Abstract
OBJECTIVE
To determine the feasibility of a detailed pain sensitivity assessment using body wide musculoskeletal tender points (TPs) in women with different types of chronic pelvic pain (CPP) and compare phenotypic differences.
METHODS
Seventy women with CPP and 35 healthy women underwent musculoskeletal evaluation of TPs in the pelvic floor, abdomen, groin, inner thigh, and all 18 fibromyalgia (FM) TPs. Subjects scored elicited pain on a numeric rating scale (NRS). Tender point pain scores were used for intergroup comparison and intragroup correlation.
RESULTS
Women with CPP were grouped as having either bladder pain syndrome (BPS, n=24) or myofascial pelvic pain (MPP, n=11) singularly or both concomitantly (BPS+MPP, n=35). Tender point pain scores for all evaluations were higher in women with CPP compared to healthy women (p<0.001). Women with BPS+MPP had elevated TP pain for each evaluation compared to women with BPS alone. Pelvic floor and FM TP scores correlated strongly in the MPP group, moderately in the BPS+MPP group, and weakly in the BPS alone group. While some moderate and strong correlations between different body locations were present in all three groups, only the BPS+MPP group showed moderate to strong correlations between all body TPs.
CONCLUSIONS
Detailed musculoskeletal evaluation of women with CPP is feasible and well tolerated. Careful phenotyping differentiated BPS, MPP, and BPS+MPP groups. Attending to the differences between these groups clinically may lead to more effective treatment strategies and improved outcomes for patients with CPP.
Keywords: bladder pain syndrome, chronic pelvic pain, tender points, myofascial pain, interstitial cystitis
INTRODUCTION
Chronic pelvic pain (CPP) affects as many as 15% of women in the United States at some time in their lives, with annual medical costs estimated at $2.8 billion (U.S.) in 1996.1,2 CPP is characterized as persistent pain in the pelvic region perceived to arise from the gynecologic, urologic, gastrointestinal, and/or musculoskeletal systems. Common and frequently coexisting CPP conditions include myofascial pelvic pain (MPP) and bladder pain syndrome (BPS). Typically presenting with symptoms of dyspareunia or lower abdominal pelvic pain, women with MPP have myofascial tender points (TPs) in the muscles of the pelvic floor upon physical examination. BPS is a chronic idiopathic visceral pain syndrome with symptoms of chronic (>6 months) pelvic pain featuring pressure or discomfort related to urinary bladder fullness and persistent urinary urgency or frequency.3
The high prevalence (78–87%) of concomitant myofascial pain, frequently extending beyond the anatomical boundaries of the pelvis in women with BPS,4,5 suggests these two disorders may share a common pathophysiology. Additionally, almost a quarter of women with CPP have musculoskeletal abnormalities,6 yet comprehensive musculoskeletal examination is seldom considered during medical evaluation.7,8 One might conceive several ways for muscle to become painful in patients with BPS. Muscle involvement could occur in response to bladder inflammation in a type of visceral or cross-organ somatic co-sensitization.9 Muscle could also constitute the primary source of pain and entrain changes in bladder function.10,11 Finally, both muscle and bladder could reflect a third deeper pathophysiologic change, such as a neural network alteration or an immunologic process. These and other mechanisms could also occur in combination or differ across individuals. Consensus guidelines for the management of CPP created in 2005 highlighted the need for a better understanding of myofascial dysfunction.12 Although body-pain mapping based on pain symptom questionnaires has revealed multiple complaints in other body locations in women with BPS,13 there is clear need to establish an objective, standardized musculoskeletal evaluation in women with CPP.
Our overarching hypothesis is that detailed examination of musculoskeletal pain sensitivity in different anatomic regions in patients with CPP can help to classify patients into potentially meaningful distinct subgroups. For example, involvement of body areas far removed from the pelvis suggests a generalized abnormality of central nervous system processing, whereas more restricted involvement of regions near the pelvis might imply more local changes. The present study aimed to 1) determine the feasibility of a detailed pain sensitivity assessment using musculoskeletal TPs in various body locations in women with CPP; and 2) compare possible phenotypic differences and correlations between body locations in patients with MPP or BPS to healthy controls (HC) to generate hypotheses regarding their pathophysiology.
MATERIALS AND METHODS
This study was approved by the University Hospitals Case Medical Center (UHCMC) Institutional Review Board (Cleveland, Ohio). Women (18–78 years) with CPP and healthy controls consented for participation in this study (2011/02 – 2014/01), part of the Interstitial Cystitis: Elucidation of Psychophysiologic and Autonomic Characteristics study (ICEPAC; ClinicalTrials.gov Identifier: NCT01616992). ICEPAC is a comprehensive, interdisciplinary approach to evaluation of women with MPP and BPS. The multidisciplinary team conducting the study consisted of a psychologist, urologist, gynecologist, urogynecologist, anesthesiologist, and neurologist. Specific details and methods of the study have been published.14 In summary, women with BPS, MPP, BPS+MPP, and healthy controls underwent detailed examination to elucidate the role of central and peripheral nervous system processing in CPP. The evaluation consisted of a structured neurologic examination of limbs and pelvis, tender point examination, autonomic testing, electrogastrography, and assessment of comorbid functional dysautonomias, assessments of stress and response to it, trauma history, and general psychological function. All subjects also underwent detailed assessment of pelvic pain symptoms, voiding patterns, gynecologic history, prior and current treatments, and present comorbidities. The primary aim of the work presented herein was to determine the feasibility and utility of a detailed pain sensitivity assessment using body wide musculoskeletal TPs examination in women with different types of CPP and compare phenotypic differences of whole body TP assessment among the groups. The primary outcome was a TP pain assessment based on a standardized numeric rating scale (NRS) pain score, with 0 meaning “pressure only” and 10 defined as “worst pain imaginable”.
Subjects were recruited from clinical practices and pelvic pain clinic. Additionally, we advertised the study through the Interstitial Cystitis Association and Interstitial Cystitis Network. Subjects were enrolled and categorized into one of the groups according to study definitions for each group when our screening evaluation confirmed the inclusion criteria. Subject screening included a pelvic floor examination and assessment of bladder pain symptoms. With the exception of a few subjects recruited from an examiner’s own clinic, examiners were unaware of pelvic pain phenotype prior to examination with the results of examinations leading to subject grouping. Inclusion criteria for study and subject classification were based on recommendations from our advisory board and evidence from available literature. Currently, there is no accepted definition or standardized means of examination for the presence of MPP.15 We included subjects into the MPP group when they reported at least 3 months of non-cyclic CPP unrelated to bladder filling or emptying and a minimum NRS pain score of at least 4 out of 10 using 2kg pressure applied with the index finger onto at least 2 of 5 examined pelvic floor TPs. Pelvic floor TPs included bilateral levator ani (puborectalis) and obturator internus muscles and a single midline perineal assessment (Figure 1). We used the modified assessment validated by Zolnoun et al.11 Initially, a pelvic muscle algometer was used for training purposes under direct supervision of Dr. Zolnoun. This algometer has a pressure sensor capable of measuring a wide spectrum of force and allows direct and isolated palpation of pelvic floor musculature. Investigators practiced placing the desired pressure on the pelvic musculature using paid, healthy volunteers. After training, the examination of subjects was performed without using the pressure sensor. Pressure was standardized against an algometer before each examination. Subjects were included in the BPS group when they complained of at least 6 months of urgency, frequency, and bladder pain clearly linked to bladder filling and emptying in accordance with 2008 European Society for the Study of Painful Bladder Syndrome recommendations.3 Pelvic TPs played no role in this definition. Since a large proportion of participants met criteria for both chronic pelvic pain disorders, they were classified as BPS+MPP. Healthy controls were included when they had no history, symptoms or signs of fibromyalgia, chronic fatigue syndrome, BPS, MPP, CPP, migraine headache, or any other putative BPS comorbid disorders and be age matched to within ±3 years of a BPS+MPP subject. The exclusion criteria are presented in Table 1.
Figure 1.
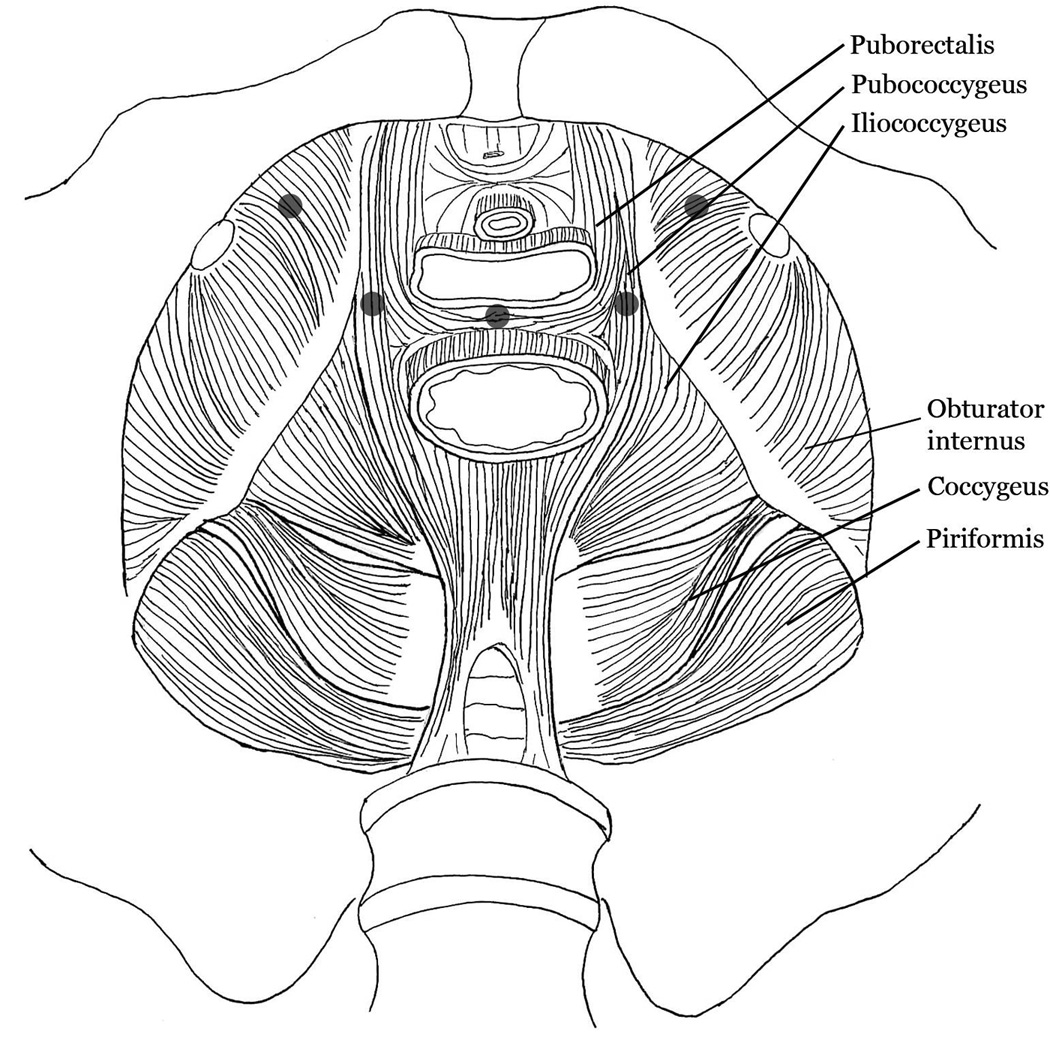
Image depicting the pelvic floor region as viewed from above with anterior being upward. The 5 shaded dots demonstrate the site of pressure application (2Kg with the examiner’s gloved index finger) in both oburator internus muscles, both levator ani muscles, and in the perineal body.
Table 1.
Exclusion criteria for study participation
| Exclusion Criteria | |
|---|---|
| 1. | Any major surgical intervention with general anesthesia, pelvic surgery within the previous 120 days |
| 2. | Drug/medical device or non-invasive treatment initiation (such as bladder instillation or pelvic floor therapy) within 30 days |
| 3. | Intravesical therapy, botox or hydrodistention within 90 days |
| 4. | Previous augmentation cystoplasty, cystectomy or cytolysis, neurectomy (i.e., hypogastric nerve plexus ablation), implanted neural stimulator in active use |
| 5. | Pelvic, bladder neoplasm/infection, recurrent cystitis, hematuria not related to BPS |
| 6. | Evidence of unstable chronic medical conditions (e.g. liver impairment, poorly controlled cardiovascular, respiratory or endocrine function, uncontrolled psychiatric illness) |
| 7. | Neuropathy, central nervous system disorder (e.g., Parkinson’s Disease, Alzheimer’s, MS, stroke, etc) |
| 8. | Use of hormones (except insulin, thyroid replacement or oral contraceptives) |
| 9. | Current substance abuse, > 10 alcoholic beverages per week |
| 10. | Currently attempting to become pregnant, pregnant (confirmed by pregnancy test), breast feeding |
| 11. | Any condition that the physician deems would interfere with the subject’s ability to participate or which would clearly confound interpretation of study results |
| Additional Exclusion Criteria for Healthy Subjects | |
| 12. | A diagnosis of BPS, MPP or chronic pelvic discomfort, or any chronic pain disorder of any type. |
| 13. | Fibromyalgia or Chronic Fatigue Syndrome. |
| 14. | Dysautonomias (e.g. Migraine Headaches, Complex Regional Pain Syndrome, Cyclic Vomiting) or a history of Psychiatric Disorders (e.g. Post-Traumatic Stress Disorder, Panic Disorder) |
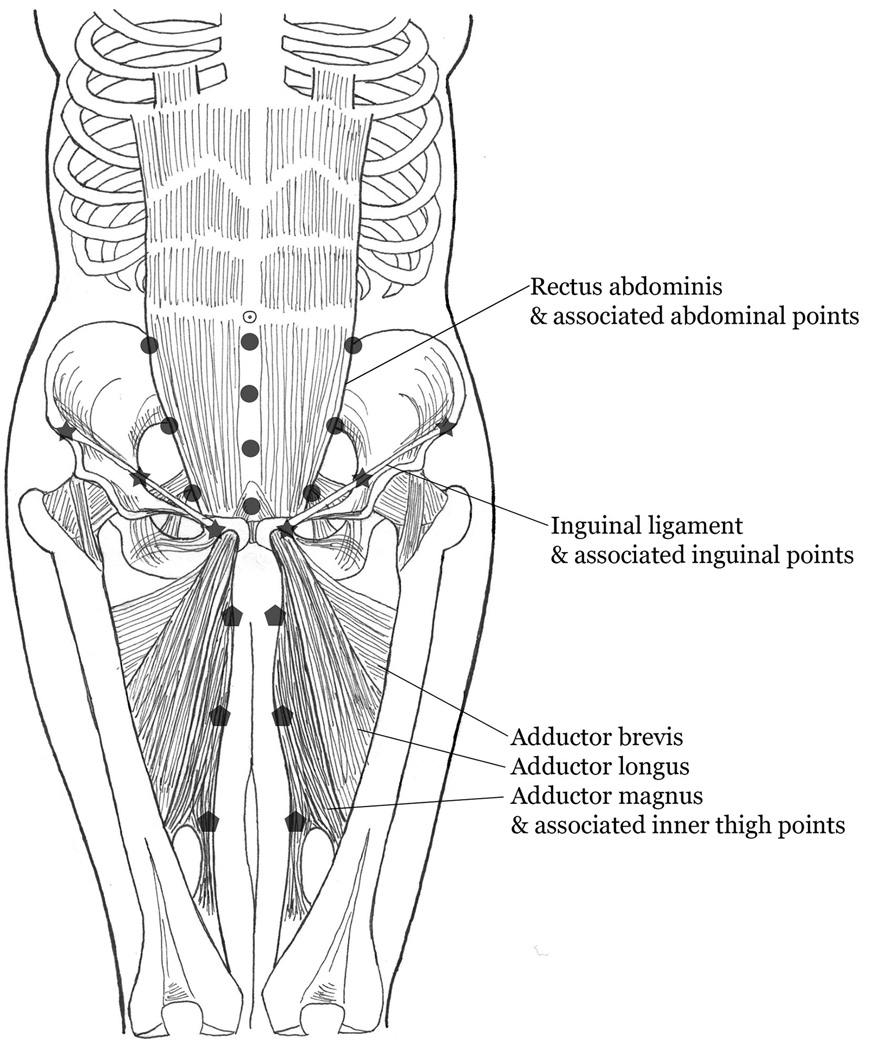
Additional pelvic adjacent and body-wide TP pain assessments were conducted using specific pressures applied with an algometer, except where indicated, so that pressure could be directly monitored during assessments. Abdominal exam (10 TPs) included 3kg pressure applied to rectus muscle midline (raphe) and lateral border muscle points (Figure 2). Palpation of the rectus muscles required the subject to actively lift their head while in the supine position. Inguinal ligaments (6 TPs total) were palpated bilaterally (3kg) along their length including origin, midpoint, and insertion (Figure 2).16 Inner thigh evaluation (6 TPs total) included bilateral (3kg) pain assessment in distal, mid-, and proximal inner thigh (Figure 2). Eighteen Fibromyalgia (FM) TPs, as described by the American College of Rheumatology,17 were palpated with the thumb using 4kg of pressure. While exerted pressure during the examination differed between body locations, we chose to apply the amount of pressure according to protocols that had been already published in the literature (4kg for FM, 3 kg for abdominal and inguinal TPs).16,17 Our advisory board recommended a 2kg pressure for pelvic muscle evaluation as there was a concern of examination tolerance in the context of myofascial pain.
Figure 2.
Image depicting the abdominal (shaded circleres), inguinal (shaded stars) and thigh (shaded pentagons) tender point palpation sites. Pressure (3 Kg) was applied at each site using an algometer.
Data were analyzed as follows. Age and BMI were compared using one-way ANOVA followed by Tukey’s multiple comparisons test (GraphPad Prism v6.1, GraphPad Software, La Jolla, California). A final “TP pain score” was defined for each examination as the average of all TPs for that subject, i.e. averages of pelvic floor, abdominal, inguinal, thigh or FM TPs. Group mean TP scores were compared using the Mann-Whitney U test and pairwise group TP score correlations and 95% confidence intervals between regions were calculated in R18 using the TP score and the logarithm of (TP score + 1). Pairwise group TP score correlations, using the TP score and the logarithm of (TP score + 1) were also calculated. Since the BPS group was significantly older than the other 3 groups, we utilized a generalized linear model (GEE) to evaluate age as a significant predictor of the pain TP scores in all five locations (pelvic floor, FM, groin, inner thighs, and abdomen). Correlations were considered to be weak, moderate and strong with r <0.3, 0.3 < r > 0.6, and r >0.6, respectively. After screening, all subjects completed the Multidimensional Pain Inventory (MPI),19 a comprehensive measure of pain, function and coping.20 This questionnaire served as a covariate in the analysis to determine the impact of higher pain levels on pain correlations across body regions.
RESULTS
Following a screening exam for subject distribution based on pelvic pain, 105 women were grouped as follows: 11 MPP, 24 BPS, 35 BPS+MPP, and 35 HC. As shown in Table 2, women with BPS alone tended to be older (F(3, 101) = 4.513, p<0.05) than other groups, whereas BMI was similar (F(3,101) = 2.012, NS).
Table 2.
Demographics
| BPS (n=24) |
MPP (n=11) |
BPS+MPP (n=35) |
HC (n=35) |
|
|---|---|---|---|---|
| Age, years | 48.5±14.1* | 32.8±9.9 | 38.6±12.8 | 38.0±15.0 |
| BMI | 28.0±8.1 | 31.9±7.5 | 31.3±9.4 | 26.9±8.5 |
| Race, n (%) | ||||
| Caucasian | 22 (92) | 7 (64) | 25 (71) | 25 (71) |
| African-American | 1 (4) | 4 (36) | 9 (26) | 5 (14) |
| Other | 1 (4) | 0 | 1 (3) | 5 (14) |
Values are mean ± SD where applicable.
BPS group is older than all other groups (p<0.05)
Mean TP scores in healthy control women were lower (Figure 3, p<0.001, all comparisons) than in CPP subjects, whereas TP scores did not differ between women with MPP and those with BPS+MPP. By definition, women with MPP (MPP or BPS+MPP) had high pelvic floor TP pain, leading to significantly higher mean pelvic floor TP scores (p<0.001) than did women with BPS alone. FM TP scores were 2.2±1.8 (SD) in subjects with BPS alone as compared to 3.7±2.4 in BPS+MPP (p<0.05) and 3.9±2.4 in MPP (p=0.05) (Appendix A). While women with BPS or MPP alone had elevated inguinal, inner thigh, and abdominal TP scores compared to control, there was no difference between these two pain groups (p>0.05). The combined group (BPS+MPP) had significantly more pain in every location than did subjects with BPS alone. In the GEE model, age was not in any model for TP scores in the five locations.
Figure 3.
Image depicting mean tender point scores in different body locations for all study groups.
Pairwise correlations and 95% confidence intervals of pelvic floor TP scores and those of FM, inguinal, inner thigh, and abdomen are shown in Table 3. Data revealed a strong positive correlation (r= 0.68, 95% CI 0.13 – 0.91) between pelvic floor and FM TP score for subjects with MPP compared to a moderate (r= 0.36, 95% CI 0.03 – 0.61) correlation for subjects with BPS+MPP. No correlation between pelvic floor and FM was found in subjects with BPS without MPP (r= −0.18, 95% CI −0.54 – 0.24). Other correlations with the pelvic floor in BPS subjects were weak as well. Moderate positive correlations between pelvic floor TP scores and other body regions were found in women with BPS+MPP. Owing to the smaller sample size, the correlations for MPP alone have much larger confidence intervals. Including MPI scores in the analysis as a covariate did not alter the correlations, suggesting that these did not simply reflect overall higher pain levels in women with BPS+MPP.
Table 3.
Pearson correlation coefficient and 95% confidence intervals of group mean tender point pain scores between the Pelvic Floor and other body areas.
| FM | Inguinal | Inner Thigh | Abdomen | |
|---|---|---|---|---|
| BPS | −0.18 | 0.11 | −0.01 | 0.06 |
| (−0.54, 0.24) | (−0.31, 0.49) | (−0.41, 0.40) | (−0.36, 0.45) | |
| MPP | 0.68 | 0.17 | 0.56 | 0.12 |
| (0.13, 0.91) | (−0.48, 0.70) | (−0.06, 0.87) | (−0.52, 0.67) | |
| BPS+MPP | 0.36 | 0.48 | 0.44 | 0.50 |
| (0.03, 0.61) | (0.18, 0.70) | (0.12, 0.67) | (0.20, 0.71) |
Pairwise correlations of TP scores for all other body regions are shown in Table 4. Data revealed moderate to strong positive correlations for TP scores between all body regions in the BPS+MPP group. The highest positive correlations were found between inguinal and abdominal TP regions in all three CPP groups.
Table 4.
Pearson correlation coefficient and 95% confidence intervals of group mean tender point pain scores among other body regions
| FM & Inguinal |
FM & Inner Thigh |
FM & Abdomen |
Inguinal & Inner Thigh |
Inguinal & Abdomen |
Inner Thigh & Abdomen |
|
|---|---|---|---|---|---|---|
| BPS | 0.34 | 0.60 | 0.59 | 0.41 | 0.71 | 0.62 |
| (−0.07, 0.65) | (0.26, 0.81) | (0.24, 0.80) | (0.01, 0.70) | (0.43, 0.86) | (0.29, 0.82) | |
| MPP | 0.64 | 0.63 | 0.50 | 0.47 | 0.88 | 0.58 |
| (−0.06, 0.89) | (0.05, 0.89) | (−0.14, 0.85) | (−0.18, 0.84) | (0.60, 0.97) | (−0.03, 0.88) | |
| BPS+MPP | 0.57 | 0.67 | 0.47 | 0.55 | 0.70 | 0.61 |
| (0.30, 0.76) | (0.43, 0.82) | (0.17, 0.70) | (0.26, 0.75) | (0.48, 0.84) | (0.35, 0.78) |
DISCUSSION
Several findings from this study emerged. First, we were able to complete a structured musculoskeletal examination of TPs in various body locations in all study subjects. This examination was feasible, tolerated by the patients, and provided a measure of deep tissue pain sensitivity in women with CPP. Second, women with CPP had significantly higher TP pain scores on musculoskeletal evaluation for all tested body regions when compared to HC. Third, careful phenotyping of women with CPP demonstrated differences in CPP subtypes, including subjects with BPS alone, MPP alone, or both BPS and MPP together. Greater pain levels occurred in all body regions when BPS and MPP were present concomitantly. While some moderate and strong correlations between different body location TPs were seen in all groups, only this BPS+MPP group consistently showed moderate to strong correlations between all body TPs, suggesting central sensitization as a more prominent feature in this group than in those with BPS alone.
This study reports on tender, not trigger, point assessment in CPP. Tender points are defined as areas of tenderness occurring in the muscle, muscle-tendon junction, bursa, or fat pad.21 As opposed to tender points, myofascial trigger points are characterized by point tenderness on a taut muscle band, local twitch response, referred pain in a different location reproduced by palpation of the trigger point, and restricted range of motion.22,23 MPP is not well defined in the existing literature. The frequent use of the terms “tender point” and “trigger point” interchangeably further complicates the definition of MPP. Given the prevalence of MPP in women, it would seem important to develop diagnostic criteria for this disorder. Based on available literature and our prior work,11 we propose that MPP criteria include the following: 1) spontaneous chronic lower abdominal and/or pelvic pain for 3 months or more; 2) pain worsened by stretching or pressure upon the pelvic muscles (e.g. insertion of a tampon, a speculum, or intercourse); 3) tenderness upon palpation (2 Kg of pressure; “moderate pressure” in a clinical setting) of at least 2 well identified pelvic floor muscles with a NRS of 4 or more on a zero to ten scale. The pelvic floor musculoskeletal examination did not extend the duration of the standard pelvic examination by more than a minute or two in our study. The presence or absence of another CPP disorder such as vulvodynia, endometriosis or BPS does not conceptually influence the diagnosis of MPP, which may or may not be related to these other disorders. This definition has the advantage of simplicity and provides a relatively uniform basis for comparing patients with additional CPP diagnoses independently of one another.
When defining MPP in this way, several properties of this syndrome emerge when compared to BPS. First, MPP is associated with higher tender point pain scores in most areas of the body. Second, the correlation of pelvic floor TPs with FM TPs is moderate to strong (Table 4) and persists even when accounting for overall pain level, suggesting that a central driver for TPs may be present in MPP with or without concomitant BPS. Third, the highest TP pain scores occurred in the BPS+MPP group for inguinal, inner thigh, and abdominal TPs, but not for FM TPs. This more localized finding is consistent with a viscero-viscero-somatic hyperalgesia phenomenon where co-existing algogenic conditions in two internal organs in the same patient (bladder and muscle) enhance pain symptoms.24 Although our findings are consistent with the previously reported high prevalence of levator muscle pain in women with bladder pain,4,5 prior reports did not provide the same broad whole-body perspective by comparing TP’s in other body locations. Tripp et al implicitly considered BPS a subset of a more generalized pain disorder, but did not distinguish the subset with MPP.13 Using the symptom-based O’Leary Sant Patient Questionnaire to diagnose BPS,25 the authors found that subjects with BPS reported more pain complaints in the thighs, abdominal region, back, and the posterior surface of the head compared to healthy control subjects. Since this questionnaire did not consider MPP and the study did not involve musculoskeletal examination, the distinction between the two disorders could not be evaluated. Thus, our findings extend and refine Tripp’s concepts by including more precise phenotypic information and a clinical examination that delineates three separate pelvic pain sub-groups.
The difference in TP pain severity between BPS and MPP (+/− BPS) groups may have several explanations. Some of these differences are clearly definitional based on study defined criteria. However, the lower TP scores for the FM examination in the BPS only group and the absence of a relationship between TP in the pelvis and FM body examination suggest some fundamental differences between BPS and MPP. The development of MPP in the context of BPS may signal the onset of central sensitization that is not present in isolated BPS, or may reflect difference in susceptibility to pain. Longitudinal studies will be required to determine if such an evolution occurs or if the phenotypic distinctions remain constant throughout the life of the disorders. If the advent of MPP signals central sensitization, one would predict greater generalized hyperalgesia in this patient group. Several reports describe decreased pain threshold (increased generalized hyperalgesia) or central sensitization with altered pain control in response to stimuli in different body locations in women with CPP with unknown MPP status.26–30 Lai et al found the presence of segmental hyperalgesia (suprapubic area) in the subjects with BPS without FM tender points as compared to age matched healthy controls.27 The reduction of pain thresholds in chronic musculoskeletal (including low back) pain suggests generalized hypersensitivity.26 Thus, if myofascial pain reflects reduction in pain threshold, it may drive or reflect central hyperalgesia and altered responses to pain stimuli in women with BPS.
The cause of the body-wide TPs remains unclear. Because they occur so diffusely, one may reasonably presume dysfunction of central nervous system pathways responsible for pain modulation. However, it is possible that pelvic TPs prime other potential TPs in some way. Testing this hypothesis would require retesting whole body TPs after the pelvic TPs have resolved, for example after an effective course of pelvic floor physical therapy. At this stage, the meaning of these body-wide TPs remains unclear from a treatment perspective as well. However, our findings do point to the utility of examining patients with CPP for diffuse body TPs as that knowledge will likely affect clinical decision making.
This study has several limitations. The subgroup consisting of women with only MPP is small and important differences in this group may not be readily apparent. Given the lack of previously defined clinical criteria for MPP, the criteria proposed herein may require further refinement. Our study protocol allowed participants to continue their pain medication treatment regimen to optimize recruitment. While this approach may have impacted pain measurements, it does not differentiate the groups and represents “real life”, reproducible assessment of CPP. Examination methodologies were standardized by our study advisory board and investigators. Applied pressure to different body locations differed as we combined the protocols evaluating myofascial pain from different published protocols. Multiple investigators performed examinations and, as such, data could be subject to examiner bias. Although a single neurologist with expertise in pain quantification trained all examiners, inter-examiner reliability was not evaluated. Finally, our cross-sectional study design does not allow any inference regarding the critical questions of causation and chronology - does bladder pain lead to tender points or vice-versa, etc. Cross-sectional design also limits our assessment of true duration of pain because of recall bias. We selected the abdominal and adductor muscle points because they are muscular and therefore similar to the pelvic muscle tender points, while the inguinal points are tendinous, and therefore more similar to the fibromyalgia points which frequently occur in tendinous insertion areas. We do not know whether these are different or not from a pathophysiologic perspective.
Data presented herein underscore the importance of a broad musculoskeletal TP evaluation. Based on our study, musculoskeletal evaluation of women with CPP is feasible, well tolerated by patients, and could be performed by a general obstetrician-gynecologist or specialist when evaluating women with CPP. If co-existing myofascial pain sensitivity (a possible sign of sensitization) is detected in patients diagnosed with BPS, the provider may want to consider approaches beyond those focused on the bladder or pelvic floor, such as whole body physical therapy, exercise programs designed to promote physical reconditioning, or other evidence-based approaches to treating generalized pain syndromes such as cognitive behavioral therapy or tricyclic agents.
Acknowledgements
Illustrations of TPs examined were by Mansi Shah. Statistical assistance at the beginning of the study was provided by Lu Zhang.
The ICEPAC Study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; R01DK083538), with additional support from Advancing a Healthier Wisconsin Endowment Grant Number 5520298
References
- 1.Apte G, Nelson P, Brismee JM, et al. Chronic female pelvic pain--part 1: clinical pathoanatomy and examination of the pelvic region. Pain Pract. 2012;12(2):88–110. doi: 10.1111/j.1533-2500.2011.00465.x. [DOI] [PubMed] [Google Scholar]
- 2.Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87(3):321–327. doi: 10.1016/0029-7844(95)00458-0. [DOI] [PubMed] [Google Scholar]
- 3.van de Merwe JP, Nordling J, Bouchelouche P, et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol. 2008;53(1):60–67. doi: 10.1016/j.eururo.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Bassaly R, Tidwell N, Bertolino S, et al. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22(4):413–418. doi: 10.1007/s00192-010-1301-3. [DOI] [PubMed] [Google Scholar]
- 5.Peters KM, Carrico DJ, Kalinowski SE, et al. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70(1):16–18. doi: 10.1016/j.urology.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 6.Tu FF, As-Sanie S, Steege JF. Prevalence of pelvic musculoskeletal disorders in a female chronic pelvic pain clinic. J Reprod Med. 2006;51(3):185–189. [PubMed] [Google Scholar]
- 7.Gyang A, Hartman M, Lamvu G. Musculoskeletal causes of chronic pelvic pain: what a gynecologist should know. Obstet Gynecol. 2013;121(3):645–650. doi: 10.1097/AOG.0b013e318283ffea. [DOI] [PubMed] [Google Scholar]
- 8.Tu FF, As-Sanie S, Steege JF. Musculoskeletal causes of chronic pelvic pain: a systematic review of diagnosis: part I. Obstet Gynecol Surv. 2005;60(6):379–385. doi: 10.1097/01.ogx.0000167831.83619.9f. [DOI] [PubMed] [Google Scholar]
- 9.Winnard KP, Dmitrieva N, Berkley KJ. Cross-organ interactions between reproductive, gastrointestinal, and urinary tracts: modulation by estrous stage and involvement of the hypogastric nerve. Am J Physiol Regul Integr Comp Physiol. 2006;291(6):R1592–R1601. doi: 10.1152/ajpregu.00455.2006. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald MP, Anderson RU, Potts J, et al. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urol. 2013;189(1 Suppl):S75–S85. doi: 10.1016/j.juro.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zolnoun D, Bair E, Essick G, et al. Reliability and reproducibility of novel methodology for assessment of pressure pain sensitivity in pelvis. J Pain. 2012;13(9):910–920. doi: 10.1016/j.jpain.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarrell JF, Vilos GA, Allaire C, et al. Consensus guidelines for the management of chronic pelvic pain. J Obstet Gynaecol Can. 2005;27(9):869–910. doi: 10.1016/s1701-2163(16)30993-8. [DOI] [PubMed] [Google Scholar]
- 13.Tripp DA, Nickel JC, Wong J, et al. Mapping of pain phenotypes in female patients with bladder pain syndrome/interstitial cystitis and controls. Eur Urol. 2012;62(6):1188–1194. doi: 10.1016/j.eururo.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Chelimsky T, Chelimsky G, McCabe NP, et al. Interstitial Cystitis - Elucidation of Psychophysiologic and Autonomic Characteristics (the ICEPAC Study): design and methods. J Pain Res. 2014;7:243–253. doi: 10.2147/JPR.S58853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams K, Gregory WT, Osmundsen B, et al. Levator myalgia: why bother? Int Urogynecol J. 2013;24(10):1687–1693. doi: 10.1007/s00192-013-2089-8. [DOI] [PubMed] [Google Scholar]
- 16.Fenton BW, Palmieri PA, Durner C, et al. Quantification of abdominal wall pain using pain pressure threshold algometry in patients with chronic pelvic pain. Clin J Pain. 2009;25(6):500–505. doi: 10.1097/AJP.0b013e31819a3cf9. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 18.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 19.Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI) Pain. 1985;23(4):345–356. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- 20.Junghaenel DU, Broderick JE. Validation of the MPI patient profiles by partners and healthcare providers. Pain. 2009;144(1–2):130–138. doi: 10.1016/j.pain.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borg-Stein J, Stein J. Trigger points and tender points: one and the same? Does injection treatment help? Rheum Dis Clin North Am. 1996;22(2):305–322. doi: 10.1016/s0889-857x(05)70274-x. [DOI] [PubMed] [Google Scholar]
- 22.Gerwin RD. Diagnosis of myofascial pain syndrome. Phys Med Rehabil Clin N Am. 2014;25(2):341–355. doi: 10.1016/j.pmr.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Simons DG, Travell JG, Simons LS. Travell and Simon’s Myofascial Pain and Dysfunction: the Trigger Point Manual: Volume I, edition 2: The Upper Body. Baltimore: Williams and Wilkins; 1999. [Google Scholar]
- 24.Giamberardino MA, Costantini R, Affaitati G, et al. Viscero-visceral hyperalgesia: characterization in different clinical models. Pain. 2010;151(2):307–322. doi: 10.1016/j.pain.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 25.O'Leary MP, Sant GR, Fowler FJ, Jr, et al. The interstitial cystitis symptom index and problem index. Urology. 1997;49(5A Suppl):58–63. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- 26.Biurrun Manresa JA, Neziri AY, Curatolo M, et al. Reflex receptive fields are enlarged in patients with musculoskeletal low back and neck pain. Pain. 2013;154(8):1318–1324. doi: 10.1016/j.pain.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Lai HH, Gardner V, Ness TJ, et al. Segmental hyperalgesia to mechanical stimulus in interstitial cystitis/bladder pain syndrome: evidence of central sensitization. J Urol. 2014;191(5):1294–1299. doi: 10.1016/j.juro.2013.11.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ness TJ, Lloyd LK, Fillingim RB. An endogenous pain control system is altered in subjects with interstitial cystitis. J Urol. 2014;191(2):364–370. doi: 10.1016/j.juro.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Twiss C, Kilpatrick L, Craske M, et al. Increased startle responses in interstitial cystitis: evidence for central hyperresponsiveness to visceral related threat. J Urol. 2009;181(5):2127–2133. doi: 10.1016/j.juro.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253–259. doi: 10.1097/GCO.0000000000000083. [DOI] [PubMed] [Google Scholar]