al., 2013). Increased sampling (e.g., 4-5 data points per participant rather than 2-3) could increase power to detect significant brain–behavior associations, thus our findings represent a conservative estimate of the association between splenium development and language performance.
In conclusion, the current study downward extends a growing body of literature implicating the splenium in language, providing new evidence that its development over the first years of life is important for the early emergence of spoken of language. The findings further highlight the importance of conducting longitudinal analyses to study developmental phenomenon.
Supplementary Material
Research Highlights.
The current study examines the development of white matter fiber pathways at 6, 12, and 24 months in relation to language at 24 months.
Results showed that infants with the greatest change over time in development of the splenium of the corpus callosum had superior language production, representing the first time a direct association between language and the splenium has been documented in infancy.
These findings illustrate the power of conducting longitudinal analyses to study developmental phenomena in infants and provide support for the conjecture that domain-general function in heteromodal regions contributes to cortical specialization.
Acknowledgements
The authors thank the IBIS children and their families for their ongoing participation in this longitudinal study.
This research was supported by grants awarded to Dr. Piven from NIH/National Institute of Child Health and Development (NICHD) (Autism Center of Excellence, R01 HD055741 and HD055741-S1; Intellectual and Developmental Disabilities Research Center, P30 HD03110 and T32 HD40127), Autism Speaks, and the Simons Foundation (SFARI Grant 140209). Dr. Swanson was supported by a National Research Service Award (T32-HD40127) from NICHD. Dr. Wolff was supported by a grant from the National Institute of Mental Health (K01-101653). Further support was provided by the National Alliance for Medical Image Computing, U54 EB005149 (PI Ron Kikinis) and NIH/National Institute of Mental Health and National Institute on Drug Abuse P01 DA022446 (PI Josephine Johns).
References
- Aeby A, De Tiège X, Creuzil M, David P, Balériaux D, Van Overmeire B, Baleriaux D. Language development at 2 years is correlated to brain microstructure in the left superior temporal gyrus at term equivalent age: a diffusion tensor imaging study. NeuroImage. 2013;78:145–151. doi: 10.1016/j.neuroimage.2013.03.076. doi:10.1016/j.neuroimage.2013.03.076. [DOI] [PubMed] [Google Scholar]
- Ashtari M. Anatomy and functional role of the inferior longitudinal fasciculus: a search that has just begun. Developmental Medicine & Child Neurology. 2012;54(1):6–7. doi: 10.1111/j.1469-8749.2011.04122.x. doi:10.1111/j.1469-8749.2011.04122.x. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance, Series B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. doi:10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. Diffusion MRI. In: Johansen-Berg H, Behrens EJT, editors. Diffusion MRI. Elsevier; 2009. pp. 105–126. doi:10.1016/B978-0-12-374709-9.00006-7. [Google Scholar]
- Breier JI, Hasan KM, Zhang W, Men D, Papanicolaou AC. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR. American Journal of Neuroradiology. 2008;29(3):483–7. doi: 10.3174/ajnr.A0846. doi:10.3174/ajnr.A0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57(1):8–16. doi: 10.1002/ana.20319. doi:10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44(8):953–961. doi: 10.1016/j.cortex.2008.04.002. doi:10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Colnat-Coulbois S, Mok K, Klein D, Pénicaud S, Tanriverdi T, Olivier A. Tractography of the amygdala and hippocampus: anatomical study and application to selective amygdalohippocampectomy. Journal of Neurosurgery. 2010;113(6):1135–43. doi: 10.3171/2010.3.JNS091832. doi:10.3171/2010.3.JNS091832. [DOI] [PubMed] [Google Scholar]
- Colombo J, Shaddy DJ, Richman WA, Maikranz JM, Blaga OM. The developmental course of habituation in infancy and preschool outcome. Infancy. 2004;5(1):1–38. [Google Scholar]
- Damaraju E, Caprihan A, Lowe JR, Allen EA, Calhoun VD, Phillips JP. Functional connectivity in the developing brain: a longitudinal study from 4 to 9months of age. NeuroImage. 2014;84:169–80. doi: 10.1016/j.neuroimage.2013.08.038. doi:10.1016/j.neuroimage.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Baillet S. A phonological representation in the infant brain. Neuroreport. 1998;9(8):1885–1888. doi: 10.1097/00001756-199806010-00040. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science (New York, N.Y.) 2002;298(5600):2013–2015. doi: 10.1126/science.1077066. doi:10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J, Mériaux S, Roche A, Sigman M, Dehaene S. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proceedings of the National Academy of Sciences. 2006;103(38):14240–14245. doi: 10.1073/pnas.0606302103. doi:10.1073/pnas.0606302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Pena M. Electrophysiological evidence for automatic phonetic processing in neonates. Neuroreport. 2001;12(14):3155–3158. doi: 10.1097/00001756-200110080-00034. [DOI] [PubMed] [Google Scholar]
- Deoni SCL, O’Muircheartaigh J, Elison JT, Walker L, Doernberg E, Waskiewicz N, Jumbe NL. White matter maturation profiles through early childhood predict general cognitive ability. Brain Structure & Function. 2014 doi: 10.1007/s00429-014-0947-x. doi:10.1007/s00429-014-0947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AS, Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135(12):3529–3550. doi: 10.1093/brain/aws222. doi:10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, Piven J. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. American Journal of Psychiatry. 2013;170(8):899–908. doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elison JT, Wolff JJ, Heimer DC, Paterson SJ, Gu H, Hazlett HC, Wright F. Frontolimbic neural circuitry at 6 months predicts individual differences in joint attention at 9 months. Developmental Science. 2013;16(2):186–197. doi: 10.1111/desc.12015. doi:10.1111/desc.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzinfar M, Li Y, Verde AR, Oguz I, Gerig G, Styner MA. DTI quality control assessment via error estimation from monte carlo simulations. Proceedings - Society of Photo-Optical Instrumentation Engineers. 2013;8669:1667549. doi: 10.1117/12.2006925. doi:10.1117/12.2006925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman HM, Campbell TF, Kurs-Lasky M, Rockette HE, Dale PS, Colborn DK, Paradise JL. Concurrent and predictive validity of parent reports of child language at ages 2 and 3 years. Child Development. 2005 doi: 10.1111/j.1467-8624.2005.00882.x. doi:10.1111/j.1467-8624.2005.00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman HM, Lee ES, Yeatman JD, Yeom KW. Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia. 2012;50(14):3348–3362. doi: 10.1016/j.neuropsychologia.2012.10.014. doi:10.1016/j.neuropsychologia.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenson L, Bates E, Dale PS, Marchman VA, Reznick JS, Thal DJ. MacArthur-Bates communicative development inventories. 2007 [Google Scholar]
- Frick JE, Colombo J, Saxon TF. Individual and developmental differences in disengagement of fixation in early infancy. Child Development. 1999;70(3):537–548. doi: 10.1111/1467-8624.00039. [DOI] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Shen D, Smith JK, Zhu H, Lin W. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cerebral Cortex. 2013;23(3):594–603. doi: 10.1093/cercor/bhs043. doi:10.1093/cercor/bhs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, Lin W. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(16):6790–5. doi: 10.1073/pnas.0811221106. doi:10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Gouttard S, Sharma A, Gu H, Styner M, Lin W, Gilmore JH. Quantitative tract-based white matter development from birth to age 2 years. NeuroImage. 2012;61(3):542–557. doi: 10.1016/j.neuroimage.2012.03.057. doi:10.1016/j.neuroimage.2012.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CB, Fletcher PT, Gilmore JH, Gerig G. Group analysis of DTI fiber tract statistics with application to neurodevelopment. NeuroImage. 2009;45(1):S133–S142. doi: 10.1016/j.neuroimage.2008.10.060. doi:10.1016/j.neuroimage.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouttard S, Styner M, Prastawa M, Piven J, Gerig G. Assessment of reliability of multi-site neuroimaging via traveling phantom study. Medical Image Computing and Computer-Assisted Intervention–MICCAI 2008. 2008;5242:263–270. doi: 10.1007/978-3-540-85990-1_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttorm TK, Leppanen PHT, Richardson U, Lyytinen H. Event-related potentials and consonant differentiation in newborns with familial risk for dyslexia. Journal of Learning Disabilities. 2001;34(6):534–544. doi: 10.1177/002221940103400606. doi:10.1177/002221940103400606. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Kamali A, Iftikhar A, Kramer LA, Papanicolaou AC, Fletcher JM, Ewing-Cobbs L. Diffusion tensor tractography quantification of the human corpus callosum fiber pathways across the lifespan. Brain Research. 2009;1249:91–100. doi: 10.1016/j.brainres.2008.10.026. doi:10.1016/j.brainres.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(14):5948–53. doi: 10.1073/pnas.0812035106. doi:10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoye L, Saint-Martin C, Cosnard G, Lee S-K, Kim J, Nassogne M-C, Hua K. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. NeuroImage. 2006;29(2):493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1-2):67–99. doi: 10.1016/j.cognition.2003.10.011. doi:10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews. Neuroscience. 2007;8(5):393–402. doi: 10.1038/nrn2113. doi:10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Statistics in Medicine. 1990;9(7):811–818. doi: 10.1002/sim.4780090710. doi:10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32(3):989–994. doi: 10.1016/j.neuroimage.2006.05.044. doi:10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hüppi PS, Dubois J. Diffusion tensor imaging of brain development. Seminars in Fetal and Neonatal Medicine. 2006;11(6):489–497. doi: 10.1016/j.siny.2006.07.006. doi:10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in infants: elements of an interactive specialization framework. Child Development. 2000;71(1):75–81. doi: 10.1111/1467-8624.00120. doi:http://dx.doi.org/10.1111/1467-8624.00120. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Interactive specialization: a domain-general framework for human functional brain development? Developmental Cognitive Neuroscience. 2011;1(1):7–21. doi: 10.1016/j.dcn.2010.07.003. doi:10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Foreword: development is not about studying children: the importance of longitudinal approaches. American Journal on Intellectual and Developmental Disabilities. 2012;117(2):87–9. doi: 10.1352/1944-7558-117.2.87. doi:10.1352/1944-7558-117.2.87. [DOI] [PubMed] [Google Scholar]
- Keehn B, Müller R-A, Townsend J. Atypical attentional networks and the emergence of autism. Neuroscience and Biobehavioral Reviews. 2013;37(2):164–83. doi: 10.1016/j.neubiorev.2012.11.014. doi:10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s Loop of the optic radiation. AJNR Am. J. Neuroradiol. 2004;25(5):677–691. Retrieved from http://www.ajnr.org/content/25/5/677.full. [PMC free article] [PubMed] [Google Scholar]
- Koegel RL, Shirotova L, Koegel LK. Brief report: using individualized orienting cues to facilitate first-word acquisition in non-responders with autism. Journal of Autism and Developmental Disorders. 2009;39(11):1587–92. doi: 10.1007/s10803-009-0765-9. doi:10.1007/s10803-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. Early language acquisition: cracking the speech code. Nature Reviews Neuroscience. 2004;5(11):831–843. doi: 10.1038/nrn1533. doi:10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Brain mechanisms in early language acquisition. Neuron. 2010;67(5):713–727. doi: 10.1016/j.neuron.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang Y, Gerig G, Gouttard S, Tao R, Fletcher T, Styner M. Quality control of diffusion weighted images. SPIE Medical Imaging. 2010:76280J–76280J–9. doi: 10.1117/12.844748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(47):20069–74. doi: 10.1073/pnas.0905314106. doi:10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PCM, Nagae-Poetscher LM. MRI atlas of human white matter. Am Soc Neuroradiology. 2005 Retrieved from http://www.ajnr.org/content/27/6/1384.2.short.
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–539. doi: 10.1016/j.neuron.2006.08.012. doi:10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Mullen EME. Mullen scales of early learning. AGS; Circle Pines, MN: 1995. [Google Scholar]
- Mundy P, Block J, Delgado C, Pomares Y, Van Hecke AV, Parlade MV. Individual differences and the development of joint attention in infancy. Child Development. 2007;78(3):938–54. doi: 10.1111/j.1467-8624.2007.01042.x. doi:10.1111/j.1467-8624.2007.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi S, Mukherjee P, Ghajar J, Mccandliss BD, Augustinack JC, Athinoula A. Individual differences in distinct components of attention are linked to anatomical variations in distinct white matter tracts. Frontiers in Neuroanatomy. 2010;4(February):2. doi: 10.3389/neuro.05.002.2010. doi:10.3389/neuro.05.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northam GB, Liégeois F, Tournier JD, Croft LJ, Johns PN, Chong WK, Baldeweg T. Interhemispheric temporal lobe connectivity predicts language impairment in adolescents born preterm. Brain. 2012;135(12):3781–3798. doi: 10.1093/brain/aws276. doi:10.1093/brain/aws276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguz I, Farzinfar M, Matsui J, Budin F, Liu Z, Gerig G, Styner MA. DTIPrep: Quality control of diffusion-weighted images. Frontiers in Neuroinformatics. 2014;8:4. doi: 10.3389/fninf.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Choe M, Flax J, Grant PE, Benasich AA. Associations between the size of the amygdala in infancy and language abilities during the preschool years in normally developing children. NeuroImage. 2010;49(3):2791–2799. doi: 10.1016/j.neuroimage.2009.10.029. doi:http://dx.doi.org/10.1016/j.neuroimage.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML. Posterior cingulate cortex: adapting behavior to a changing world. Trends in Cognitive Sciences. 2011;15(4):143–51. doi: 10.1016/j.tics.2011.02.002. doi:10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. doi:10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Pietrasanta M, Restani L, Caleo M. The corpus callosum and the visual cortex: plasticity is a game for two. Neural Plasticity. 2012;2012:838672. doi: 10.1155/2012/838672. doi:10.1155/2012/838672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel D, Hickok G. Towards a new functional anatomy of language. Cognition. 2004;92(1-2):1–12. doi: 10.1016/j.cognition.2003.11.001. doi:10.1016/j.cognition.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Pogribna U, Burson K, Lasky RE, Narayana PA, Evans PW, Parikh NA. Role of diffusion tensor imaging as an independent predictor of cognitive and language development in extremely low-birth-weight infants. AJNR.American Journal of Neuroradiology. 2014;35(4):790–796. doi: 10.3174/ajnr.A3725. doi:10.3174/ajnr.A3725; 10.3174/ajnr.A3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett JR, Kandala S, Hoertel S, Snyder AZ, Elison JT, Nishino T, Piven J. Accurate age classification of 6 and 12 month-old infants based on resting-state functional connectivity magnetic resonance imaging data. Developmental Cognitive Neuroscience. 2015 doi: 10.1016/j.dcn.2015.01.003. doi:10.1016/j.dcn.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam MC, Steven MS, Doron KW, Riggall AC, Gazzaniga MS. Cortical projection topography of the human splenium: hemispheric asymmetry and individual differences. Journal of Cognitive Neuroscience. 2010;22(8):1662–1669. doi: 10.1162/jocn.2009.21290. [DOI] [PubMed] [Google Scholar]
- Redcay E, Haist F, Courchesne E. Functional neuroimaging of speech perception during a pivotal period in language acquisition. Developmental Science. 2008;11(2):237–252. doi: 10.1111/j.1467-7687.2008.00674.x. doi:10.1111/j.1467-7687.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- Sadeghi N, Prastawa M, Fletcher PT, Wolff JJ, Gilmore JH, Gerig G. Regional characterization of longitudinal DT-MRI to study white matter maturation of the early developing brain. NeuroImage. 2013;68:236–47. doi: 10.1016/j.neuroimage.2012.11.040. doi:10.1016/j.neuroimage.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, Weiller C. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. doi:10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. doi:10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(14):3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. doi:10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz S, Vouloumanos A, Bennett RH, Pelphrey K. Neural specialization for speech in the first months of life. Developmental Science. 2014;17(5):766–74. doi: 10.1111/desc.12151. doi:10.1111/desc.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales (VABS-II) American Guidance Service; Circle Pines, MN: 2005. [Google Scholar]
- Vandermosten M, Poelmans H, Sunaert S, Ghesquière P, Wouters J. White matter lateralization and interhemispheric coherence to auditory modulations in normal reading and dyslexic adults. Neuropsychologia. 2013;51(11):2087–99. doi: 10.1016/j.neuropsychologia.2013.07.008. doi:10.1016/j.neuropsychologia.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nature Reviews. Neuroscience. 2009;10(11):792–802. doi: 10.1038/nrn2733. doi:10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Verde AR, Budin F, Berger J-B, Gupta A, Farzinfar M, Kaiser A, Hazlett HC. UNC-Utah NA-MIC framework for DTI fiber tract analysis. Frontiers in Neuroinformatics. 2013;7(51) doi: 10.3389/fninf.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouloumanos A, Curtin S. Foundational tuning: how infants’ attention to speech predicts language development. Cognitive Science. 2014;38(8):1675–86. doi: 10.1111/cogs.12128. doi:10.1111/cogs.12128. [DOI] [PubMed] [Google Scholar]
- Warren JE, Wise RJS, Warren JD. Sounds do-able: auditory-motor transformations and the posterior temporal plane. Trends in Neurosciences. 2005;28(12):636–43. doi: 10.1016/j.tins.2005.09.010. doi:10.1016/j.tins.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. a postmortem morphological study. Brain: A Journal of Neurology. 1989;112(3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Gerig G, Lewis JD, Soda T, Styner MA, Vachet C, Piven J. Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain. 2015 doi: 10.1093/brain/awv118. awv118–. doi:10.1093/brain/awv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Piven J. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. American Journal of Psychiatry. 2012;169(6):589–600. doi: 10.1176/appi.ajp.2011.11091447. doi:10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.