Abstract
Background
Depression and anxiety are common mental health problems in transplant populations. There is mixed evidence concerning whether they increase morbidity and mortality risks post-transplant. If such associations exist, additional risk reduction strategies may be needed.
Methods
Four bibliographic databases were searched from 1981 through September, 2014 for studies prospectively examining whether depression or anxiety (determined with diagnostic evaluations or standardized symptom scales) affected risk for post-transplant mortality, graft loss, acute graft rejection, chronic rejection, cancer, infection, and rehospitalization.
Results
Twenty-seven studies (10 heart, total n=1,738; 6 liver, n=1,063; 5 kidney, n=49,515; 4 lung, n=584; 1 pancreas, n=80; 1 mixed recipient sample, n=205) were identified. In each, depression and/or anxiety were typically measured pre- or early post-transplant. Follow-up for outcomes was a median of 5.8 years (range:0.50–18.0). Depression increased the relative risk (RR) of mortality by 65% (RR=1.65, 95% CI:1.34,2.05; 20 studies). Meta-regression indicated that risk was stronger in studies that did (v. did not) control for potential confounders(p=.032). Risk was unaffected by type of transplant or other study characteristics. Depression increased death-censored graft loss risk (RR=1.65, CI:1.21,2.26, 3 studies). Depression was not associated with other morbidities (each morbidity assessed in 1–4 studies). Anxiety did not significantly increase mortality risk (RR=1.39, CI:0.85,2.27, 6 studies) or morbidity risks (assessed in single studies).
Conclusions
Depression increases risk for post-transplant mortality. Few studies considered morbidities; the depression-graft loss association suggests that linkages with morbidities deserve greater attention. Depression screening and treatment may be warranted, although whether these activities would reduce post-transplant mortality requires study.
Keywords: organ transplantation, depression, anxiety, mortality, graft loss, morbidities
Introduction
Organ transplantation promotes survival(1). It can foster improvements in other domains such as patient mental health and emotional well-being, and these too are recognized as salient outcomes during the transplantation process(2–6). Their importance as outcomes would alone justify the need for timely identification and treatment of common psychiatric conditions such as depression and anxiety in transplant patients. But it is possible that such mental health problems could have their own negative consequences, increasing both morbidity and mortality risks post-transplantation. Depressive and anxiety-related conditions each serve as risk factors for morbidities and mortality in community-based and nontransplant chronic disease populations(7–18). However, whether such associations occur in the context of transplantation is unclear.
On the one hand, one might argue that any impact of depression or anxiety on outcomes would be attenuated in transplant populations, given routine use of psychiatric evaluation protocols designed to screen out or identify transplant candidates requiring mental health intervention before transplantation (2,4,6,19–21). On the other hand, despite such protocols, prevalence rates of clinically significant depression and anxiety in transplant recipients remain substantially elevated over rates in the general population, and they equal or exceed rates in other chronic disease cohorts. For example, up to 63% of transplant recipients experience depression or anxiety during the first several years post-transplant(2,6,22–24), while rates during comparable time periods are 3–10% in the general population (9,25,26) and 10–40% in individuals with arthritis, cancers, heart disease, diabetes, kidney disease and lung disease(25–30). The elevated rates in transplant recipients may arise from stressors associated with the recovery and rehabilitation process, the need to follow a complex medical regimen, and adjustment to the prospect of new health threats including acute and chronic graft rejection, infections, and malignancies (2,6,19). In this context, it would be surprising if prevalent mental health problems such as depression and anxiety did not increase risk for poor outcomes in transplant populations, just as they do in other general population and patient groups(7–18).
To date, findings on whether either depression or anxiety predicts transplant-related outcomes appear mixed. For example, across different types of organ transplantation, some studies report that depression and/or anxiety occurring pre- or early post-transplant increases patients’ risk for morbidities and mortality(31–35), with speculation that psychiatric distress results in behavioral problems (e.g., poorer medical adherence) and/or pathophysiological abnormalities that contribute to poor health outcomes. Other studies fail to find significant associations(36–38), or even report that greater depression or anxiety predicts more favorable post-transplant health outcomes(39,40), potentially due to behavioral factors (e.g., increased care-seeking among depressed individuals and/or increased symptom vigilance among anxious individuals, leading to quicker identification of transplant-related complications). It is also unclear whether depression is the more important risk factor, or whether anxiety plays an equally strong role(41). Reviews summarizing these effects have been narrative rather than systematic reviews. With few exceptions(4), they focus on narrow portions of the literature—considering, for example, only certain types of transplantation, or only reports published during brief time periods such as the 12–18 months before the review(22,23,41–46). The reviews note that differences across studies are difficult to reconcile due to variations in study methodology, including the timing and nature of assessments of predictors and outcomes, and the duration of follow-up. Other factors (e.g., type of transplantation, age group studied), may also affect observed associations, although their impact is unknown.
A clearer understanding of whether depression and/or anxiety affect risk for post-transplant morbidity and mortality, as well as factors moderating these associations’ strength, could provide the foundation necessary for (a) identification of patients for whom mental health monitoring and care are particularly critical, and (b) clinical trials testing interventions to lessen any impact of depression and anxiety, thereby potentially reducing morbidities and mortality post-transplant. We thus conducted a systematic review and meta-analysis to achieve several goals. First, we sought to summarize and describe the literature across all types of organ transplantation, and encompassing both adult and pediatric samples. Second, we aimed to determine how strongly depression and anxiety were each associated with post-transplant mortality and with common transplant-related morbidities. Third, we aimed to examine whether any observed associations varied depending on key study characteristics, including type of population evaluated (e.g., organ transplant type, age group), study methodology (e.g., approach to psychiatric assessment, follow-up duration), and study quality.
Methods
We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines(47).
Search Strategy and Study Selection
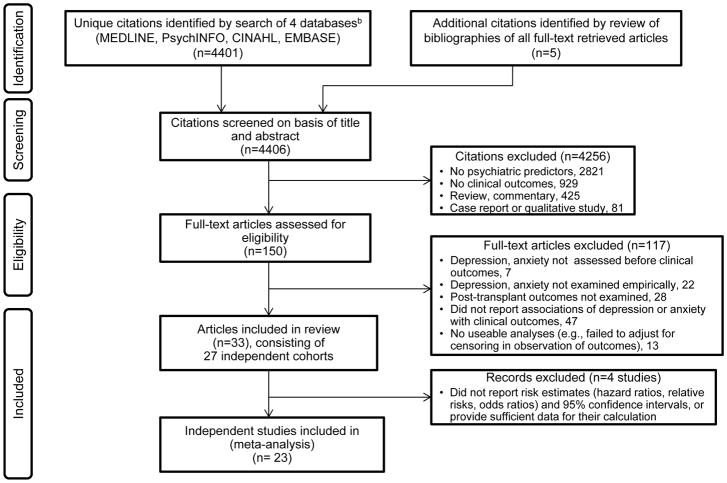
Following a protocol designed by the authors, eligible studies were sought from multiple sources (Figure 1). Table 1 lists inclusion/exclusion criteria; the search included publications through September, 2014. Pairs of authors (one of whom was M.A.D.) independently evaluated titles and abstracts of identified citations and, for those deemed potentially relevant by either member of the pair, full-text articles were retrieved.
Figure 1.
PRISMAa flow diagram of study selection.
a Adapted from PRISMA guidelines (47).
b The search algorithm was (kidney transplant* or pancreas transplant* or heart transplant* or lung transplant* or heart-lung transplant* or liver transplant*) AND (psych* or mental or depress* or anxiety or mood) AND (survival or morbidity or mortality or cancer or rejection or infection or hospitalization or health), AND limit = 1981 – current (September, 2014) AND limit = human. Although an exclusion criterion to retrieved citations was that they were published in a language other than English, Spanish, French or German, none of the identified citations was excluded due to this criterion. See Table 1 for full list of inclusion/exclusion criteria.
Table 1.
Inclusion and exclusion criteria for studies for the systematic review and meta-analysis.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
| |
For inclusion in both the
systematic review and meta-analysis:
|
For exclusion from both the
systematic review and meta-analysis:
Among the final pool of studies in the systematic review, exclusion from the meta-analysis:
|
Data Extraction
Pairs of authors (one of whom was M.A.D.) independently reviewed and extracted data from each study, and then met to reconcile any differences in data extracted.
Predictor-outcome associations
The primary information extracted pertained to prospective associations of depression and anxiety with any of 8 post-transplant outcomes listed in Table 1 (see inclusion criteria). Relative risks (RRs) and 95% Confidence IntervaIs (CIs), expressed in terms of risk for a poor post-transplant outcome as a function of a given psychiatric predictor, were extracted. We selected results from the full statistical model that adjusted for the largest number of potential confounders.
Assessment-related and other study characteristics
For each predictor (depression, anxiety), we recorded the assessment method used (standardized clinical interview v. standardized self-report symptom rating scale v. retrieval of medical records information on a clinical evaluation). We recorded whether the predictor was defined in terms of the presence/absence of caseness (i.e., individuals met criteria for diagnosable disorder or had a score exceeding a clinically-validated threshold establishing caseness on a symptom scale) or, alternatively, as degree of symptomatology along a continuous scale. We recorded whether the predictor was assessed pre- or post-transplant.
For transplant-related outcomes, we recorded their source (medical/registry records v. patient report). We extracted descriptive data about each study (e.g., publication date, type of transplantation).
To evaluate study methodologic quality and risk of bias(47,50,51), pairs of authors independently rated each study on 7 components of methodology using a validated scoring system for each(52). We employed a consensus approach where any disagreements were resolved before assigning a final rating (see Appendix Table A1, footnote). The 7 components (each rated yes/no/cannot be determined) were whether (a) the sample was clearly described (e.g., including demographics, dates of transplant); (b) the patients approached for enrollment were representative of the study site’s transplant population, (c) the sample enrolled was representative of those approached; (d) characteristics of patients lost to follow-up were clearly described; (e) outcome measures were clearly described; (f) all analyses adjusted for any differences in follow-up duration (censoring); and (g) all analyses of psychiatric variable-outcome variable associations adjusted for potential demographic and clinical confounders (i.e., factors that, if not controlled, could lead to erroneous conclusions about the true size of the associations)(52,53). A total quality score—a count of the components rated “yes” (score range, 0–7)—was computed for each study.
Statistical analysis
Descriptive statistics (e.g., percentages, means, standard deviations) were computed to characterize the studies in the systematic review. Among studies included in the meta-analysis, for each transplant-related outcome (e.g., mortality), we calculated the pooled estimate of the RR for the outcome given the presence (v. absence) of patient depression at baseline. We similarly calculated the RR given baseline anxiety. For studies examining depression (or anxiety) only as a continuous predictor, we separately calculated the pooled RR for the outcome in relation to incremental levels of depression symptomatology and the RR in relation to incremental anxiety symptomatology.
Across studies, the pooled RR is a weighted average that takes within-study variance into account. We generated it under a random effects model to allow generalizability beyond the retrieved studies(54). If the pooled RR was statistically significant, we evaluated the impact of publication bias, i.e., that studies finding predictor-outcome associations may have been more likely to be published. We did this by (a) examining Begg’s funnel plot of study size by effect size and the accompanying Begg and Mazumdar rank correlation(54), and (b) calculating the “fail-safe N”(54,55), i.e., the number of missing studies obtaining null findings that would need to be added to the analysis so the pooled RR is no longer statistically significant.
When there was significant variability across studies in size of their individual RRs (based on the Q test for heterogeneity), we performed additional analyses. First, we performed a “leave-one-out” sensitivity analysis (wherein each study is individually removed from the analysis and the pooled RR is computed across remaining studies) to examine the pooled RR’s stability and determine if any single study primarily accounted for its size(56). Next, we used random effects meta-regression(54,57) to determine whether RR variability across studies could be explained by 8 study characteristics: type of transplant, publication year, age group studied, maximum duration of follow-up, whether psychiatric status was assessed pre- or post-transplant, method used to assess psychiatric status, study methodologic quality, and whether the associations of outcomes with depression and anxiety were examined after controlling for potential confounders. (Although this latter variable was a component of the methodologic quality rating, we also examined it individually because of its potentially critical role in influencing robustness and interpretation of any psychiatric status-outcome association.)
Results
Search results
As shown in Figure 1, 4,401 citations were identified, and 150 articles underwent full-text review. Of these articles, 33 were included in the systematic review(31–40,58–79), representing 27 studies of independent cohorts.
Description of studies
Table 2 summarizes descriptive information for the 27 studies; details for each investigation are in Table 3. The largest number of studies (n=10, 37%) focused on heart recipients. Studies were almost exclusively from North America or Europe. Across all studies, over 53,000 patients were included, contributing almost 164,000 person-years of observation. One registry-based report (with 47,899 kidney recipients)(32) greatly increased the volume of observations. Even so, Table 2 shows that studies of heart and liver transplantation each contributed over 1,000 patients. Fewer lung recipients have been studied. Most studies focused exclusively on adults. Two reports included transplant recipients of all ages; none focused solely on children. Follow-up duration was a median of almost 6 years across studies, ranging up to 18 years.
Table 2.
Descriptive information for 27 independent studies examining whether depression or anxiety affected risk for transplant-related mortality and morbidities.
| Characteristic | Total | Type of Organ Transplantation
|
||||
|---|---|---|---|---|---|---|
| Heart | Liver | Kidney | Lung | Othera | ||
| Number of studies | 27 | 10 | 6b | 5b | 4 | 2 |
|
| ||||||
| Year of earliest relevant publication | 1989 | 1989 | 1997 | 2008 | 1998 | 1993 |
|
| ||||||
| Study location, % (n) | ||||||
| North America | 59.3 (16) | 60.0 (6) | 83.3 (5) | 20.0 (1) | 75.0 (3) | 50.0 (1) |
| Europe | 37.0 (10) | 40.0 (4) | 16.7 (1) | 60.0 (3) | 25.0 (1) | 50.0 (1) |
| Other | 3.7 (1) | 0.0 (0) | 0.0 (0) | 20.0 (1) | 0.0 (0) | 0.0 (0) |
|
| ||||||
| Total number of countries represented | 10 | 4 | 2 | 5 | 3 | 2 |
|
| ||||||
| Age group studied, % (n) | ||||||
| Adult only | 92.6 (25) | 90.0 (9) | 100.0 (6) | 80.0 (4) | 100.0 (4) | 100.0 (2) |
| Adult and child | 7.4 (2) | 10.0 (1) | 0.0 (1) | 20.0 (1) | 0.0 (0) | 0.0 (0) |
|
| ||||||
| Total number of patients studied | 53,105 | 1,738 | 1,063 | 49,515 | 584 | 205 |
|
| ||||||
| Sample size | ||||||
| Median (IQR) | 147 (103–201) | 107 (101–188) | 160 (125–224) | 527 (105–24,389) | 153 (84–201) | 102 (80–102) |
| Absolute range | 23–47,899 | 58–555 | 60–358 | 23–47,899 | 76–201 | 80–125 |
|
| ||||||
| Total person years of observation | 163,713 | 6,879 | 4,564 | 148,247 | 3,820 | 203 |
|
| ||||||
| Duration of observation of follow-up, years | ||||||
| Median (IQR) | 5.8 (3.0–9.6) | 5.4 (3.1–8.4) | 7.4 (4.4–12.0) | 3.0 (1.0–8.3) | 10.8 (8.8–13.5) | 2.6 (1.0–2.6) |
| Absolute range | 0.5–18.0 | 1.0–18.0 | 2.7–13.0 | 0.5–8.7 | 8.5–14.0 | 1.0–4.3 |
|
| ||||||
| Study focus on depression or anxiety as risk factors | ||||||
| Depression only | 51.9 (14) | 30.0 (3) | 83.3 (5) | 80.0 (4) | 25.0 (1) | 50.0 (1) |
| Anxiety only | 3.7 (1) | 0.0 (0) | 0.0 (0) | 20.0 (1) | 0.0 (0) | 0.0 (0) |
| Both depression and anxiety | 44.4 (12) | 70.0 (7) | 16.7 (1) | 0.0 (0) | 75.0 (3) | 50.0 (1) |
|
| ||||||
| Study timing of assessment of depression and/or anxiety | ||||||
| Pre-transplant | 59.3 (16) | 40.0 (4) | 83.3 (5) | 40.0 (2) | 75.0 (3) | 100.0 (2) |
| Post-transplant | 33.3 (9) | 50.0 (5) | 16.7 (1) | 60.0 (3) | 0.0 (0) | 0.0 (0) |
| Both pre- and post-transplant | 7.4 (2) | 10.0 (1) | 0.0 (0) | 0.0 (0) | 25.0 (1) | 0.0 (0) |
|
| ||||||
| Study assessment method for depression and/or anxiety | ||||||
| Standardized clinical interview | 11.1 (3) | 20.0 (2) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 50.0 (1) |
| Standardized self-report scale | 55.6 (15) | 30.0 (3) | 50.0 (3) | 80.0 (4) | 100.0 (4) | 50.0 (1) |
| Both standardized interview and scale | 7.4 (2) | 20.0 (2) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Medical record review | 25.9 (7) | 30.0 (3) | 50.0 (3) | 20.0 (1) | 0.0 (0) | 0.0 (0) |
|
| ||||||
| Study quality evaluation, % (n) | ||||||
| Low (score 1–3) | 25.9 (7) | 30.0 (3) | 16.7 (1) | 20.0 (1) | 25.0 (1) | 0.0 (0) |
| Moderate (4–5) | 55.6 (15) | 60.0 (6) | 33.3 (2) | 80.0 (4) | 50.0 (2) | 100.0 (2) |
| High (6–7) | 18.5 (5) | 10.0 (1) | 50.0 (3) | 0.0 (0) | 25.0 (1) | 0.0 (0) |
includes one study of pancreas recipients and one study with a mixed sample of recipients of different types of organ transplant recipients.
One investigation reported on both a liver and a kidney recipient cohort(39). Because findings for each were provided separately, we considered this investigation as contributing two independent cohorts (i.e., two of the 27 cohorts in the systematic review).
Table 3.
Characteristics of individual studies included in the systematic review.
| Study first author, year, related publications |
Country | Sample size |
Baseline age |
Male gender |
Timing
of baseline assessment |
Post-tx followup duration |
Psychiatric risk factor measures | Risk
factor-outcome relationships examineda |
Methodo- logic Qualityc |
|---|---|---|---|---|---|---|---|---|---|
| Heart | |||||||||
| Maricle, 1989,58 199159 | U.S.A. | 58 | Adults | 86.2% | Evaluation for tx | ≥ 3 mo post-tx; M 2.1±1.1 y | DSM-III depressive disorder diagnoses in
medical record of tx evaluation Depression and anxiety subscales, SCL-90, mean scoreb |
Depression
with Mortality Depression, anxiety with Acute rejectionb Infectionb |
1 |
| Dew, 199960 | U.S.A. | 145 | Aged ≥18, 46.9% < 50 y | 84.1% | Across first year post-tx | 2 y after baseline year | First year post-tx DSM-III-R depressive or
anxiety disorders by SCID or CIDI Depression and anxiety subscales, SCL-90, score>1 SD of normative mean |
Depression, anxiety
with Mortality Acute rejection Chronic rejection |
6 |
| Skotzko, 199938 | U.S.A. | 107 | Adults | --- | Evaluation for tx | Max 1 y post-tx | DSM-III-R depression and anxiety disorder diagnoses recorded in medical record of tx evaluation | Depression, anxiety
with Mortality Acute rejectionb Infectionb Hospitalizationb |
5 |
| Zipfel, 200261 | Germany | 103 | Adults aged ≥18 | --- | After listing for tx | M 4.4 y post-tx, max ~5 y | Zerssen Depression Scale, >
median State subscale, STAI, score > median |
Depression, anxiety
with Mortality |
4 |
| Grigioni, 200562 Sirri, 201063 |
Italy | 95 | Adults ≥ 18, M 56.0±10.1 y | 83.2% | M 4.4±3.2 y post-tx, range 0.5–14.2 | Max 6.2 y after baseline | Anxiety, Depression subscales, Kellner Symptom Questionnaire, score > median | Depression, anxiety
with Mortality |
1 |
| Owen, 200664 | U.S.A. | 108 | Adults, M 53.3+12.9 y | 73.1% | Evaluation for tx | M 2.7 y post-tx, range 1 day- 5.7 y | DSM depressive and anxiety disorder diagnoses recorded in medical record at tx evaluation | Depression, anxiety
with Mortality Infectionb Hospitalizationb |
4 |
| Havik, 200733 | Norway | 147 | Adults, M 53.4±12.4 y, range 18–73 | 76.9% | M 5.6±3.9 y post-tx, range 1–16 | M 6.0±0.3 after baseline | BDI, score ≥10 | Depression with Mortality |
4 |
| van de Beek, 200865 | U.S.A. | 313 | Adults and children, median 52 y, IQR 38–59 | --- | Post-tx before hospital discharge | Median 5.5 y post tx, IQR 2.2–9.9, max 18 y | Diagnosis of depression recorded in medical record | Depression with Mortality |
4 |
| Favaro, 201166 | Italy | 107 | Adults, M 58.1±11.8 y, range 18–75 | 79.4% | M 3.4±1.4 y post-tx, range 1–5 | M 7.8±0.5, y after baseline, range 7.2–8.9 | DSM-IV post-tx current depression, PTSD
diagnoses by SCID interview DSM-IV pre-tx life depression, PTSD diagnoses by SCID interview |
Depression with Acute rejectionb Depression, anxiety with Mortality Cancer |
4 |
| Farmer, 201367 | U.S.A. | 555 | Adults, M 59.4 y | 79% | 5–10 y post-tx | M 2.5 y after baseline, max 3.5 y | Cardiac Depression Scale, mean score | Depression with Mortality | 3 |
| Liver | |||||||||
| Singh, 199768 | U.S.A. | 60 | Adults | --- | While on wait list for tx | Max 2.7 y post-tx | BDI-1, score >10 | Depression with Mortality |
3 |
| Gedaly, 200869 | U.S.A. | 147 | Adults, median 52 y, range 26–69 | 89.4% | Pre-tx | Median 3.4 y post-tx, max 11.7 | History of depression recorded in pre-tx medical record | Depression with Mortality |
7 |
| Rowley, 201037 | U.S.A. | 358 | Adults, M 50.2±9.7 y, range 18–73 | 62.3% | Evaluation for tx | Median 6.2 y post-tx, range 0–13 | DSM-IV-TR depressive disorder diagnoses in medical record of transplant evaluation | Depression with Mortality |
6 |
| Corruble, 201139,2011,70 201271 | France | 134 152 (overlaps with 134) |
For n=134: Adults, ≥18, M
50.3±10.6 y For n=152: adults, ≥18 |
For n=134, 63.4% | While on wait list for tx | n=134: median 3.6 y post-tx, range
=0.5–5.0 n=152: max 1.5 y post-tx |
For n=134: BDI, short form, mean
score State subscale, STAI, mean score For n=152: BDI, short form, score ≥4 |
Depression with Graft loss Depression, anxiety with Mortality |
4 |
| DiMartini, 201131 Rogal, 201372 |
U.S.A. | 167 | Adults, M 50±8 y | 84% | Across first year post-tx | 9 y after baseline year | BDI-1, groups of patients with consistently low symptoms during year (scores<10), increasing symptoms, or consistently high symptoms (scores>15) | Depression with Mortality |
5 |
| Rogal, 201136 | U.S.A. | 179 | Adults, aged ≥18, M 52.1 y | 71.5% | Evaluation for tx and while on wait list for tx | M 2.8 y post-tx, max 5.8 y | History of depression, antidepressant medication use in pre-tx medical record | Depression
with Mortality Acute rejection Graft loss Infection Hospitalization |
7 |
| Kidney | |||||||||
| * Burke, 200840 | South Africa | 23 | Adults, M 29.7 y | 52.2% | While on wait list for tx | All at 6 mo post-tx | State subscale, STAI, mean scoreb | Anxiety with Acute rejectionb |
1 |
| Dobbels, 200832 | U.S.A. | 47,899 | Adults and children, 3% aged 0–17, 97% aged ≥18 | 60.0% | 0–3 y post-tx | Max 3 yr post-tx | Medicare claim for ICD-9 diagnosis of depression not elsewhere classified recorded in United States Renal Data System database | Depression
with Mortality Graft loss Graft loss (death censored) |
5 |
| Novak, 201034 Molnar-Varga, 201173 | Hungary | 879 | Adults, ≥18, M 49±13 y | 58.4% | ≥ 3 mo post-tx, median 4.5 y | Median 7.8 y after baseline | Center for Epidemiologic Studies Depression scale, mean score and score ≥18 | Depression
with Mortality Graft loss (death censored) |
5 |
| Corruble, 201139 | France | 187 | Adults, ≥18 y | --- | While on tx wait list | Max 1.5 y post-tx | BDI, short form, score ≥4 | Depression
with Mortality Graft loss |
4 |
| Zelle, 201274 | The Netherlands | 527 | Adults, M 51 ±12 y | 55.0% | Median 6.0 y post-tx, IQR 2.6–11.4 | Median 7.0 y after baseline, IQR 6.2–7.5 | Depression subscale, SCL-90, summed score ≥25 | Depression
with Mortality Graft loss (death censored) |
5 |
| Lung | |||||||||
| * Cohen, 199875 | Canada | 107 | Adults, ≥23 y | --- | Evaluation for tx | Max ~8.5 y post-tx | BDI, mean scoreb and score >13 State subscale, STAI, mean scoreb |
Depression, anxiety
with Mortalityb Acute rejectionb Chronic rejectionb Infectionb |
1 |
| Vermeulen, 200876 | The Netherlands | 200 | Adults, M 45 y, range 20–68 | 50.0% | While on tx wait list, assessment closest to tx (M 2.1 mo pre-tx) | Max ~14 y post-tx | ZDS, mean score State subscale, STAI, mean score |
Depression, anxiety
with Mortality |
5 |
| Evon, 201077 | U.S.A. | 76 | Adults, M 34.8 y | 43.4% | Evaluation for tx | Max > 9 y post-tx | BDI, mean score | Depression with Mortality |
4 |
| Smith, 201435 | U.S.A. | 201 | Adults, M 49.1±13.2 y | 38.8% | While on wait list for tx | M 9.2±1.5 y post-tx, range 4–12 y | BDI-II, mean score and score
>13 State subscale, STAI, mean score |
Depression, anxiety
with Mortality |
6 |
| Other | |||||||||
| * Popkin, 199378 (pancreas) | U.S.A. | 80 | Adults, M 31.6 y | 28.8% | Evaluation for tx | M 3.8 y post-tx, range 0–4.3 y | DSM-III depressive and anxiety disorder diagnoses from Diagnostic Interview Schedule conducted at tx evaluationb | Depression, anxiety with Graft lossb |
5 |
| * Dobbels, 200979 (heart, liver, or lung recipients) | Belgium | 125 | Adults, ≥18 y | --- | While on wait list for tx | 0.5–1 yr post-tx, | Depression, anxiety subscales, Hospital Anxiety and Depression Inventory, categorized into 4 levels of severityb | Depression with Graft lossb |
3 |
Study included in systematic review but not in the meta-analysis because insufficient information was provided in order to calculate any effect size measure.
For each study, only risk factor-outcomes that were examined with appropriate analyses are listed (i.e., those that did not adjust for censoring in follow-up time, or provide sufficient data to perform such calculations, were excluded). Transplant outcomes were determined in all studies from medical record or registry reviews, with the exception of Burke(40), in which the outcome was based on patient self-report.
Although appropriate analyses were performed to examine this variable, it could not be included in the meta-analysis because risk estimates and 95% CIs could not be determined from the information provided in the study.
1=low, 7=high; see text for description of ratings.
Abbreviations: BDI, Beck Depression Inventory; CIDI, Composite International Diagnostic Instrument; DSM, Diagnostic and Statistical Manual of Mental Disorders; ICD, International Classification of Disease; IQR, interquartile range; PTSD, post-traumatic stress disorder; SCID, Structured Clinical Interview for DSM; SCL-90, Symptom Check List 90; STAI, Spielberger State Trait Anxiety Inventory; tx, transplant; ZDS, Zung Depression Scale.
Table 2 shows that a majority of studies (59%) examined only depression as a risk factor, while 44% examined both depression and anxiety. (Among the latter, only two studies(60,75) attempted to determine independent effects of depression v. anxiety on outcome; none considered whether the occurrence of both conditions together had synergistic effects on outcomes.) In terms of assessment, in 74% of studies, depression and anxiety were measured via standardized diagnostic assessments (e.g., the Structured Clinical Interview for DSM-III or DSM-IV and/or psychometrically validated clinical scales)(see Table 3). Remaining studies utilized data extracted from mental health diagnostic evaluations in medical records.
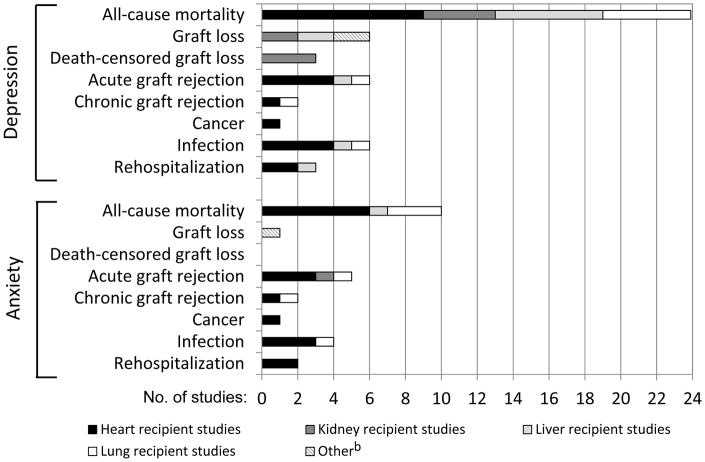
Figure 2 shows the numbers of studies examining depression or anxiety in relation to each post-transplant outcome. Mortality was the most common outcome, examined in 24 studies in relation to depression and 10 studies in relation to anxiety. Morbidity outcomes were examined more rarely, with some areas (e.g., chronic graft rejection, cancer) receiving consideration in only 1–2 studies each. For most outcomes, studies of heart, liver, and lung recipients were most common. Exceptions were graft loss outcomes where kidney recipient studies predominated.
Figure 2.
Numbers of studies examining depression or anxiety in relation to each clinical outcome (across 27 studies in systematic review)a
a Post-transplant outcomes were determined in all studies from medical record or registry reviews, with the exception of Burke(40), in which the outcome was based on patient self-report.
b For depression, this category included one study of a mixed sample of heart recipients, liver recipients, and lung recipients, and one study of pancreas recipients. For anxiety, this category included the study of the mixed sample.
As shown in the last row of Table 2, the 27 studies varied in methodologic quality. Those with low quality scores (26% of studies) failed to meet quality standards in the majority of areas rated. (See Table 3 for each study’s total quality score; Appendix A provides individual ratings used to calculate total scores). The majority of studies received scores in the moderate to high quality range. Studies of liver recipients were most likely to receive high quality scores.
Meta-analysis
Twenty-three of the 27 studies provided sufficient quantitative information for meta-analysis.
Depression and risk for post-transplant mortality
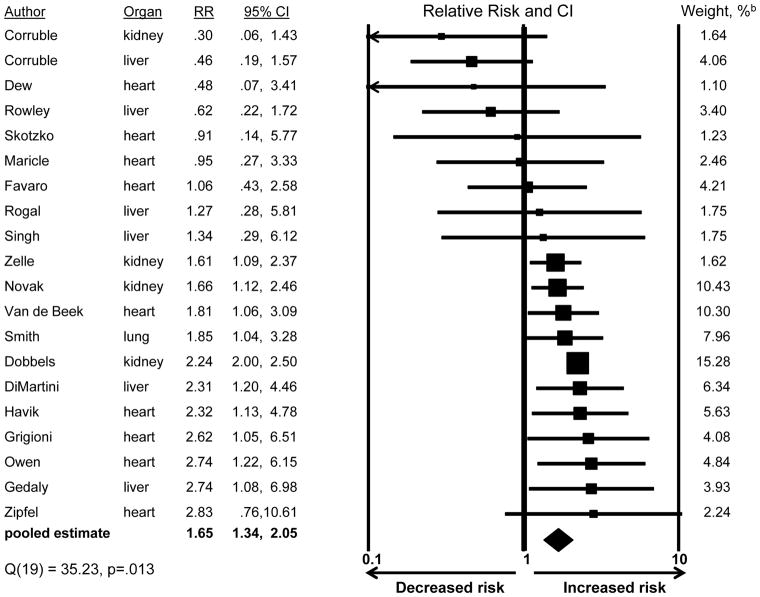
Figure 3 shows the risk estimate from each of 20 studies that examined whether depression affected mortality risk: estimates ranged from an RR of 0.30, indicating a decreased mortality risk among patients with depression, to an RR of 2.83, indicating an almost threefold increased mortality risk. Across all studies, the pooled RR was 1.65 (CI:1.34,2.05), indicating a 65% greater mortality risk among patients with depression.
Figure 3.
Association of depression with post-transplant mortalitya
a The studies classified patients according to depression status (present/absent). The presence of depression was defined as either diagnosable major depressive disorder or depression symptom levels exceeding an established threshold for caseness on a standardized symptom scale.
b Pooled estimate is weighted to take into account the precision of the effect within each study; larger weights are assigned to studies with greater precision.
We found no evidence suggesting that publication bias accounted for the pooled RR’s size, based on both a visual review of funnel plot evidence, and the finding that the plot’s accompanying Begg and Mazumdar rank correlation was not significant (τ = −.20, p=.218)(50). Furthermore, the fail-safe N was 251: this is the number of unpublished/unretrieved studies obtaining null findings that would need to be added to the analysis so that the pooled RR would become nonsignificant. The large fail-safe N suggests that the pooled RR is not an artifact of publication bias favoring reports of large, significant RRs.
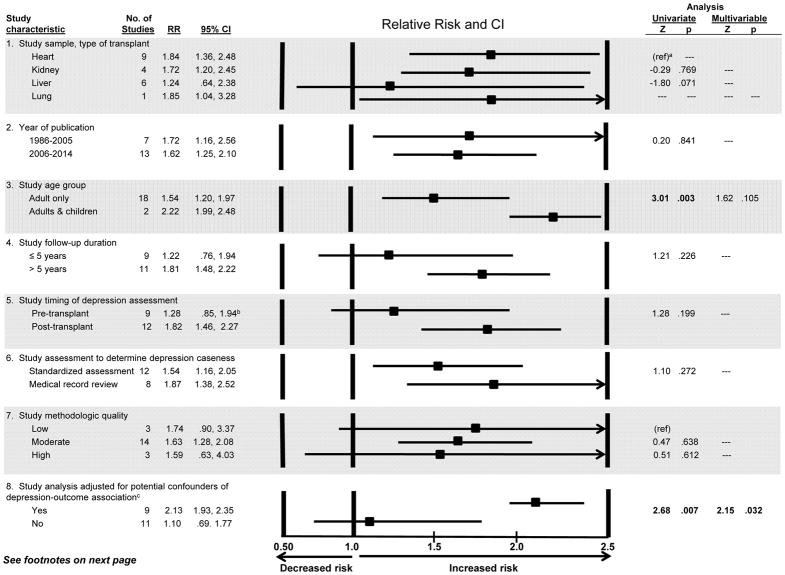
As evident in Figure 3, the size of the risk estimates varied across the 20 studies; this heterogeneity was significant (Q=35.23, df=19, p=.013). “Leave-one-out” analysis showed that the pooled RR changed relatively little no matter which of the 20 studies was omitted (ranging from pooled RR=1.57, CI:1.25,1.96, to pooled RR=1.80, CI:1.50, 2.16), suggesting that no one study unduly influenced the results. We next examined whether study characteristics, such as type of transplant examined, accounted for the effect sizes’ heterogeneity. We first considered the impact of each characteristic shown in Figure 4 individually (i.e., one predictor at a time in a meta-regression equation). We began this way because the total number of studies was not large enough to permit meta-regression with all characteristics included simultaneously(54,80). As shown in Figure 4 (third subsection), studies that included children found that depression more strongly increased mortality risk (RR=2.22) than did studies of adults only (RR=1.54); this difference was significant (Z=3.01, p=.003; univariate analysis). The depression-mortality association was also significantly stronger in studies that controlled for potential confounders than in studies that did not (Figure 4). There were no other significant differences. When the two significant characteristics from univariate analyses were included in a single meta-regression, study age group became nonsignificant (Z=1.62, p=.105; rightmost column of Figure), while the difference between studies that did v. did not control for potential confounders remained significant (Z=2.15, p=.032).
Figure 4.
Study characteristics potentially explaining variability in the size of the depression - post-transplant mortality association (relative risk): Meta-regression results
a Because there was only one lung recipient study and the relative risk was similar to heart recipient samples, the studies were combined to create a referent group of thoracic transplant recipients.
b Favaro et al.(66) examined pre- and post-transplant depression separately in relation to mortality. We report our analysis that includes both effect sizes (RRs) from this study. However, because the two effect sizes are not independent, we performed a sensitivity analysis by including only the effect based on pre-transplant depression, and then repeated the analysis including only the effect based on post-transplant depression. The separate results were indistinguishable from those reported here.
c Note that the comparison of studies that did vs. did not adjust for confounders was a between-studies comparison designed to test a question of effect modification, i.e., whether studies using more rigorous, stringent procedures (i.e., analyses that would reduce the possibility of drawing erroneous conclusions about the true size of the depression-mortality association) produced effect sizes that, on average, differed from those in studies that did not use such an analytic approach(50,52,80). At the same time, one might expect that within a given study, smaller effects would be observed after controlling for confounders compared to before such adjustment(80). Among the nine studies that reported controlling for confounders, 5 reported only results from multivariable models. Of the four studies that reported univariate results, followed by multivariable results, three observed that controlling for confounders did attenuate the size of the association of depression with mortality(33,34,74). The fourth study showed little difference in the size of depression-mortality associations between univariate and multivariable analyses(36).
Anxiety and risk for post-transplant mortality
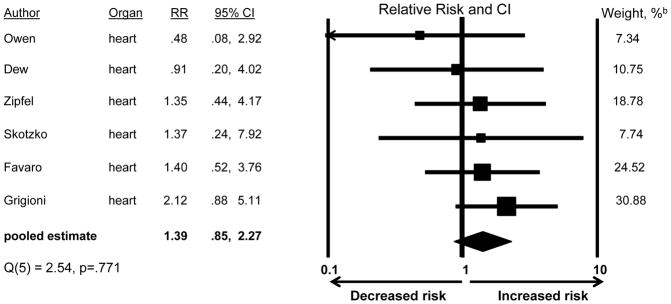
Figure 5 shows results for the 6 studies examining whether patient anxiety increased mortality risk. The pooled RR was 1.39, but was not statistically significant (CI:0.85,2.27). The studies’ effect sizes did not show significant heterogeneity (Q=2.54, df=5, p=.771), precluding the need to examine differences by study characteristics.
Figure 5.
Association of anxiety with post-transplant mortalitya
a The studies classified patients according to anxiety status (present/absent). The presence of anxiety was defined as either any diagnosable anxiety disorder or anxiety symptom levels exceeding an established threshold for caseness on a standardized symptom scale.
b Pooled estimate is weighted to take into account the precision of the effect within each study; larger weights are assigned to studies with greater precision.
Depression or anxiety as risk factors for post-transplant morbidities
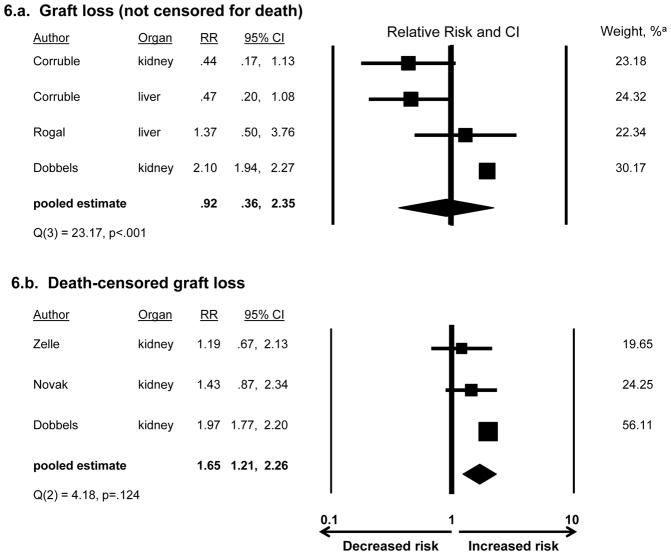
Considering first depression as a risk factor, Figure 6a shows that, among the four studies examining overall graft loss (not censored for death), the pooled RR of 0.92 was not significant (CI:0.36,2.35). Despite significant heterogeneity across studies (Q=23.17, df=3, p<.001), there were too few studies to explore factors explaining the heterogeneity(50). Leave-one-out analysis showed that the pooled RR was consistently nonsignificant (range: pooled RR=0.63, CI:0.32,1.26 to RR=1.17, CI:0.44,3.06).
Figure 6.
Association of depression with post-transplant graft lossa
a See Figure 3 for definition of depression and explanation of weights
As shown in Figure 6b, among the three studies examining depression in relation to death-censored graft loss, the pooled RR of 1.65 was significant (CI:1.21,2.26). Concerning the potential for publication bias influencing these results, while there were too few studies to usefully examine the funnel plot, the fail-safe N of 50 suggests that the RR is robust to the discovery of many additional studies with null results. The studies did not show significant heterogeneity (Q=4.18, df=2, p=.124).
Two studies(36,60) examined depression in relation to acute graft rejection; neither found a significant association (RR=0.42, CI:0.08,2.14; RR=0.90, CI:0.48,1.69, respectively). The pooled RR of 0.81 was not significant (CI:0.45,1.47). There was no evidence of heterogeneity (Q=0.72, df=1, p=.395).
Remaining morbidities were examined relative to depression in only one study each, all with nonsignificant findings: depression did not increase heart recipients’ risk of chronic graft rejection (RR=1.66, CI:0.57,4.86)(60) or cancer (RR=1.42, CI:0.57,3.54)(66), or liver recipients’ risk of infection or rehospitalization (RR=1.29, CI:0.82,2.05; RR=1.19, CI:0.81,1.75, respectively)(36).
With respect to anxiety and post-transplant morbidities, no studies examined overall graft loss or death-censored graft loss. Only single studies examined any of the other outcomes: anxiety did not significantly increase heart recipients’ risk of acute rejection (RR=0.85, CI:0.30,2.37)(60), chronic rejection (RR=1.50, CI:0.54,4.18)(60), or cancer (RR=1.40, CI:0.49,4.01)(66).
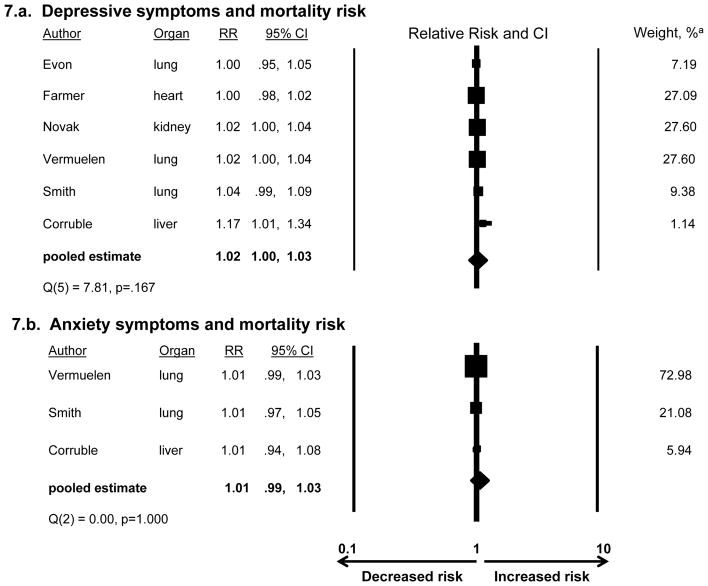
Continuous measures of depressive and anxiety symptomatology, and risk for post-transplant mortality and morbidities
Among studies examining depression scale scores (with no imposed threshold defining clinically significant depression), we examined the pooled RR for mortality for each 1-point increment on the scale. Across 6 studies, the RR of 1.02 was not significant (CI:1.00,1.03)(Figure 7a). Studies’ RRs did not show significant heterogeneity (Q=7.81, df=5, p=.167). Among 3 studies examining continuous anxiety scale scores, the pooled RR for mortality was 1.01 and was not significant (CI:0.99,1.03)(Figure 7b). There was no heterogeneity (Q=0.00, df=2, p=1.00). No studies examined continuous depression or anxiety scale scores in relation to post-transplant morbidities.
Figure 7.
Association of continuous measures of depression and anxiety symptoms and post-transplant mortality
a See Figure 3 for explanation of weights
Discussion
We conducted the first systematic review with meta-analysis of a growing literature examining whether depression and anxiety increase morbidity and mortality risks after transplantation. The review is timely given the continued challenge to identify modifiable risk factors in order to improve post-transplant outcomes. Most studies focused on depression, typically assessed pre- or early post-transplant, and its potential impact on post-transplant mortality risk. Few studies considered whether depression predicts transplant-related morbidities. Even fewer considered anxiety relative to either mortality or morbidity post-transplant.
We found that the presence of depression was associated with a 65% increased risk of post-transplant mortality. This effect size is well within the range of depression-mortality associations noted in community-based populations(8,9,11,12), and in cohorts with lung, heart or kidney disease(7,9–12,16,17,81–84), cancers(14,85), and diabetes(86). The risk effects found in these reports typically range between 20%-90%. In fact, in our meta-analysis, studies conducting the most stringent, rigorous analyses (by adjusting for potential confounders and thereby reducing the possibility of drawing erroneous conclusions about effect sizes)(50,52) found depression-mortality associations even stronger than those in other populations: depression more than doubled the risk of post-transplant mortality (RR=2.13).
In contrast, although anxiety appeared to bear a modest association with increased mortality risk post-transplant, this association was not significant. Anxiety has been found to increase mortality risk in other chronic disease populations(7,9,16–18). It is noteworthy, however, that we identified only six studies examining this relationship, and thus our estimate of the pooled effect size was less precise (wider CI) than the estimated effect for depression. In turn, although study methodologic quality was equivalent across investigations of depression and anxiety (see ratings, Table 3), the CIs around the estimates from individual studies examining anxiety tended to be slightly wider, indicating less precision, than those from studies examining depression (Figures 3 and 5). Given fewer and less precise estimates in the transplant literature, it remains premature to draw strong conclusions regarding anxiety’s role in relation to mortality.
Given the larger pool of studies focused on depression and post-transplant mortality, we could examine whether depression’s impact on risk varied depending on specific study characteristics. In addition to the difference noted above between studies that did vs. did not adjust for confounding factors, we found some evidence that depression more strongly predicted mortality in studies that included children than in those that did not. Although significant in univariate analysis, this effect was diminished in multivariable analysis. However, only two of 20 studies assessing mortality in the meta-analysis included children; none directly compared children to adults. Given evidence that psychosocial factors such as emotional well-being have heightened impact on health and behavioral outcomes after pediatric transplantation(87,88), establishing whether depression plays any role in pediatric samples should be a future research priority.
We found no evidence that the depression-mortality association’s size was affected by other study-related characteristics, including type of organ transplant studied, era of study publication, method of determining depression caseness, timing of depression assessment (pre- v. post-transplant), and follow-up duration. The absence of significant differences based on assessment timing is particularly noteworthy because it suggests that any occurrence of depression, whether pre- or post-transplant, has the potential to increase mortality risk. Depression is a readily treatable disorder and many pharmacologic and psychotherapeutic interventions exist(89,90). Although there is concern regarding the level of evidence and the safety of utilizing many interventions—particularly pharmacologic strategies—pre-transplant in individuals with severely compromised organ function(28,39,91,92), there is a large psychosomatic medicine practice-focused literature showing that pharmacologic and psychotherapeutic options can be utilized safely and effectively with transplant recipients who have stable organ function(6,23,44,93–96). Hence, ongoing screening (with treatment) for depression at routine post-transplant follow-up may be warranted, and has also been recommended pre-transplant(4,23,24,95), but with the caveat that we continue to lack the rigorous clinical trial evidence essential to assert that depression screening and treatment are effective. Moreover, any potential harms of screening also merit rigorous study.
But—if programs decide to undertake screening given the current state of the evidence—how best to screen for depression? We note that it was clinically significant depression—i.e., depression meeting diagnostic criteria or exceeding a threshold for caseness on a self-report scale—that increased mortality risk. In contrast, among studies considering depressive symptomatology along a continuum (assessed by scales, with no threshold imposed), there was no large or statistically significant association with mortality. This suggests that, rather than aiming to detect small and likely subclinical increments to symptomatology, screening efforts should focus identifying clinically significant depression, using validated assessment instruments with thresholds for caseness (e.g., Beck Depression Inventory-II(97), Patient Health Questionnaire-9(98)).
Our meta-analyses of post-transplant morbidity risks found that the largest numbers of studies considered graft loss in relation to depression. Risk for overall graft loss was not significantly increased by depression. However, death-censored graft loss allows for a more focused consideration of factors affecting graft function independent of patient mortality(48). Depression elevated risk for this outcome by 65%. All studies, however, focused on kidney recipients, who can receive dialysis or retransplantation after graft failure. Other organ recipients have no equivalent of dialysis and would require retransplantation. Retransplantation rates remain relatively low(1). Thus, although potentially important, it may be challenging to examine death-censored graft loss in relation to depression beyond kidney transplantation.
We found little evidence that risk for any other post-transplant morbidity was increased by either depression or anxiety. However, each outcome was examined in only 1–2 studies. Given that depression and anxiety are associated with increased risk for many morbidities in the general population (e.g., diabetes, cancers, cardiac events)(7,11,13,14,81–83), more extensive examination of associations with morbidities in transplant populations is needed.
Both behavioral and pathophysiological mechanisms may explain why depression would increase risk for post-transplant mortality and, at least in kidney recipients, death-censored graft loss. For example, depression can lead to poorer adherence to the post-transplant immunosuppressive medication regimen(99–102) which, in turn, can increase mortality and graft loss(100,102,103). In multiple populations, depression is linked to poorer lifestyle behaviors including substance use, and inadequate diet and exercise(104–107). Depressed individuals frequently suffer from reduced social support and increased social isolation, both of which increase mortality risk(108–110). As noted above, depression increases risk for many medical conditions; these in turn contribute to mortality. Depression also appears to lead to reduced heart rate variability, and elevated levels of C-reactive protein and pro-inflammatory cytokines, each of which increase mortality risk in general population and advanced organ disease cohorts(111–115).
Such potential mechanisms have received little to no study in transplant populations, thus precluding consideration in our review. Other limitations of our review reflect the state of the research we synthesized: this literature provides little consideration of some patient subgroups (e.g., children); it focuses on all-cause rather than cause-specific mortality; and there is a dearth of work on post-transplant morbidities. There are other domains of outcomes that our review does not address, including patient-reported physical functional status, role function, and other components of quality of life.
We also could not examine combined effects of depression and anxiety for any evidence of synergistic effects on outcomes because no individual studies considered this issue. In addition, a potential criticism of our decision to employ meta-analysis is that meta-analysis combines results of studies that differ in their characteristics and it may ignore such differences(54,57). However, consistent with best practices for meta-analysis(50,54,56,80), we examined whether a variety of study characteristics moderated the size of the observed depression-mortality associations. In other instances (e.g., the anxiety-mortality association, the association of depression and death-censored graft loss), studies’ effect sizes showed no evidence of heterogeneity, thus precluding a search for effect moderators. Nevertheless, we note that, overall, the relevant literature remains small, and there may be additional unmeasured factors that could influence the strength of observed risk factor-outcome associations. One such factor is receipt of mental health treatment. With one exception focused on depression treatment (72; discussed below), the studies we examined did not report whether patient outcomes differed as a function of psychiatric treatment. It is possible, for example, that anxiety bore a weaker, nonsignificant relationship to mortality in our meta-analysis because it was treated more aggressively than depression. However, studies examining psychiatric treatment in transplant populations suggest that anxiety is treated at similar rates or is even undertreated relative to depression (116–119). Finally, our review did not include “gray literature” (i.e., unpublished data and reports produced by government, academics, or industry that are not peer-reviewed or included in standard bibliographic databases)(50). However, whether the inclusion of gray literature leads to more accurate effect size estimates is unclear(50,120). Moreover, our results appear robust to publication bias.
Our review’s limitations suggest issues important for future work. At the same time, our findings—particularly those showing a depression-post-transplant mortality association—provide an important foundation and justification for our recommendations of depression screening and focused treatment, not only pre- but post-transplant. We need not await an understanding of mechanisms by which depression increases mortality risk before proceeding with risk-reduction activities. An observational study hints at the potential impact of such work within transplantation: Rogal et al.(72), in additional analyses of the DiMartini et al. cohort(31) included in the present meta-analysis, found that liver recipients who received adequate, evidence-based pharmacotherapy for early post-transplant depression had long-term survival equivalent to that of nondepressed recipients, while depressed recipients receiving inadequate or no treatment had poorer survival. Important next steps include randomized trials to determine if such effects are causal and robust. In the meantime, clinical attention to the mental health of transplant patients seems warranted, not only for maximizing quality of life but because of its potential to affect post-transplant survival.
Acknowledgments
Funding: Preparation of this article was supported in part by Grant TL1TR000145 from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH), Rockville, MD, and a grant from the International Transplant Nurses Society (ITNS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the ITNS.
Abbreviations
- RR
relative risk
- CI
confidence interval
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- DSM-III
DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Fourth Edition
Appendix
Table A1.
Ratings of individual components of methodologic quality of studies included in the systematic review.*
| Study (First author, year, related publications) |
1. Patients
in sample clearly described? |
2.
Patients approached representative of entire population? |
3.
Patients enrolled representative of entire population? |
4. Characteris- tics of patients lost to follow-up described? |
5. Is
each outcome measure clearly described? |
6. Do analyses adjust for different lengths of follow-up? |
7. Adjustment for con- founding in analyses of outcomes? |
Total Score |
|---|---|---|---|---|---|---|---|---|
| Heart | ||||||||
| Maricle, 1989,58 199159 | N | ? | ? | Y | N | N | N | 1 |
| Dew, 199960 | Y | Y | Y | Y | Y | Y | N | 6 |
| Skotzko, 199938 | N | Y | Y | Y | Y | Y | N | 5 |
| Zipfel, 200261 | N | Y | ? | Y | Y | Y | N | 4 |
| Grigioni, 2005,62 Sirri, 201063 | N | ? | ? | Y | ? | N | N | 1 |
| Owen, 200664 | N | Y | Y | Y | N | Y | N | 4 |
| Havik, 200733 | N | ? | N | Y | Y | Y | Y | 4 |
| van de Beek, 200865 | Y | ? | ? | N | Y | Y | Y | 4 |
| Favaro, 201166 | Y | ? | ? | Y | Y | Y | N | 4 |
| Farmer, 201367 | Y | ? | ? | ? | Y | Y | N | 3 |
| Liver | ||||||||
| Singh, 199768 | N | ? | ? | Y | Y | Y | N | 3 |
| Gedaly, 200869 | Y | Y | Y | Y | Y | Y | Y | 7 |
| Rowley, 201037 | Y | Y | Y | Y | Y | Y | N | 6 |
| Corruble, 201139,2011,70 201271 | Y | ? | ? | Y | Y | Y | N | 4 |
| DiMartini, 2011,6 31 Rogal, 201372 | Y | Y | Y | N | N | Y | Y | 5 |
| Rogal, 201136 | Y | Y | Y | Y | Y | Y | Y | 7 |
| Kidney | ||||||||
| Burke, 200840 | N | ? | ? | N | N | Y | N | 1 |
| Dobbels, 200832 | N | Y | Y | N | Y | Y | Y | 5 |
| Novak, 201034 Molnar-Varga, 201173 |
N | Y | Y | N | Y | Y | Y | 5 |
| Corruble, 201139 | Y | ? | ? | Y | Y | Y | N | 4 |
| Zelle, 201274 | Y | Y | ? | N | Y | Y | Y | 5 |
| Lung | ||||||||
| Cohen, 199875 | N | ? | ? | N | ? | Y | ? | 1 |
| Vermeulen, 200876 | Y | Y | N | Y | Y | Y | N | 5 |
| Evon, 201077 | N | ? | ? | Y | Y | Y | Y | 4 |
| Smith,201435 | Y | Y | ? | Y | Y | Y | Y | 6 |
| Other | ||||||||
| Popkin, 199378 | Y | Y | Y | Y | N | Y | N | 5 |
| Dobbels, 200979 | Y | Y | ? | N | Y | N | N | 3 |
For items 1–4 and 6–7, Y = yes, N = no, ? = cannot be determined. For item 5, Y = yes, N = no, ? = mixed evidence across measures included in report. Total score is a count of items scored as yes (52). Interrater reliability (kappa) of item ratings prior to consensus discussions to reach final rating determinations averaged .78 across the 7 items (range, .63 to 1.00). (Benchmarks: kappas of .61–.80 indicate substantial agreement; kappas of .81 to 1.00 indicate near perfect to perfect agreement[121]). Disagreements were resolved by re-review of contents of articles and discussion to pinpoint specific evidence that most strongly supported assigning a specific rating. There were no cases in which the pair of authors rating a given study failed to resolve disagreements and reach final consensus.
Footnotes
The authors contributed to the research design (Dew, Myaskovsky, DiMartini, DeVito Dabbs, Switzer, Greenhouse), the writing of the paper (Dew, Greenhouse), review and editing the paper (Dew, Rosenberger, Myaskovsky, DiMartini, DeVito Dabbs, Posluszny, Steel, Switzer, Shellmer, Greenhouse), the performance of the research (Dew, Rosenberger, Myaskovsky, DiMartini, DeVito Dabbs, Posluszny, Steel, Switzer, Shellmer, Greenhouse), and the data analysis (Dew, Greenhouse).
Disclosure: The authors declare no conflicts of interest.
Contributor Information
Mary Amanda Dew, Departments of Psychiatry, Psychology, Epidemiology, Biostatistics, and Clinical and Translational Science, University of Pittsburgh.
Emily M. Rosenberger, Clinical and Translational Science Institute, and Department of Medicine, University of Pittsburgh.
Larissa Myaskovsky, Department of Medicine, University of Pittsburgh, and Center for Health Equity Research and Promotion, Veterans Administration Pittsburgh Healthcare System.
Andrea F. DiMartini, Departments of Psychiatry and Surgery, University of Pittsburgh.
Annette J. DeVito Dabbs, Department of Acute and Tertiary Care, University of Pittsburgh School of Nursing.
Donna M. Posluszny, Department of Medicine, University of Pittsburgh.
Jennifer Steel, Departments of Surgery, Psychiatry and Psychology, University of Pittsburgh.
Galen E. Switzer, Department of Medicine, University of Pittsburgh, and Center for Health Equity Research and Promotion, Veterans Administration Pittsburgh Healthcare System.
Diana A. Shellmer, Department of Surgery, University of Pittsburgh.
Joel B. Greenhouse, Department of Statistics, Carnegie Mellon University Department of Psychiatry, University of Pittsburgh.
References
References marked with an asterisk were included in the systematic review.
- 1.Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients (OPTN/SRTR) 2013 Annual Data Report. Dept. of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: 2014. [Google Scholar]
- 2.Dew MA, DiMartini AF. Organ transplantation. In: Friedman HS, editor. Oxford Handbook of Health Psychology. New York: Oxford University Press; 2011. p. 522. [Google Scholar]
- 3.Annunziato RA, Jerson B, Seidel J, Glenwick DS. The psychosocial challenges of solid organ transplant recipients during childhood. Pediatric Transplant. 2012;16(7):803. doi: 10.1111/j.1399-3046.2012.01749.x. [DOI] [PubMed] [Google Scholar]
- 4.Corbett C, Armstrong MJ, Parker R, Webb K, Neuberger JM. Mental health disorders and solid-organ transplant recipients. Transplantation. 2013;96(7):593. doi: 10.1097/TP.0b013e31829584e0. [DOI] [PubMed] [Google Scholar]
- 5.Cupples SA, Dew MA, Grady KL, et al. The present status of research on psychosocial outcomes in cardiothoracic transplantation: review and recommendations for the field. J Heart Lung Transplant. 2006;25:716. doi: 10.1016/j.healun.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 6.DiMartini A, Dew MA, Crone C. Organ transplantation. In: Sadock BJ, Sadock VA, Ruiz P, editors. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. 9. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2009. p. 2441. [Google Scholar]
- 7.Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144(3):766. doi: 10.1378/chest.12-1911. [DOI] [PubMed] [Google Scholar]
- 8.Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Is excess mortality higher in depressed men than in depressed women? A meta-analytic comparison. J Affect Disord. 2014;161:47. doi: 10.1016/j.jad.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Eaton WW, Martins SS, Nestadt G, Blenvenu OJ, Clarke D, Alexandre P. The burden of mental disorders. Epidemiol Rev. 2008;30:1. doi: 10.1093/epirev/mxn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan H, Yu W, Zhang Q, et al. Depression after heart failure and risk of cardiovascular and all-cause mortality: a meta-analysis. Prev Med. 2014;63:36. doi: 10.1016/j.ypmed.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Gross AL, Gallo JJ, Eaton WW. Depression and cancer risk: 24 years of follow-up of the Baltimore Epidemiologic Catchment Area sample. Cancer Causes Control. 2010;21(2):191. doi: 10.1007/s10552-009-9449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessler RC. The costs of depression. Psychiatr Clin North Am. 2012;35(1):1. doi: 10.1016/j.psc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubzansky LD, Koenen KC, Jones C, Eaton WW. A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychol. 2009;28(1):125. doi: 10.1037/0278-6133.28.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oerlemans ME, van den Akker M, Schuurman AG, et al. A meta-analysis on depression and subsequent cancer risk. Clin Pract Epidemiol Ment Health. 2007;3:29. doi: 10.1186/1745-0179-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan A, Lucas M, Sun Q, et al. Increased mortality risk in women with depression and diabetes mellitus. Arch Gen Psychiatry. 2011;68(1):42. doi: 10.1001/archgenpsychiatry.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J COPD. 2014;9:315. doi: 10.2147/COPD.S53255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watkins LL, Koch GG, Sherwood A, et al. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc. 2013;2(2) doi: 10.1161/JAHA.112.000068. epub 000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wrenn KC, Mostofsky E, Tofler GH, Muller JE, Mittleman MA. Anxiety, anger, and mortality risk among survivors of myocardial infarction. Am J Medicine. 2013;126(12):1107. doi: 10.1016/j.amjmed.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olbrisch ME, Benedict SM, Ashe K, Levenson JL. Psychological assessment and care of organ transplant patients. J Consult Clin Psychol. 2002;70(3):771. doi: 10.1037//0022-006x.70.3.771. [DOI] [PubMed] [Google Scholar]
- 20.Steinman TI, Becker BN, Frost AE, et al. Guidelines for the referral and management of patients eligible for solid organ transplantation. Transplantation. 2001;71(9):1189. doi: 10.1097/00007890-200105150-00001. [DOI] [PubMed] [Google Scholar]
- 21.Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53(2):123. doi: 10.1016/j.psym.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Dew MA, DiMartini AF. Psychological disorders and distress after adult cardiothoracic transplantation. J Cardiovasc Nurs. 2005;20(5 Suppl):S51. doi: 10.1097/00005082-200509001-00007. [DOI] [PubMed] [Google Scholar]
- 23.Fusar-Poli P, Lazzaretti M, Ceruti M, et al. Depression after lung transplantation: causes and treatment. Lung. 2007;185(2):55. doi: 10.1007/s00408-006-0093-1. [DOI] [PubMed] [Google Scholar]
- 24.Zalai D, Szeifert L, Novak M. Psychological distress and depression in patients with chronic kidney disease. Semin Dial. 2012;25(4):428. doi: 10.1111/j.1525-139X.2012.01100.x. [DOI] [PubMed] [Google Scholar]
- 25.Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54:216. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- 26.Polsky D, Doshi JA, Marcus S, Oslin D, Rothbard A, Thomas N, Thompson CL. Long-term risk for depressive symptoms after a medical diagnosis. Arch Intern Med. 2005;165:1260. doi: 10.1001/archinte.165.11.1260. [DOI] [PubMed] [Google Scholar]
- 27.Dew MA. Psychiatric disorder in the context of physical illness. In: Dohrenwend BP, editor. Adversity, Stress and Psychopathology. New York: Oxford University Press; 1998. p. 177. [Google Scholar]
- 28.Hedayati SS, Finkelstein FO. Epidemiology, diagnosis, and management of depression in patients with CKD. Am J Kidney Dis. 2009;54(4):741. doi: 10.1053/j.ajkd.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14:721. doi: 10.1016/S1470-2045(13)70244-4. [DOI] [PubMed] [Google Scholar]
- 30.Whooley MA, Wong JM. Depression and cardiovascular disorders. Ann Rev Clin Psychol. 2013;9:327. doi: 10.1146/annurev-clinpsy-050212-185526. [DOI] [PubMed] [Google Scholar]
- 31*.DiMartini A, Dew MA, Chaiffetz D, Fitzgerald MG, deVera ME, Fontes P. Early trajectories of depressive symptoms after liver transplantation for alcoholic liver disease predict long-term survival. Am J Transplant. 2011;11:1287. doi: 10.1111/j.1600-6143.2011.03496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Dobbels F, Skeans MA, Snyder JJ, Tuomari AV, Maclean JR, Kasiske BL. Depressive disorder in renal transplantation: an analysis of Medicare claims. Am J Kidney Dis. 2008;51(5):819. doi: 10.1053/j.ajkd.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 33*.Havik OE, Siversten B, Relbo A, et al. Depressive symptoms and all-cause mortality after heart transplantation. Transplantation. 2007;84:97. doi: 10.1097/01.tp.0000268816.90672.a0. [DOI] [PubMed] [Google Scholar]
- 34*.Novak M, Molnar MZ, Szeifert L, et al. Depressive symptoms and mortality in patients after kidney transplantation: a prospective prevalent cohort study. Psychosom Med. 2010;72:527. doi: 10.1097/PSY.0b013e3181dbbb7d. [DOI] [PubMed] [Google Scholar]
- 35*.Smith PJ, Blumenthal JA, Carney RM, et al. Neurobehavioral functioning and survival following lung transplantation. Chest. 2014;145(3):604. doi: 10.1378/chest.12-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Rogal SS, Landsittel D, Surman O, Chung RT, Rutherford A. Pretransplant depression, antidepressant use, and outcomes of orthotopic liver transplantation. Liver Transplant. 2011;17:251. doi: 10.1002/lt.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Rowley AA, Hong BA, Chapman W, Crippin JS. The psychiatric diagnosis of alcohol abuse and the medical diagnosis of alcoholic related liver disease: effects on liver transplant survival. J Clin Psychol Med Settings. 2010;17:195. doi: 10.1007/s10880-010-9201-8. [DOI] [PubMed] [Google Scholar]
- 38*.Skotzko CE, Rudis R, Kobashigawa JA, Laks H. Psychiatric disorders and outcome following cardiac transplantation. J Heart Lung Transplant. 1999;18(10):952. doi: 10.1016/s1053-2498(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 39*.Corruble E, Barry C, Varescon I, et al. Report of depressive symptoms on waiting list and mortality after liver and kidney transplantation: a prospective cohort study. BMC Psychiatry. 2011;11:182. doi: 10.1186/1471-244X-11-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Burke A. Could anxiety, hopelessness and health locus of control contribute to the outcome of a kidney transplant? South African J Psychol. 2006;38(3):527. [Google Scholar]
- 41.Rosenberger EM, Dew MA, Crone C, DiMartini AF. Psychiatric disorders as risk factors for adverse medical outcomes after solid organ transplantation. Curr Opin Organ Transplant. 2012;17(2):188. doi: 10.1097/MOT.0b013e3283510928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobbels F, Verleden G, Dupont L, Vanhaecke J, De Geest S. To transplant or not? The importance of psychosocial and behavioural factors before lung transplantation. Chron Resp Dis. 2006;3(1):39. doi: 10.1191/1479972306cd082ra. [DOI] [PubMed] [Google Scholar]
- 43.Huang E, Segev DL, Rabb H. Kidney transplantation in the elderly. Sem Nephrol. 2009;29(6):621. doi: 10.1016/j.semnephrol.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mullish BH, Kabir MS, Thursz MR, Dhar A. Review article: depression and the use of antidepressants in patients with chronic liver disease or liver transplantation. Aliment Pharmacol Ther. 2014;40:880. doi: 10.1111/apt.12925. [DOI] [PubMed] [Google Scholar]
- 45.Rook M, Rand E. Predictors of long-term outcome after liver transplant. Curr Opin Organ Transplant. 2011;16(5):499. doi: 10.1097/MOT.0b013e32834a945d. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberger EM, Fox KR, DiMartini AF, Dew MA. Psychosocial factors and quality-of-life after heart transplantation and mechanical circulatory support. Curr Opin Organ Transplant. 2012;17(5):558. doi: 10.1097/MOT.0b013e3283564f45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moher D, Liberati A, Tetzlaff J, Altman DG The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Floege J, Johnson RJ, Feehally J. Comprehensive Clinical Nephrology. 4. New York: Elsevier; 2011. [Google Scholar]
- 49.Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statist Med. 1998;17:2815. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 50.Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. updated March 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- 51.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology (MOOSE): a proposal for reporting. JAMA. 2000;283(15):2008. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 52.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reports of observational studies in epidemiology (STROBE): explanation and elaboration. PloS Medicine. 2007;4(10):1628. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. New York: Wiley; 2009. [Google Scholar]
- 55.Rosenthal R. The “file-drawer problem” and tolerance for null results. Psychol Bull. 1979;86:638. [Google Scholar]
- 56.Greenhouse JB, Iyengar S. Sensitivity analysis and diagnostics. In: Cooper H, Hedges LV, Valentine JC, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. NY: Russell Sage Foundation; 2009. p. 417. [Google Scholar]
- 57.Bailar JC. The promise and problems of meta-analysis. N Engl J Med. 1997;337:559. doi: 10.1056/NEJM199708213370810. [DOI] [PubMed] [Google Scholar]
- 58*.Maricle RA, Hosenpud JD, Norman DJ, et al. Depression in patients being evaluated for heart transplantation. Gen Hosp Psychiatry. 1989;11:418. doi: 10.1016/0163-8343(89)90137-0. [DOI] [PubMed] [Google Scholar]
- 59*.Maricle RA, Hosenpud JD, Norman DJ, Pantley GA, Cobanoglu AM, Starr A. The lack of predictive value of preoperative psychologic distress for postoperative medical outcome in heart transplant recipients. J Heart Lung Transplant. 1991;10(6):942. [PubMed] [Google Scholar]
- 60*.Dew MA, Kormos RL, Roth LH, Murali S, DiMartini A, Griffith BP. Early post-transplant medical compliance and mental health predict physical morbidity and mortality one to three years after heart transplantation. J Heart Lung Transplant. 1999;18(6):549. doi: 10.1016/s1053-2498(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 61*.Zipfel S, Schneider A, Wild B, et al. Effect of depressive symptoms on survival after heart transplantation. Psychosom Med. 2002;64:740. doi: 10.1097/01.psy.0000031575.42041.6a. [DOI] [PubMed] [Google Scholar]
- 62*.Grigioni F, Musuraca AC, Tossani E, et al. Relationship between psychiatric disorders and physical status during the course of a heart transplantation program: a prospective, longitudinal study. Ital Heart J. 2005;6:900. [PubMed] [Google Scholar]
- 63*.Sirri L, Potena L, Masetti M, Tossani E, Magelli C, Grandi S. Psychological predictors of mortality in heart transplanted patients: a prospective, 6-year follow-up study. Transplantation. 2010;89(7):879. doi: 10.1097/TP.0b013e3181ca9078. [DOI] [PubMed] [Google Scholar]
- 64*.Owen JE, Bonds CL, Wellisch DK. Psychiatric evaluations of heart transplant candidates: predicting post-transplant hospitalizations, rejection episodes, and survival. Psychosomatics. 2006;47(3):213. doi: 10.1176/appi.psy.47.3.213. [DOI] [PubMed] [Google Scholar]
- 65*.van de Beek D, Kremers W, Daly RC, et al. Effect of neurologic complications on outcome after heart transplant. Arch Neurol. 2008;65(2):226. doi: 10.1001/archneurol.2007.52. [DOI] [PubMed] [Google Scholar]
- 66*.Favaro A, Gerosa G, Caforio ALP, et al. Posttraumatic stress disorder and depression in heart transplantation recipients: the relationship with outcome and adherence to medical treatment. Gen Hosp Psychiatry. 2011;33:1. doi: 10.1016/j.genhosppsych.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 67*.Farmer SA, Grady KL, Wang E, McGee EC, Cotts WG, McCarthy PM. Demographic, psychosocial, and behavioral factors associated with survival after heart transplantation. Ann Thorac Surg. 2013;95:876. doi: 10.1016/j.athoracsur.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 68*.Singh N, Gayowski T, Wagener MM, Marino IR. Depression in patients with cirrhosis: impact on outcome. Dig Dis Sci. 1997;42(7):1421. doi: 10.1023/a:1018898106656. [DOI] [PubMed] [Google Scholar]
- 69*.Gedaly R, McHugh PP, Johnston TD, et al. Predictors of relapse to alcohol and illicit drugs after liver transplantation for alcoholic liver disease. Transplantation. 2008;86:1090. doi: 10.1097/TP.0b013e3181872710. [DOI] [PubMed] [Google Scholar]
- 70*.Corruble E, Barry C, Varescon I, Falissard B, Castaing D, Samuel D. Depressive symptoms predict long-term mortality after liver transplantation. Psychosom Res. 2011;71:32. doi: 10.1016/j.jpsychores.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 71*.Corruble E, Barry C, Verescon I, Castaing D, Samuel D, Falissard B. The Transplanted Organ Questionnaire: a validation study. J Psychosom Res. 2012;73:319. doi: 10.1016/j.jpsychores.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 72.Rogal SS, Dew MA, Fontes P, DiMartini AF. Early treatment of depressive symptoms and long-term survival after liver transplantation. Am J Transplant. 2013;13:928. doi: 10.1111/ajt.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73*.Molnar-Varga M, Molnar MZ, Szeifert L, et al. Health-related quality of life and clinical outcomes in kidney transplant recipients. Am J Kidney Dis. 2011;58(3):444. doi: 10.1053/j.ajkd.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 74*.Zelle DM, Dorland HF, Rosmalen JGM, et al. Impact of depression on long-term outcome after renal transplantation: a prospective cohort study. Transplantation. 2012;94(10):1033. doi: 10.1097/TP.0b013e31826bc3c8. [DOI] [PubMed] [Google Scholar]
- 75*.Cohen L, Littlefield C, Kelly P, Maurer J, Abbey S. Predictors of quality of life and adjustment after lung transplantation. Chest. 1998;113:633. doi: 10.1378/chest.113.3.633. [DOI] [PubMed] [Google Scholar]
- 76*.Vermeulen KM, TenVergert E, Verschuuren EAM, Erasmus ME, van der Bij W. Pre-transplant quality of life does not predict survival after lung transplantation. J Heart Lung Transplant. 2008;27(6):623. doi: 10.1016/j.healun.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 77*.Evon DM, Burker EJ, Galanko JA, Egan TM. Depressive symptoms and mortality in lung transplant. Clin Transplant. 2010;24:E201. doi: 10.1111/j.1399-0012.2010.01236.x. [DOI] [PubMed] [Google Scholar]
- 78*.Popkin MK, Callies AL, Colon EA, Lentz RD, Sutherland DE. Psychiatric diagnosis and the surgical outcome of pancreas transplantation in patients with type I diabetes mellitus. Psychosomatics. 1993;34(3):251. doi: 10.1016/S0033-3182(93)71887-3. [DOI] [PubMed] [Google Scholar]
- 79*.Dobbels F, Vanhaecke J, Dupont L, et al. Pretransplant predictors of post-transplant adherence and clinical outcome: an evidence base for pretransplant psychosocial screening. Transplantation. 2009;87(10):1497. doi: 10.1097/TP.0b013e3181a440ae. [DOI] [PubMed] [Google Scholar]
- 80.Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. updated March 2011. Available from www.cochrane-handbook.org ADD AS NEW 75. [Google Scholar]; Rothman KJ. Epidemiology: An introduction. 2. NY: Oxford University Press; 2012. [Google Scholar]
- 81.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 82.Meijer A, Conradi HJ, Bos EH, Thombs BD, van Melle JP, de Jonge P. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33(3):203. doi: 10.1016/j.genhosppsych.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 83.Meijer A, Conradi HJ, Bos EH, et al. Adjusted prognostic association of depression following myocardial infarction with mortality and cardiovascular events: individual patient data meta-analysis. Brit J Psychiatry. 2013;203(2):90. doi: 10.1192/bjp.bp.112.111195. [DOI] [PubMed] [Google Scholar]
- 84.Palmer SC, Vecchio M, Craig JC, et al. Association between depression and death in people with CKD: a meta-analysis of cohort studies. Am J Kidney Dis. 2013;62(3):493. doi: 10.1053/j.ajkd.2013.02.369. [DOI] [PubMed] [Google Scholar]
- 85.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psycholog Med. 2010;40(11):1797. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kimbro LB, Mangione CM, Steers NW, et al. Depression and all-cause mortality in persons with diabetes mellitus: Are older adults at higher risk? Results from the Translating Research Into Action for Diabetes study. J Am Geriatr Soc. 2014;62:1017. doi: 10.1111/jgs.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oliva M, Singh TP, Gauvreau K, Vanderpluym CJ, Bastardi HJ, Almond CS. Impact of medication non-adherence on survival after pediatric heart transplantation in the U.S.A. J Heart Lung Transplant. 2013;32(9):881. doi: 10.1016/j.healun.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 88.Dew MA, DeVito Dabbs A, Myaskovsky L, et al. Meta-analysis of medical regimen adherence outcomes in pediatric solid organ transplantation. Transplantation. 2009;88(5):736. doi: 10.1097/TP.0b013e3181b2a0e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li M, Rodin G. Depression. In: Levenson JL, editor. The American Psychiatric Publishing Textbook of Psychosomatic Medicine : Psychiatric Care of the Medically Ill. 2. Washington, DC: American Psychiatric Publishing, Inc; 2010. p. 175. [Google Scholar]
- 90.Rush AJ, Nierenberg AA. Mood disorders: treatment of depression. In: Sadock BJ, Sadock VA, Ruiz P, editors. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. 9. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2009. p. 1734. [Google Scholar]
- 91.Harris J, Heil JS. Managing depression in patients with advanced heart failure awaiting transplantation. Am J Health Syst Pharm. 2013;70:867. doi: 10.2146/ajhp110738. [DOI] [PubMed] [Google Scholar]
- 92.Nagler EV, Webster AC, Vanholder R, Zoccali C. Antidepressants for depression in stage 3–5 chronic kidney disease: a systematic review of pharmacokinetics, efficacy and safety with recommendations by European Renal Best Practice (ERBP) Nephrol Dial Transplant. 2012;27:3736. doi: 10.1093/ndt/gfs295. [DOI] [PubMed] [Google Scholar]
- 93.Crone CC, Gabriel GM. Treatment of anxiety and depression in transplant patients: pharmacokinetic considerations. Clin Pharmacokinet. 2004;43(6):361. doi: 10.2165/00003088-200443060-00002. [DOI] [PubMed] [Google Scholar]
- 94.DiMartini AF, Crone C, Fireman M, Dew MA. Psychiatric aspects of organ transplantation in critical care. Crit Care Clinics. 2008;24(4):949. doi: 10.1016/j.ccc.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fusar-Poli P, Picchioni M, Martinelli V, et al. Anti-depressive therapies after heart transplantation. J Heart Lung Transplant. 2006;25:785. doi: 10.1016/j.healun.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 96.Heinrich TW, Marcangelo M. Psychiatric issues in solid organ transplantation. Harv Rev Psychiatry. 2009;17:398. doi: 10.3109/10673220903463259. [DOI] [PubMed] [Google Scholar]
- 97.Beck AT, Steer RA, Brown CK. Beck Depression Inventory—Second Edition Manual. San Antonio, TX: Psychological Corp, Harcourt Brace Jovanovich; 1986. [Google Scholar]
- 98.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Griva K, Davenport A, Harrison M, Newman SP. Non-adherence to immunosuppressive medications in kidney transplantation: intent vs. forgetfulness and clinical markers of medications intake. Ann Behav Med. 2012;44(1):85. doi: 10.1007/s12160-012-9359-4. [DOI] [PubMed] [Google Scholar]
- 100.De Geest S, Dobbels F, Fluri C, Paris W, Troosters T. Adherence to the therapeutic regimen in heart, lung, and heart-lung transplant recipients. J Cardiovasc Nurs. 2005;20(5 Suppl):S88. doi: 10.1097/00005082-200509001-00010. [DOI] [PubMed] [Google Scholar]
- 101.Rodrigue JR, Nelson DR, Hanto DW, Reed AI, Curry MP. Patient-reported immunosuppression nonadherence 6 to 24 months after liver transplant: association with pretransplant psychosocial factors and perceptions of health status change. Prog Transplant. 2013;23(4):319. doi: 10.7182/pit2013501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fine RN, Becker Y, De Geest S, et al. Nonadherence consensus conference summary report. Am J Transplant. 2009;9(1):35. doi: 10.1111/j.1600-6143.2008.02495.x. [DOI] [PubMed] [Google Scholar]
- 103.Denhaerynck K, Dobbels F, Cleemput I, et al. Prevalence, consequences, and determinants of nonadherence in adult renal transplant patients: a literature review. Transplant International. 2005;18(10):1121. doi: 10.1111/j.1432-2277.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 104.Pagato SL, Ma Y, Bodenlos JS, et al. Association of depressive symptoms and lifestyle behaviors among Latinos at risk of type 2 diabetes. J Am Dietetic Assoc. 2009;109(7):1246. doi: 10.1016/j.jada.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Granner ML, Mburia-Mwalili A. Correlates of television viewing among African American and Caucasian women. Women Health. 2010;50(8):783. doi: 10.1080/03630242.2010.533090. [DOI] [PubMed] [Google Scholar]
- 106.Duivis HE, de Jonge P, Penninx BW, Na BY, Cohen BE, Whooley MA. Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: prospective findings from the heart and soul study. Am J Psychiatry. 2011;168(9):913. doi: 10.1176/appi.ajp.2011.10081163. [DOI] [PubMed] [Google Scholar]
- 107.Strine TW, Mokdad AH, Dube SR, et al. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen Hosp Psychiatry. 2008;30(2):127. doi: 10.1016/j.genhosppsych.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 108.Pantell M, Rehkopf D, Jutte D, Syme SL, Balmes J, Adler N. Social isolation: a predictor of mortality comparable to traditional clinical risk factors. Am J Public Health. 2013;103(11):2056. doi: 10.2105/AJPH.2013.301261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barger SD. Social integration, social support and mortality in the US National Health Interview Survey. Psychosom Med. 2013;75(5):510. doi: 10.1097/PSY.0b013e318292ad99. [DOI] [PubMed] [Google Scholar]
- 110.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaptoge S, Di Angelantonio E, Lowe G, et al. C-Reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hung KC, Wu CC, Chen HS, et al. Serum IL-6, albumin and co-morbidities are closely correlated with symptoms of depression in patients on maintenance haemodialysis. Nephrol Dial Transplant. 2011;26(2):658. doi: 10.1093/ndt/gfq411. [DOI] [PubMed] [Google Scholar]
- 113.Vaccarino V, Johnson BD, Sheps DS, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE Study. J Am Coll Cardiol. 2007;50(21):2044. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 114.Cashion AK, Holmes SL, Arheart KL, Acchiardo SR, Hathaway DK. Heart rate variability and mortality in patients with end stage renal disease. Nephrol Nurs J. 2005;32(2):173. [PubMed] [Google Scholar]
- 115.Kojima M, Hayano J, Fukuta H, et al. Loss of fractal heart rate dynamics in depressive hemodialysis patients. Psychosom Med. 2008;70(2):177. doi: 10.1097/PSY.0b013e31816477a1. [DOI] [PubMed] [Google Scholar]
- 116.Berney-Martinet S, Key F, Bell L, Lepine S, Clermont MJ, Fombonne E. Psychological profile of adolescents with a kidney transplant. Pediatr Transplant. 2009;13:701. doi: 10.1111/j.1399-3046.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- 117.Dew MA, DiMartini AF, DeVito Dabbs AJ, et al. Onset and risk factors for anxiety and depression during the first 2 years after lung transplantation. Gen Hosp Psychiatry. 2012;34:127. doi: 10.1016/j.genhosppsych.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dew MA, Kormos RL, DiMartini AF, et al. Prevalence and risk of depression and anxiety-related disorders during the first three years after heart transplantation. Psychosomatics. 2001;42:300. doi: 10.1176/appi.psy.42.4.300. [DOI] [PubMed] [Google Scholar]
- 119.Woodman CL, Geist LJ, Vance S, Laxson C, Jones K, Kline JN. Psychiatric disorders and survival after lung transplantation. Psychosomatics. 1999;40(4):293. doi: 10.1016/S0033-3182(99)71221-1. [DOI] [PubMed] [Google Scholar]
- 120.White HD. Scientific communication and literature retrieval. In: Cooper H, Hedges LV, Valentine JC, editors. Handbook of Research Synthesis and Meta-Analysis. 2. New York: Russell Sage; 2009. pp. 51–72. [Google Scholar]
- 121.Fleiss JL. Statistical Methods for Rates and Proportions. 2. New York: Wiley; 1981. [Google Scholar]