Abstract
Objective
Current treatment options for lupus are far from optimal. Previously, we reported that PI3K/AKT/mTOR, MEK1/Erk1,2, p38, STAT3, STAT5, NF-κB, multiple Bcl-2 family members, and various cell cycle molecules were over-expressed in splenic B-cells in an age-dependent and gene-dose-dependent manner in mouse strains with spontaneous lupus. As the synthetic triterpenoid methyl-2-cyano-3,12-dioxooleana-1,9-dien-28-oate (CDDO-Me) has been shown to inhibit AKT, MEK1/2, and NF-κB, and to induce caspase-mediated apoptosis, we tested the therapeutic potential of this agent in murine lupus nephritis.
Methods
The synthetic triterpenoid CDDO-Me, or placebo, was administered to two month old B6.Sle1.Sle3 mice or MRL.lpr mice, which develop spontaneous lupus. All mice were phenotyped for disease.
Results
CDDO-Me-treated mice exhibited significantly reduced splenic cellularity, with decreased CD4+ T-cells and activated CD69+/CD4+ T-cells compared to the placebo-treated mice. These mice also exhibited significant reduction in serum autoantibody levels, including anti-dsDNA and anti-glomerular antibodies. Finally, CDDO-Me treatment attenuated renal disease in mice, as marked by reduced 24-hour proteinuria, blood urea nitrogen, and glomerulonephritis. At the mechanistic level, CDDO-Me treatment dampened MEK1/2, ERK, and STAT3 signaling within lymphocytes and oxidative stress. Importantly, the NF-E2-Related Factor 2 (Nrf2) pathway was activated after CDDO-Me treatment, indicating that CDDO-Me may be modulating renal damage in lupus via the inhibition of oxidative stress.
Conclusion
These findings underscore the importance of AKT/MEK1/2/NF-κB signaling in engendering murine lupus. Our studies reveal that the blockade of multiple signaling nodes and oxidative stress may effectively prevent and reverse the hematological, autoimmune and pathological manifestations of lupus.
Keywords: SLE, Lymphoproliferation, alternative medicine, oxidative stress, signaling
Introduction
Systemic lupus erythematosus (SLE) is a highly complex autoimmune disease, characterized by hyperproliferation and hyperactivation of lymphocytes, autoantibody production, and eventually end organ damage. Several lines of evidence have indicated that specific signaling pathways are involved in the pathogenesis of lupus. For example, overexpression of both phosphoinositide 3-kinase (PI3K) (1) and the anti-apoptotic molecule Bcl-2 (2), as well as haplo-insufficiency of the tumor suppressor PTEN (Pten+/−) (3) have been shown to cause lymphoproliferative lupus. Our previous studies demonstrated that multiple signaling pathways are upregulated in lupus B cells, including the AKT pathway, MAPK pathway, Jak/STAT pathway, CDK pathway, NF-κB pathway, and pathways downstream of some anti-apoptotic Bcl-2 family members (4). Given that lupus is associated with the activation of multiple signaling axes, therapies targeting multiple activated signaling cascades may prove to be more effective in curtailing this disease.
2-cyano-3, 12-dioxoolean-1, 9-dien-28-oic acid (CDDO), a novel synthetic triterpenoid derived from oleanolic acid, has been shown to be a more potent anti-tumor and anti-inflammatory agent than its natural plant-derived analogues (5, 6). The C-28 methyl ester of CDDO, CDDO-Me, also known as bardoxolone methyl, has been shown to inactivate STAT3 signaling (7, 8), inhibit mitochondrial electron transport via perturbations in inner mitochondrial membrane integrity (9), block the NF-kB pathway (10), induce apoptosis by disrupting intracellular redox balance (11), induce the proapoptotic Bax protein (12), inhibit the activation of Erk1,2 (13), and block Bcl-2 phosphorylation (12). Additionally, CDDO-Me protects against LPS-induced inflammatory responses via activation of the NF-E2-Related Factor 2 (Nrf2)-dependent anti-oxidative pathway (14). Very recently CDDO-Me has been shown to effectively sustain increases in the estimated glomerular filtration rate of patients with advanced chronic kidney disease and type 2 diabetes in a Phase II clinical trial (15). Given the fact that CDDO-Me can target multiple signaling pathways in multiple cell types, we examined whether it had the potential to suppress lymphoproliferation, autoantibody production and renal inflammation in murine lupus.
Results
Hyperactivation of B cells and CD4+ T cells was suppressed by CDDO-Me
Given the previous demonstration of CDDO-Me in suppressing cell proliferation, we asked whether CDDO-Me could suppress the development of splenomegaly in mice with lupus. To address this, 2-mo-old B6.Sle1.Sle3 female mice were treated for 60 days with CDDO-Me or placebo, then splenic size and cellularity were assessed in both groups. Strikingly, the overall splenic weights in this group were decreased almost 50% compared to the placebo-treated group (Fig. 1A). Consistently, the total number of splenocytes was also decreased in the CDDO-Me treated group, compared to those treated with placebo (Fig. 1B). Next, we asked which cell populations were significantly suppressed by CDDO-Me. As expected, among splenic T cells, the percentage of CD4+ T cells was decreased (12.1 ± 0.35% vs 15.1 ± 1.2%, P = 0.021), while the percentage of CD8+ T cells was increased (9.73 ± 0.4% vs 6.8 ± 1.1%, P = 0.023) in the CDDO-Me treated group (Fig. 1C). The absolute number of total splenic CD4+ T cells was also decreased (18.7 ± 3.8 million vs 39.0 ± 2.0 million, P < 0.0001) in the CDDO-Me treated group (Table 1). Within the CD4+ T cell compartment, the activated population (CD69+) was significantly deceased in the CDDO-Me treated group compared to controls (Fig. 1 and Table 1). Of note, besides the dramatic reduction and deactivation of CD4+ T cells, the absolute cell numbers (if not percentages) of splenic B220+ B cells (both mature and immature B cells, and B1a cells) were also decreased with CDDO-Me treatment (Table 1). The activation status of the B-cells, as gauged by surface CD86 expression was also markedly reduced following CDDO-Me treatment (6.48±0.42 vs 8.72±0.45 MFI units, P < 0.002; data not plotted). Importantly, after CDDO-Me treatment, the cell number and activation status of splenic B cells and T cells and their subsets were reversed to normal, similar to the phenotypes seen in healthy B6 mice previously reported (16).
Figure 1.
CDDO-Me attenuates disease in B6.Sle1.Sle3 spontaneous lupus mice. 2-month-old female B6.Sle1.Sle3 (N = 20/group) were treated with CDDO-Me or placebo (sesame oil) as indicated. CDDO-Me ameliorated splenomegaly (A-B) and suppressed expansion of activated CD4+ T cells in B6.Sle1.Sle3 mice examined 60 days following treatment (C). The flow cytometry plots shown in the figure are from one representative experiment, while data from all experiments are summarized in Table 1. H&E staining of kidney shown is representative of several similar experiments (D, n = 10). Proteinuria (E), glomerulonephritis scores (F) and BUN (G) were reduced in CDDO-Me-treated B6.Sle1.Sle3 mice after two months of treatment. CDDO-Me treatment attenuated serum IgG autoantibody levels in B6.Sle1.Sle3 mice (H-K). Each dot represents the autoantibody level of an individual mouse at the age of 2 mo (D0) or 4 mo (D60). AU = Arbitrary Unit. Serially diluted B6.Sle1.lpr sera were used for plotting a standard curve, and the highest standard was set as 100 AU. Student’s t-test was used to assess statistical significance.
Table 1. Activation status and lymphocyte subsets in B6.Sle1.Sle3 mice treated with CDDO-Me or placebo (10 mice per group). All mice were sacrificed at the age of 4 mo.
| Placebo | CDDO-Me | P value | |
|---|---|---|---|
| Total spleen cell count (×106) | 146.8 ± 2.5 | 89.1 ± 2.3 | 0.0005 |
| Splenic T Cells | |||
| CD4 T cells | 39.0 ± 2.0 | 18.7 ± 3.8 | <0.0001 |
| CD8 T cells | 9.9 ± 1.3 | 8.6 ± 2.4 | NS |
| CD69+/CD4 T cells | 14.5 ± 0.9 | 5.1 ± 0.6 | <0.0001 |
| CD69+/CD8 T cells | 1.4 ± 0.3 | 0.6 ± 0.1 | 0.008 |
| Splenic B cells | |||
| B220+, AA4.1− (Mature) | 64.8 ± 4.5 | 45.3 ± 3.2 | 0.013 |
| Follicular B cells | 40.8 ± 2.7 | 30.9 + 1.6 | 0.017 |
| Marginal zone B cells | 8.3 ± 0.5 | 4.0 ± 0.2 | 0.062 |
| B1a cells | 7.5 ± 0.9 | 4.0 ± 0.3 | 0.013 |
| B220+, AA4.1+ (Immature) | 14.0 ± 1.3 | 10.4 ± 1.1 | 0.032 |
| T1 Immature | 2.0 ± 0.2 | 1.0 ± 0.1 | 0.02 |
| T2 Immature | 4.5 ± 0.3 | 3.1 ± 0.2 | NS |
| T3 Immature | 4.2 ± 0.2 | 4.0 ± 0.2 | 0.061 |
CDDO-Me treatment of B6.Sle1.Sle3 mice ameliorated kidney disease as manifested by reduced proteinuria, blood urea nitrogen (BUN), and glomerulonephritis (GN)
Next, we examined if the administration of CDDO-Me subdues renal damage in murine lupus nephritis. At D60 after placebo or CDDO-Me treatment, urine was collected and proteinuria was examined. Compared to the placebo-treated group, the CDDO-Me-treated group showed significantly reduced proteinuria (Fig. 1E). Kidney pathology analysis clearly demonstrated that administration of CDDO-Me resulted in lower GN scores compared to placebo treatment. By microscopic analysis, we noted increased cellularity in the glomeruli of mice treated with placebo compared to those administered CDDO-Me, indicating the presence of more inflammation and greater numbers of infiltrating cells in the placebo mice. CDDO-Me treatment also led to reduced BUN levels, further indicating that renal function was improved in these mice (Fig. 1F-G). Most importantly, all parameters of renal disease were reversed to normal, similar to the phenotypes see in healthy B6 mice previously reported (16).
CDDO-Me treatment ameliorated autoantibody production in B6.Sle1.Sle3 mice
After 60 days of CDDO-Me treatment, the serum levels of IgG anti-dsDNA, anti-ssDNA, anti-histone, and anti-glomerular antibodies were all significantly decreased compared to the placebo group. Prior to the initiation of CDDO-Me treatment (D0), basal levels of IgG anti-dsDNA, anti-ssDNA, anti-histone, and anti-glomerular antibodies were measured and found to be comparable (Fig. 1H-K). It is important to note that after CDDO-Me treatment, all the IgG antibody levels listed above were reversed to normal, similar to the phenotypes seen in healthy B6 mice previously reported (16).
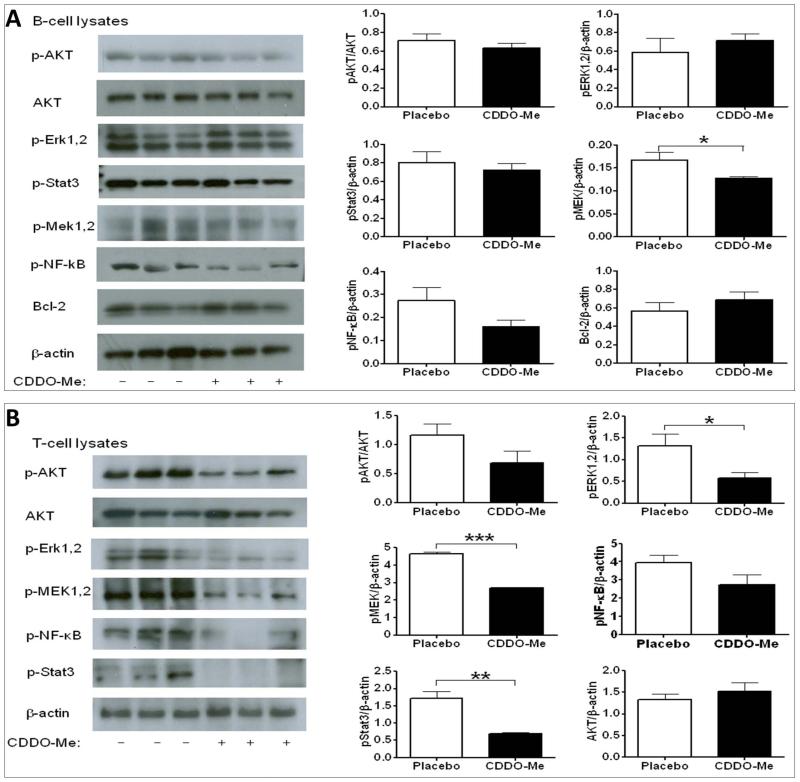
CDDO-Me treatment reduced MEK activation in splenic B cells from lupus-prone mice
Currently, the major therapeutic use of CDDO-Me is to inactivate signaling pathways underlying cell growth and cell proliferation in cancer. To address the effects of CDDO-Me administration on lymphocyte signaling, splenic B220+ B cells were purified with magnetic beads, and B cell lysates were analyzed by western blot analysis for several signaling axes. Our results demonstrate that Mek1/2 activation was significantly dampened in splenic B cells from the CDDO-Me treated group (Fig. 2A). In addition, CDDO-Me treatment also appeared to diminish the activation of NF-κB, STAT3, and to a lesser extent AKT, although these differences were not statistically significant.
Figure 2.
CDDO-Me inhibited selected signaling axes in splenic B cells (A) or T cells (B) of B6.Sle1.Sle3 mice (female, n = 10 each). At the end of the 60-day CDDO-Me treatment period (i.e., all mice were at 4 mo age), splenic B or T cells were purified using magnetic beads, and cell lysates were examined for signaling status by western blot. Quantification of western blot results was done with ImageJ software (NIH) and plotted in the bar charts. Each bar is representative of band intensities observed in 10 mice. Student’s t-test was used to assess statistical significance. (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
CDDO-Me treatment reduced the activation of selected signaling pathways in T cells
To determine if CDDO-Me treatment similarly impacted signaling cascades in splenic T cells, splenic CD4+ T cells were isolated and their lysates were analyzed as described above. The activation/phosphorylation of Erk1/2, Mek1/2, and STAT3 were significantly ameliorated by CDDO-Me treatment in CD4+ T cells (Fig. 2B). Both AKT and NF-κB showed a trend towards reduced phosphorylation, but these differences did not reach statistical significance. Collectively, the data in Figures 2A and 2B provide strong evidence that multiple signaling pathways were inhibited in both splenic B cells and CD4+ T cells following CDDO-Me administration.
CDDO-Me may also confer disease protection by altering the redox pathway
Kidney damage can be triggered via oxidative or inflammatory signals. In this context, CDDO-Me has previously been shown to protect against LPS-induced inflammatory responses via activation of the NF-E2-Related Factor 2 (Nrf2)-dependent anti-oxidative pathway (14). Hence, we examined the CDDO-Me treated mice for evidence of enhanced Nrf2 activation in flash-frozen renal tissues. As shown in Figure 3, the anti-oxidative modulator, Nrf2, and its target, NQO1, were both significantly increased after CDDO-Me treatment within the kidneys. The Nrf2 regulator GCLC also showed a trend towards enhanced expression after CDDO-Me treatment. These results suggest that CDDO-Me may also protect against renal damage in murine lupus nephritis by altering the intra-renal redox balance.
Figure 3.
The Nrf2 signaling pathway was upregulated in the kidneys of B6.Sle1.Sle3 mice after CDDO-Me treatment. Kidneys were collected from both CDDO-Me-treated and placebo-treated B6.Sle1.Sle3 mice (female, n = 4-5 each), and kidney lysates were used for western blots. Quantification of western blot results was done with ImageJ (NIH) and plotted in the bar charts; each bar is representative of band intensities observed in 4-5 mice. Student’s t-test was used to assess statistical significance. (***, p < 0.001)
Validating the efficacy of CDDO-Me in another lupus mouse model: MRL.lpr
To confirm if CDDO-Me can attenuate disease in other murine lupus strains that are genetically different from B6.Sle1.Sle3, we administered CDDO-Me to 2-month-old MRL.lpr, another strain that develops spontaneous lupus. In addition, to clarify if the vehicle sesame oil by itself has any modulatory effects on murine lupus, we included another control group where only water was administered. In this validation study, we also included a “positive control” group for drug treatment, where the lupus-prone mice were treated with dexamethasone. Dexamethasone is a glucocorticoid, and some glucocorticoids are standard therapy for human lupus (17). Once again, we found CDDO-Me significantly reduced splenomegaly, compared to placebo (sesame) group (Fig 4A). As expected, we observed a significant reduction of anti-dsDNA antibody levels (Fig. 4B), proteinuria and GN scores (Fig. 4C) in the CDDO-Me treated group compared to the control groups. The drug control group administered dexamethasone also demonstrated disease improvement after treatment, comparable to the efficacy noted with CDDO-Me. These studies also demonstrated that sesame oil vehicle by itself did not have any significant impact on the disease since the mice treated with sesame oil exhibited phenotypes that were comparable to the mice fed with water. We further examined the signaling status in both B cells and T cells in the spleen after CDDO-Me treatment. The phosphorylation of MEK and Erk1,2 were downregulated whereas the anti-oxidative molecule Nrf2 was upregulated in both lymphocyte compartments (Fig. 4D).
Figure 4.
CDDO-Me ameliorated disease in another lupus mouse model-MRL.lpr. After 2 mo of treatment, splenomegaly was significantly reduced in CDDO-Me treated mice, compared to the water-treated group or sesame-treated group (N = 8 per group). Significantly reduced spleens were also noted in the dexamethasone-treated group (A). Compared to water-treated or sesame-treated mice, anti-dsDNA antibody levels (B), proteinuria and GN score (C) were also significantly decreased after CDDO-me treatment. Compared to the placebo group, the phosphorylation of MEK and Erk1,2 were reduced while Nrf2 was increased in both splenic B cells and T cells after CDDO-Me treatment.
CDDO-Me mitigated oxidative stress in splenic CD4+ T cells and CD11b+ cells
To investigate if CDDO-Me has any modulatory effect on oxidative stress in murine lupus, we examined the level of Reactive Oxygen Species (ROS) in the splenocytes using a DCFDA kit as detailed in Materials and Methods. We found the ROS levels as indicated by DCFDA+ cells (%) were significantly reduced among both CD4+ T cells and CD11b+ cells in the CDDO-Me treated group, compared to the other control groups, including the dexamethasone treated mice (Fig 5A-C, Fig S1). We also found in CD4+ T cells, the ROS levels positively correlated with the phosphorylation of Erk1,2 and MEK, but negatively correlated with Nrf2 levels, suggesting that the pathways by which CDDO-Me influences lymphocyte signaling may be mechanistically linked to the pathways that generate ROS (Fig 5D-F). To determine if the upregulation or phosphorylation of the signaling molecules MEK1 and STAT3 may be mechanistically linked to altered ROS in murine lupus, we measured DCFDA+ (%) cells among splenocytes following in vitro inhibition of MEK1 or STAT3; however, we did not find any significant association between ROS levels and MEK1 or STAT3 activation (Fig S2).
Figure 5.
CDDO-Me treatment significantly reduced cellular ROS in both splenic CD4+ T cells and CD11b+ cells but not B220+ B cells isolated from 4-month-old MRL.lpr mice (A-C). The reduction of ROS positively correlates with the decreased phosphorylation of Erk1,2 and MEK (D-E), but negatively correlates with the increase in Nrf2.
CDDO-Me could also attenuate lupus phenotypes after disease onset in NZM2410 mice
Finally, to test the therapeutic efficacy of CDDO-Me after disease onset, we carried out an additional treatment study by administering CDDO-Me to a third strain of lupus mice (NZM2410, age = 7mo; N = 4-7 per group) for a period of 2 months. These mice were already proteinuric at the beginning of the study. Once again, CDDO-Me was effective in improving survival, and reducing cellularity, circulating antibodies and proteinuria (Fig. S3). Thus, CDDO-Me appears to be therapeutically effective even when administered after disease onset.
CDDO-Me treatment did not cause any apparent side effects in lupus mice
Since this was a relatively long-term study, and since CDDO-Me cripples multiple signaling axes, one concern may be potential side effects. After 60 days of drug administration (either in the preventative or treatment study), body weights of mice from both the CDDO-Me group and the placebo group were similar.Likewise, all blood cell counts including WBC, RBC, HGB, HCT, MCV, MCH, MCHC, RDW, MPV, NEUT, LYMPH, EOS remained similar between the two groups (Figure S4). Platelet counts in the two groups were also comparable. These results indicate that the mice suffered no apparent hematological side effects or weight loss as a result of CDDO-Me administration. In addition, we monitored liver function by assaying aspartate aminotransferase (AST); however, there were no significant changes in the CDDO-Me treated group (AST activity: 75.7 ± 6.5 U/L) compared to the placebo group (74.9 ± 5.8 U/L).
DISCUSSION
Lupus is a highly complex autoimmune disease, where B cells, T cells, and even myeloid cells are hyperproliferative and hyperactive. These hyperactivated immune cells can infiltrate organs, causing tissue damage, resulting in end organ problems such as nephritis. Previous studies have demonstrated that despite the distinct genetic backgrounds of mouse models used for studying spontaneous lupus, these different strains share the upregulation of similar cell signaling pathways involving PI3K/AKT/mTOR, MAP kinases, STAT3/5, NF-κB, multiple Bcl-2 family members, and various cell cycle molecules in B cells (4). Several key signaling molecules including NF-κB (18), STAT3 (19), CaMKIV (20), Syk (21) have also been observed to be altered in lupus T cells. Furthermore, several of these signaling intermediates are positive regulators of a number of inflammatory cytokines and chemokines. Hence, intervention in leukocyte signaling pathways might be beneficial in the treatment of lupus.
Triterpenoids are natural plant products generated by the cyclization of squalene and are used for medicinal purpose in many Asian countries, as they have been reported to have anti-carcinogenic activity (22-25). Because the biological activities of some of the natural triterpenoids are not strong enough, new analogues of these molecules have been chemically synthesized in an attempt to produce more potent agents. One of these analogues, CDDO was found to inhibit proliferation of many human cancer cells and to suppress the ability of various inflammatory cytokines such as IFN-γ, interleukin-1, and tumor necrosis factor-α. CDDO-Me is a methyl derivative of CDDO that was found to be as active as CDDO in suppressing the increased production of nitric oxide by IFN-γ in mouse macrophages (26). Further, there are a number of studies showing CDDO-Me can block selected signaling pathways. For example, others have identified CDDO-Me as a potent caspase-mediated apoptosis inducer in human lung cancer in acute myelogenous leukemia (12, 27). CDDO-Me has also been shown to directly inhibit both JAK1 and STAT3 (7) and to inhibit the NF-κB pathway through direct inhibition of IKKβ on Cys-179 (10). Further, this compound has also been shown to inhibit IκB kinase and to enhance apoptosis induced by TNF and chemotherapeutic agents through downregulation of NF-κB-regulated gene products in human leukemic cells (28).
Our findings are consistent with the above reports since CDDO-Me treatment diminished the activation of MEK1/2 in B cells, and ERK, MEK and STAT3 in T cells. In both T and B cells, NF-κB showed a trend towards reduced activation following CDDO-Me treatment, but these differences did not reach statistical significance. These findings suggest that CDDO-Me can suppress cell activation and inflammatory signals mediated via multiple signaling axes not only in cancer cells, but also in immune cells and possibly in other tissues (including renal cells).
Indeed this is the first report demonstrating that CDDO-Me is beneficial in suppressing hyperactivation of immune cells, particularly CD4+ T cells (Fig. 1 and Table 1). In a murine acute graft-versus-host disease model, CDDO-Me exhibited an increased ability to inhibit allogeneic T cell responses and induce cell death of alloreactive T cells in vitro (29). In a transgenic adenocarcinoma of the mouse prostate (TRAMP) cancer model, CDDO-Me induced apoptosis in TRAMPC-1 cells, as revealed by the increased expression of annexin V and cleavage of procaspases 3, −8, and −9; CDDO-Me also inhibited NF-kappaB-regulated antiapoptotic Bcl-2, Bcl-xL, and XIAP (30, 31). Additionally, CDDO-Me participates in the induction of apoptosis in acute myeloid leukemia (32). In this study, both splenic B cells and T cells were decreased in the CDDO-me treated group compared to the placebo controls, suggesting that CDDO-Me might be inducing apoptosis in splenic B and T cells, thereby subduing autoimmunity.
The reduced activation of lymphocytes was associated with a reduction in the production of autoantibodies such as anti-dsDNA, anti-ssDNA, anti-histone, and anti-GBM in B6.Sle1.Sle3 mice following CDDO-Me treatment (Fig. 1). Importantly, the most prominent benefit of this drug lies in its effective prevention of renal damage, as marked by the dramatic reduction in proteinuria, BUN, GN score, and other renal pathology measures (Fig. 1). A recent clinical trial of Bardoxolone methyl (another name for CDDO-Me) carried out in patients with advanced chronic kidney disease and type 2 diabetes has demonstrated its capacity in sustaining an increase of the estimated glomerular filtration rate (15). Our findings are consistent with that report, and suggest that CDDO-Me might be of therapeutic benefit in chronic renal disease arising from multiple initial triggers.
Besides dampening cell signaling, triterpenoids may also improve disease outcomes through other mechanisms. CDDO and its derivatives have been found to induce Nrf2 signaling, which in turn induces cytoprotective and anti-oxidative genes (38, 39). The transcription factor Nrf2 binds and activates the antioxidant response element ARE (40), a cis-acting sequence found in the 5′ flanking region of genes encoding many cytoprotective enzymes, including the NAD(P)H:quinone oxidoreductase NQO1 (41-43). It has been demonstrated that reactive oxygen species (ROS) are present at higher levels during lupus nephritis (44), therefore, antioxidant molecules such as Nrf2/NQO1 might be beneficial in protecting against ROS-induced kidney damage. In this study, we have shown that Nrf2 and its partner NQO1 were significantly induced in the kidneys of B6.Sle1.Sle3 mice after CDDO-Me treatment (Fig. 3). Our results suggest that renal damage and potentially other tissue damage may be ameliorated by CDDO-Me, in part via the activation of the anti-oxidant pathway.
The importance of Nrf2 in protecting against lupus nephritis has been reported before. Interestingly, Nrf2-deficient female mice develop lupus-like autoimmune nephritis (45). Similar to the current study, other natural agents that are beneficial in lupus nephritis have also been associated with renal Nrf2 elevation. Antroquinonol, a purified compound and major effective component of Antrodia camphorate, inhibited the production of reactive oxygen species and nitric oxide, but increased the activation of Nrf2 within the kidneys in an accelerated mouse model of severe lupus nephritis. This was associated with significantly reduced infiltrating T cell proliferation and renal lesions (46). Epigallocatechin-3-gallate (EGCG), the major bioactive polyphenol in green tea has also been shown to increase Nrf2 and ameliorate renal disease in NZB/W F1 mice (47).
The present studies reveal that CDDO-Me could abrogate reactive oxygen species both in vivo and in vitro (Fig. 5, Fig S1). However, our studies suggest that the impact of CDDO-Me on ROS levels and lymphocyte signaling may be independent events (Fig. S3), though this needs to be examined more carefully. There is some evidence in the literature linking both these molecular phenomena. Thus, the inactivation of STAT3 has been observed to suppress load-driven mitochondrial activity, leading to elevated levels ROS in cultured primary osteoblasts (48). Conversely, ROS activates STAT3 and induces IL6 production in cancer cells (49). Also, treating rat sympathetic neurons with a MEK1 inhibitor greatly decreased cellular concentrations of glutathione, a major cellular antioxidant (50). Clearly, the mechanistic links between ROS production and lymphocyte signaling warrants further study, particularly in the context of autoimmunity.
There is a very interesting relationship between oxidative stress and one particular cell signaling pathways in lupus. The anti-oxidant N-acetyl cysteine (NAC) has been shown to inhibit mTOR activity in vitro, and also confer therapeutic benefit in murine lupus (33, 34). In resonance with those earlier reports, more recent work by Lai and co-workers has also demonstrated that NAC confers therapeutic benefit in patients with SLE, once again associated with mTOR inhibition and enhanced lymphocyte apoptosis (35). Although mTOR was not directly assessed in the present study, our finding that AKT phosphorylation is reduced in T-cells following CDDO-me treatment is consistent with the above literature reports. Although further mechanistic studies are warranted, collectively these findings suggest that one important mechanism of action of anti-oxidants in lupus might be reduced signaling via the AKT/mTOR axis coupled with elevated apoptosis of immune effector cells. Indeed, there is recent evidence that mTOR is a direct target of CDDO-me (36). The relationship between oxidative stress and mTOR, and its implications for the pathogenesis and treatment of SLE are elegantly discussed in a recent review by Perl (37).
In summary, CDDO-Me, a drug known to inhibit cell growth, has a reproducible impact on suppressing murine lupus and lupus nephritis when administered pre-disease, and more importantly, post-disease. This agent appears to be operating by suppressing multiple cell signaling axes in leukocytes (and possibly other tissues) and countering oxidative stress. Given the efficacy of this agent in modulating immune cell signaling as well as lupus nephritis, this may be an attractive option to pursue in the context of human lupus therapeutics.
Materials and Methods
Mice
C57BL/6 (B6), MRL.lpr and NZM2410 mice were obtained from The Jackson Laboratory and bred in our animal facility. The derivation of B6 congenic mice bearing different NZM2410-derived lupus susceptibility intervals has been detailed previously (51). B6.Sle1.Sle3 mice, bicongenic two lupus susceptibility intervals Sle1 and Sle3, were previously derived by intercrossing the respective monocongenic strains (4) . All mice used for this study were bred and housed in a specific-pathogen-free colony at UT Southwestern Medical Center Department of Animal Resources, in Dallas, TX. Both male and female mice were used, and any observed sex differences are noted. Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at UT Southwestern Medical Center at Dallas.
Flow cytometric analysis and antibodies
Splenocytes were depleted of red blood cells using a lysis buffer (containing 0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA, pH 7.2), and single-cell suspensions were prepared for flow cytometric analysis. Flow cytometric analysis was performed as described previously (16).
Purification of splenic B220+ B-cells and CD4+ T-cells
Spleens were harvested from euthanized mice and single cell suspensions were prepared by crushing spleens between frosted glass slides. RBC were lysed using ACK lysis buffer (Invitrogen) followed by two washes in PBS + 0.5 % BSA. B220+ B-cells were purified by positive selection from this total splenocyte suspension with B220 microbeads (Miltenyi Biotech). CD4+ T-cells were purified by positive selection from total splenocytes with CD4 microbeads (Miltenyi Biotech) using the manufacturer-recommended protocol.
ELISA for autoantibodies
The anti-dsDNA, anti-histone, and anti-histone/DNA ELISA assays were carried out as described before (16). Raw OD was converted to Units/ml (U/ml), using a positive control serum derived from a B6.Sle1.lpr mouse, arbitrarily setting the reactivity of a 1:100 dilution of this serum to 100 U/ml. Test sera with reactivity stronger than the standard were diluted further and re-assayed. The glomerular-binding ELISA was performed as described previously (16), using sonicated rat glomeruli as the substrate.
Western Blot analysis
Purified B cells or T-cells were lysed using 20 mM Tris-HCl (pH7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 μg/ml leupetin, 1% Triton X-100, 1 mM PMSF and 1 mM Na3VO4. Total protein was quantified by the Bradford assay, and 10 μg was loaded per lane onto SDS-PAGE gels. The following primary Abs were obtained from Cell Signaling Technology: p-STAT3S727 (#9134), P-Erk1,2T202/Y204 (#4376), Erk1,2 (#4695), p-NF-κBS536 (#3031), p-MEK1/2S217/221 (#9121), MEK1/2 (#9122), AKT (#9272), p-AKTT308 (#9275), α-tubulin (#2144). β-actin (Advanced Immunochemical Inc, catalog # RGM2), Bcl-2 (Santa Cruz Biotechnology, Inc., # sc-23960). Antibodies to Nrf2 (ab71890), NQO1 (ab2346), GCLC (ab53179) and GCLM (ab81445) were purchased from Abcam. HRP-conjugated secondary antibodies and the ECL-plus detection kit (Amersham) were used to develop the blot. For western blot analysis of purified B and T-cells, cells were pooled from 3-4 mice for each lane. The respective band intensities were measured using ImageJ software version 1.37 (http://rsb.info.nih.gov/ij), and normalized against the corresponding β-actin or α-tubulin levels. Where samples from different strains were compared, normalized band intensities were expressed as ratios, relative to the corresponding B6 levels.
In vivo Studies
In the first preventive study, CDDO-Me was diluted in sesame oil and administered to 2-month-old B6.Sle1.Sle3 mice (N = 20) at a final dose of 3 mg/kg CDDO-Me or vehicle alone (i.e., “placebo”), by oral gavage 3x/week for a period of 2 mo. In a confirmatory preventive study, 2-month-old MRL.lpr mice (n = 8 per group) were orally administered CDDO-Me (3mg/kg), or sesame oil or water or treated with dexamethasone (1mg/kg) for 2 months, all reagents being administered 3 times/week. Serum and 24-h urine samples were obtained on D0, D14, and D60. All serum samples were assayed for autoantibodies by ELISA, and the urine samples were assayed for total protein as described before (16, 52). On D60, upon sacrifice, the cellular composition of the spleen and lymph nodes were determined by flow cytometry, and the kidneys were examined for pathology, as described. In addition, the expression of various signaling molecules in the spleens and kidneys of the treated mice was assayed by Western blot, as described above. In the Treatment study, proteinuric 7mo NZM2410 mice (N = 4-7) were treated with CDDO-Me (3mg/kg) or placebo by oral gavage 5x/week for a period of 2 month. Proteinuria, autoantibody production, spleen weights and survival rates were assessed.
Cellular Reactive Oxygen Species Detection
Cellular Reactive Oxygen Species Detection was performed using a Flow Cytometry based method with a DCFDA-Cellular Reactive Oxygen Species Detection Assay Kit (Abcam, #ab113851). For the in vivo studies, splenocytes were harvested from MRL.lpr mice after treatment with CDDO-me or placebos or dexamethasone. An aliquot of 1.5 × 105 cells was stained with 20 μM DCFDA or antibodies to cell surface markers (B220, CD4 and CD11b). DCF was excited by the 488 nm laser and detected at 535 nm. For the in vitro studies, splenocytes were isolated from 4-month-old MRL.lpr mice and treated with a STAT3 inhibitor (1 μM of Cucurbitacin I, Santa Cruz Biotechnology) or MEK1 inhibitor (50 μM of PD98059, Cell Signaling Technology) for 4 hours. These cells were then stained with 20 μM DCFDA or antibodies to cell surface markers (B220, CD4 and CD11b) for flow cytometric analysis.
Renal disease
24-h urine samples were collected using metabolic cages. The total amount of urinary protein was assayed using a Coomassie-based assay (Pierce). Upon sacrifice, kidneys were fixed, sectioned, and stained with hematoxylin and eosin, and periodic acid schiff. At least 100 glomeruli were examined per section by light microscopy for evidence of inflammation and/or tissue damage, and graded as described before (52), in a blinded fashion. The occurrence of any mesangiopathic, capillary hyaline, proliferative, membranous, or crescentic, glomerular changes was also noted.
Statistics
Statistical comparisons were performed using the paired or unpaired Students’t test, as appropriate, using SigmaStat (Jandel scientific). For all experiments, the mean/SEM is also depicted. P < 0.05 was considered significant.
Acknowledgments
Financial support statement: This work was supported by National Institutes of Health grant AR50812.
Footnotes
There are no financial conflicts of interest associated with this work.
References
- 1.Borlado LR, Redondo C, Alvarez B, Jimenez C, Criado LM, Flores J, et al. Increased phosphoinositide 3-kinase activity induces a lymphoproliferative disorder and contributes to tumor generation in vivo. FASEB J. 2000;14(7):895–903. doi: 10.1096/fasebj.14.7.895. [DOI] [PubMed] [Google Scholar]
- 2.Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991;88(19):8661–5. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 1999;285(5436):2122–5. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 4.Wu T, Qin X, Kurepa Z, Kumar KR, Liu K, Kanta H, et al. Shared signaling networks active in B cells isolated from genetically distinct mouse models of lupus. J Clin Invest. 2007;117(8):2186–96. doi: 10.1172/JCI30398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sporn MB, Liby KT, Yore MM, Fu L, Lopchuk JM, Gribble GW. New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod. 2011;74(3):537–45. doi: 10.1021/np100826q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petronelli A, Pannitteri G, Testa U. Triterpenoids as new promising anticancer drugs. Anticancer Drugs. 2009;20(10):880–92. doi: 10.1097/CAD.0b013e328330fd90. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad R, Raina D, Meyer C, Kufe D. Triterpenoid CDDO-methyl ester inhibits the Janus-activated kinase-1 (JAK1)-->signal transducer and activator of transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer Res. 2008;68(8):2920–6. doi: 10.1158/0008-5472.CAN-07-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling X, Konopleva M, Zeng Z, Ruvolo V, Stephens LC, Schober W, et al. The novel triterpenoid C-28 methyl ester of 2-cyano-3, 12-dioxoolen-1, 9-dien-28-oic acid inhibits metastatic murine breast tumor growth through inactivation of STAT3 signaling. Cancer Res. 2007;67(9):4210–8. doi: 10.1158/0008-5472.CAN-06-3629. [DOI] [PubMed] [Google Scholar]
- 9.Samudio I, Konopleva M, Pelicano H, Huang P, Frolova O, Bornmann W, et al. A novel mechanism of action of methyl-2-cyano-3,12 dioxoolean-1,9 diene-28-oate: direct permeabilization of the inner mitochondrial membrane to inhibit electron transport and induce apoptosis. Mol Pharmacol. 2006;69(4):1182–93. doi: 10.1124/mol.105.018051. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem. 2006;281(47):35764–9. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda T, Sporn M, Honda T, Gribble GW, Kufe D. The novel triterpenoid CDDO and its derivatives induce apoptosis by disruption of intracellular redox balance. Cancer Res. 2003;63(17):5551–8. [PubMed] [Google Scholar]
- 12.Konopleva M, Tsao T, Ruvolo P, Stiouf I, Estrov Z, Leysath CE, et al. Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood. 2002;99(1):326–35. doi: 10.1182/blood.v99.1.326. [DOI] [PubMed] [Google Scholar]
- 13.Konopleva M, Contractor R, Kurinna SM, Chen W, Andreeff M, Ruvolo PP. The novel triterpenoid CDDO-Me suppresses MAPK pathways and promotes p38 activation in acute myeloid leukemia cells. Leukemia. 2005;19(8):1350–4. doi: 10.1038/sj.leu.2403828. [DOI] [PubMed] [Google Scholar]
- 14.Thimmulappa RK, Fuchs RJ, Malhotra D, Scollick C, Traore K, Bream JH, et al. Preclinical evaluation of targeting the Nrf2 pathway by triterpenoids (CDDO-Im and CDDO-Me) for protection from LPS-induced inflammatory response and reactive oxygen species in human peripheral blood mononuclear cells and neutrophils. Antioxid Redox Signal. 2007;9(11):1963–70. doi: 10.1089/ars.2007.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–36. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 16.Shi X, Xie C, Kreska D, Richardson JA, Mohan C. Genetic dissection of SLE: SLE1 and FAS impact alternate pathways leading to lymphoproliferative autoimmunity. J Exp Med. 2002;196(3):281–92. doi: 10.1084/jem.20010955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luijten RK, Fritsch-Stork RD, Bijlsma JW, Derksen RH. The use of glucocorticoids in systemic lupus erythematosus. After 60 years still more an art than science. Autoimmunity reviews. 2013;12(5):617–28. doi: 10.1016/j.autrev.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Wong HK, Kammer GM, Dennis G, Tsokos GC. Abnormal NF-kappa B activity in T lymphocytes from patients with systemic lupus erythematosus is associated with decreased p65-RelA protein expression. Journal of immunology. 1999;163(3):1682–9. [PubMed] [Google Scholar]
- 19.Harada T, Kyttaris V, Li Y, Juang YT, Wang Y, Tsokos GC. Increased expression of STAT3 in SLE T cells contributes to enhanced chemokine-mediated cell migration. Autoimmunity. 2007;40(1):1–8. doi: 10.1080/08916930601095148. [DOI] [PubMed] [Google Scholar]
- 20.Juang YT, Wang Y, Solomou EE, Li Y, Mawrin C, Tenbrock K, et al. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J Clin Invest. 2005;115(4):996–1005. doi: 10.1172/JCI200522854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnan S, Juang YT, Chowdhury B, Magilavy A, Fisher CU, Nguyen H, et al. Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. Journal of immunology. 2008;181(11):8145–52. doi: 10.4049/jimmunol.181.11.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang MT, Badmaev V, Ding Y, Liu Y, Xie JG, Ho CT. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13(1-4):225–30. doi: 10.1002/biof.5520130135. [DOI] [PubMed] [Google Scholar]
- 23.Takayasu J, Tanaka R, Matsunaga S, Ueyama H, Tokuda H, Hasegawa T, et al. Anti-tumor-promoting activity of derivatives of abieslactone, a natural triterpenoid isolated from several Abies genus. Cancer Lett. 1990;53(2-3):141–4. doi: 10.1016/0304-3835(90)90206-d. [DOI] [PubMed] [Google Scholar]
- 24.Yu LJ, Ma RD, Wang YQ, Nishino H, Takayasu J, He WZ, et al. Potent anti-tumorigenic effect of tubeimoside 1 isolated from the bulb of Bolbostemma paniculatum (Maxim) Franquet. Int J Cancer. 1992;50(4):635–8. doi: 10.1002/ijc.2910500425. [DOI] [PubMed] [Google Scholar]
- 25.Nishino H, Nishino A, Takayasu J, Hasegawa T, Iwashima A, Hirabayashi K, et al. Inhibition of the tumor-promoting action of 12-O-tetradecanoylphorbol-13-acetate by some oleanane-type triterpenoid compounds. Cancer Res. 1988;48(18):5210–5. [PubMed] [Google Scholar]
- 26.Honda T, Rounds BV, Bore L, Favaloro FG, Jr., Gribble GW, Suh N, et al. Novel synthetic oleanane triterpenoids: a series of highly active inhibitors of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett. 1999;9(24):3429–34. doi: 10.1016/s0960-894x(99)00623-x. [DOI] [PubMed] [Google Scholar]
- 27.Kim KB, Lotan R, Yue P, Sporn MB, Suh N, Gribble GW, et al. Identification of a novel synthetic triterpenoid, methyl-2-cyano-3,12-dioxooleana-1,9-dien-28-oate, that potently induces caspase-mediated apoptosis in human lung cancer cells. Mol Cancer Ther. 2002;1(3):177–84. [PubMed] [Google Scholar]
- 28.Shishodia S, Sethi G, Konopleva M, Andreeff M, Aggarwal BB. A synthetic triterpenoid, CDDO-Me, inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin Cancer Res. 2006;12(6):1828–38. doi: 10.1158/1078-0432.CCR-05-2044. [DOI] [PubMed] [Google Scholar]
- 29.Li M, Sun K, Redelman D, Welniak LA, Murphy WJ. The triterpenoid CDDO-Me delays murine acute graft-versus-host disease with the preservation of graft-versus-tumor effects after allogeneic bone marrow transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(6):739–50. doi: 10.1016/j.bbmt.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deeb D, Gao X, Dulchavsky SA, Gautam SC. CDDO-Me inhibits proliferation, induces apoptosis, down-regulates Akt, mTOR, NF-kappaB and NF-kappaB-regulated antiapoptotic and proangiogenic proteins in TRAMP prostate cancer cells. Journal of experimental therapeutics & oncology. 2008;7(1):31–9. [PubMed] [Google Scholar]
- 31.Deeb D, Gao X, Jiang H, Dulchavsky SA, Gautam SC. Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells by independently targeting pro-survival Akt and mTOR. The Prostate. 2009;69(8):851–60. doi: 10.1002/pros.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmad R, Liu S, Weisberg E, Nelson E, Galinsky I, Meyer C, et al. Combining the FLT3 inhibitor PKC412 and the triterpenoid CDDO-Me synergistically induces apoptosis in acute myeloid leukemia with the internal tandem duplication mutation. Molecular cancer research : MCR. 2010;8(7):986–93. doi: 10.1158/1541-7786.MCR-10-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Loghlen A, Perez-Morgado MI, Salinas M, Martin ME. N-acetyl-cysteine abolishes hydrogen peroxide-induced modification of eukaryotic initiation factor 4F activity via distinct signalling pathways. Cellular signalling. 2006;18(1):21–31. doi: 10.1016/j.cellsig.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Suwannaroj S, Lagoo A, Keisler D, McMurray RW. Antioxidants suppress mortality in the female NZB x NZW F1 mouse model of systemic lupus erythematosus (SLE) Lupus. 2001;10(4):258–65. doi: 10.1191/096120301680416940. [DOI] [PubMed] [Google Scholar]
- 35.Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64(9):2937–46. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yore MM, Kettenbach AN, Sporn MB, Gerber SA, Liby KT. Proteomic analysis shows synthetic oleanane triterpenoid binds to mTOR. PloS one. 2011;6(7):e22862. doi: 10.1371/journal.pone.0022862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perl A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nature reviews Rheumatology. 2013;9(11):674–86. doi: 10.1038/nrrheum.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65(11):4789–98. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 39.Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci U S A. 2005;102(12):4584–9. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313–22. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 41.Jaiswal AK. Human NAD(P)H:quinone oxidoreductase (NQO1) gene structure and induction by dioxin. Biochemistry. 1991;30(44):10647–53. doi: 10.1021/bi00108a007. [DOI] [PubMed] [Google Scholar]
- 42.Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J. 2003;374(Pt 2):337–48. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Favreau LV, Pickett CB. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991;266(7):4556–61. [PubMed] [Google Scholar]
- 44.Moroni G, Novembrino C, Quaglini S, De Giuseppe R, Gallelli B, Uva V, et al. Oxidative stress and homocysteine metabolism in patients with lupus nephritis. Lupus. 2010;19(1):65–72. doi: 10.1177/0961203309346906. [DOI] [PubMed] [Google Scholar]
- 45.Yoh K, Itoh K, Enomoto A, Hirayama A, Yamaguchi N, Kobayashi M, et al. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney international. 2001;60(4):1343–53. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 46.Tsai PY, Ka SM, Chang JM, Lai JH, Dai MS, Jheng HL, et al. Antroquinonol differentially modulates T cell activity and reduces interleukin-18 production, but enhances Nrf2 activation, in murine accelerated severe lupus nephritis. Arthritis Rheum. 2012;64(1):232–42. doi: 10.1002/art.33328. [DOI] [PubMed] [Google Scholar]
- 47.Tsai PY, Ka SM, Chang JM, Chen HC, Shui HA, Li CY, et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free radical biology & medicine. 2011;51(3):744–54. doi: 10.1016/j.freeradbiomed.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 48.Zhou H, Newnum AB, Martin JR, Li P, Nelson MT, Moh A, et al. Osteoblast/osteocyte-specific inactivation of Stat3 decreases load-driven bone formation and accumulates reactive oxygen species. Bone. 2011;49(3):404–11. doi: 10.1016/j.bone.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Yoon S, Woo SU, Kang JH, Kim K, Kwon MH, Park S, et al. STAT3 transcriptional factor activated by reactive oxygen species induces IL6 in starvation-induced autophagy of cancer cells. Autophagy. 2010;6(8):1125–38. doi: 10.4161/auto.6.8.13547. [DOI] [PubMed] [Google Scholar]
- 50.Kirkland RA, Franklin JL. Prooxidant effects of NGF withdrawal and MEK inhibition in sympathetic neurons. Antioxid Redox Signal. 2003;5(5):635–9. doi: 10.1089/152308603770310301. [DOI] [PubMed] [Google Scholar]
- 51.Morel L, Rudofsky UH, Longmate JA, Schiffenbauer J, Wakeland EK. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1994;1(3):219–29. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 52.Xie C, Zhou XJ, Liu X, Mohan C. Enhanced susceptibility to end-organ disease in the lupus-facilitating NZW mouse strain. Arthritis Rheum. 2003;48(4):1080–92. doi: 10.1002/art.10887. [DOI] [PubMed] [Google Scholar]