Abstract
La Paz River in Andean highlands is heavily polluted with urban run-off and further contaminates agricultural lowlands and downstream waters at the Amazon watershed. Agricultural produce at this region is the main source of vegetables for the major Andean cities of La Paz and El Alto. We conducted a 1 year study, to evaluate microbial quality parameters and occurrence of multiple enteropathogenic bacteria (Enterohemorrhagic E. coli—EHEC, Enteroinvasive E. coli or Shigella—EIEC/Shigella, Enteroaggregative E. coli—EAEC, Enteropathogenic E. coli—EPEC Enterotoxigenic E. coli—ETEC and Salmonella) and its resistance to 11 antibiotics. Four sampling locations were selected: a fresh mountain water reservoir (un-impacted, site 1) and downstream sites receiving wastewater discharges (impacted, sites 2–4). River water (sites 1–4, N = 48), and soil and vegetable samples (site 3, N = 24) were collected during dry (April–September) and rainy seasons (October–March). Throughout the study, thermotolerant coliform density values at impacted sites greatly exceeded the guidelines for recreational and agricultural water uses. Seasonal differences were found for thermotolerant coliform density during dry season in water samples nearby a populated and hospital compound area. In contrast to the un-impacted site, where none of the tested enteropathogens were found, 100 % of surface water, 83 % of soil and 67 % of vegetable samples at impacted sites, were contaminated with at least one enteropathogen, being ETEC and Salmonella the most frequently found. ETEC isolates displayed different patterns of toxin genes among sites. The occurrence of enteropathogens was associated with the thermotolerant coliform density. At impacted sites, multiple enteropathogens were frequently found during rainy season. Among isolated enteropathogens, 50 % were resistant to at least two antibiotics, with resistance to ampicillin, nalidixic acid, trimethoprim–sulfamethoxazole and tetracycline commonly present. Moreover, some Salmonella isolates were distinguished by their multi-resistance to ≥8 antibiotics, within soil and vegetable samples. Overall, this study demonstrates that La Paz River—an affluent of the Amazon macrobasin—is heavily polluted along the year with a high density of thermotolerant coliforms and is a reservoir of multiple antibiotic resistant enteropathogens, present in river water, soil and vegetables. These data highlight health risk associated with food and waterborne diseases at the region.
Keywords: Thermotolerant coliforms, Enteropathogenic bacteria, Antibiotic multi-resistance, Sewage water, River contamination, Contamination of soil and vegetables, Food and waterborne diseases risk, Amazon macrobasin
Background
Worldwide, the contamination of fresh water resources due to urbanization affects food security and ecosystem sustainability (WWAP 2012). This situation is exacerbated in developing countries and more so in dry regions such as the Bolivian Highlands, where population growth, urban expansion and widespread malnutrition give rise to an increasing demand for water that is crucial for food security (Buxton et al. 2013). In this region, along with a shortage of water, urban wastewater directly drains into fresh water bodies that flow downstream into the Amazon macrobasin, contributing to its aquatic ecosystem degradation. Moreover, within agricultural dry regions, vegetable production is enhanced by the use of river surface water and sludge as sources of irrigation and organic nutrients (Duran et al. 2003). This is the case of the agricultural production along La Paz River basin, where river effluents are highly valued by farmers, allowing for a continuous crop production throughout the year, to respond to the growing food demand of the nearby cities of La Paz and El Alto, which are the biggest urban centers in the Bolivian Andean highlands (MMAyA 2012).
Water pollution is largely associated with the incidence of water and food borne diseases and the origin of many outbreaks (Buchholz et al. 2011; Juliana et al. 2008; Little and Gillespie 2008; Marcheggiani et al. 2015). Moreover, water–borne diseases are among the most common causes of mortality and morbidity around the world, accounting for approximately 3 % of all deaths, affecting primarily children in developing countries. It is estimated that each year around 2 million people die from diarrheal diseases, likely associated to contaminated food and water (WHO 2014). This situation may likely be exacerbated by climate change in the coming years, increasing human exposure to environmental pathogens (Boxall et al. 2009).
In Bolivia it is estimated that around 25 % of children less than five years of age suffer from acute diarrhea, and more than 870 thousand diarrheal cases are registered in all ages annually (SNIS 2013). It is unknown however, how many of these cases have a water and/or food borne origin.
In Bolivia, cholera epidemics started in 1992 at the agricultural area of La Paz River basin and extended to most of the country, reaching 44,000 reported cases. The cholerae outbreak was associated with the consumption of fresh vegetables irrigated with sewage polluted water (CDC 1993; MS 2000). Later in 1997, Ohno et al. reported the occurrence of fecal pollution and enteric pathogens in the study area (Ohno et al. 1997). Although no further large outbreaks were reported (INE 2011; SNIS 2013), consumption of raw vegetables cultivated in the area may be associated with a risk of diarrhea.
Pollution of La Paz River contributes to the environmental degradation of the Amazon watershed and may increase the health risk of downstream agricultural rural settlements and riverine communities, which are affected by water borne diseases (Confalonieri and Fonseca 2013; Rede Interagencial de Informações Para a Saúde 2012). Fecal pollution indicators associated with urban contamination and occurrence of enteric pathogens have already been described in the Amazon watershed (De Paula et al. 2007; Diniz-Mendes et al. 2008; Miagostovich et al. 2008; Pereira et al. 2010).
The aim of this study was to evaluate the microbial river water quality at un-impacted and impacted sites, as well as the occurrence of multiple enteropathogenic bacteria (Enterohemorrhagic E. coli—EHEC, Enteroinvasive E. coli or Shigella—EIEC/Shigella pathovar, Enteroaggregative E. coli—EAEC, Enteropathogenic E. coli—EPEC Enterotoxigenic E. coli and Salmonella) and its resistance to antibiotics in river water, soil and vegetable samples of the La Paz River basin, throughout a 1-year period.
Methods
Study area
La Paz River basin network is located in the northeast region of the high plateau in La Paz, Bolivia, extending from mountain glaciers towards urban and agricultural areas with altitudes ranging from 2400 to 5500 meters above sea level. La Paz River is part of the Amazon macrobasin, being one of the main Andean tributaries of the Madeira River (UNEP 2004; Goulding et al. 2003). Along the river’s course in La Paz city, urban and industrial wastewater discharges are directly released into surface water without prior treatment. Furthermore, at lowland agricultural areas, river water and sediments are used to irrigate and to flood vegetable crops.
Sampling sites and sample collection
Four sampling sites along the La Paz River basin were selected based on water quality, predominant source of pollution and use. Site 1, Incachaca, is located at the upstream un-impacted region, right at the exit point of the fresh water reservoir. Site 2, Holguín, is located 17.4 km downstream from site 1, at the impacted and heavily populated urban area right next to the hospital compound. Site 3, Mecapaca, is located at the agricultural lowland impacted region, placed 40 km from site 1. Site 4, Jillusaya River, is an impacted urban side affluent of the La Paz River (Fig. 1). Soil and vegetable samples were only collected at Mecapaca river shorelines (site 3).
Fig. 1.
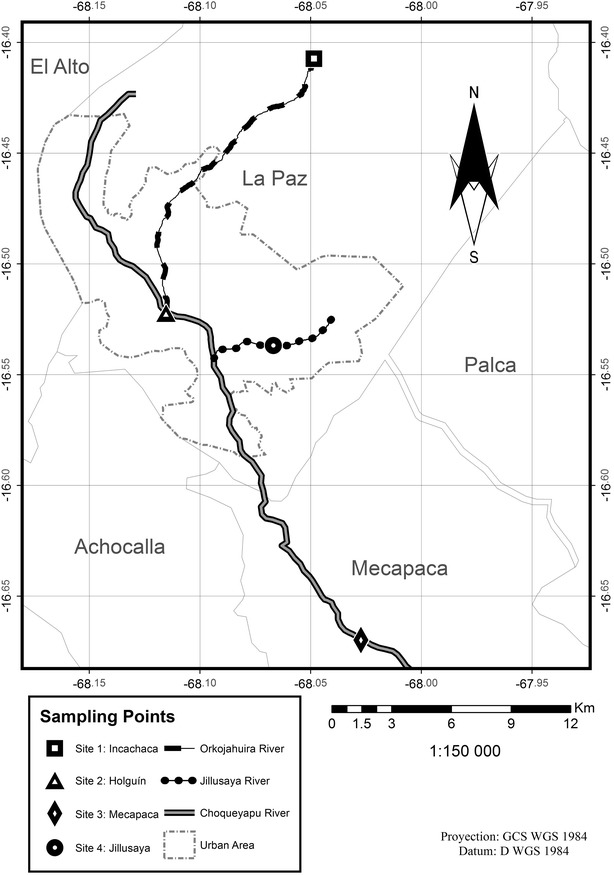
Sampling study area. Site 1 Incachaca; site 2 Holguin; site 3 Mecapaca and site 4 Jillusaya
Surface water, vegetable and soil samples were collected monthly between April 2013 and March 2014 at each sampling point, comprising dry (April–September) and rainy (October–March) seasons were collected at each site along the year (1 sample/month). The pH, temperature (°C), conductivity (μS/cm) and redox potential (mV) were measured in situ (Oakton Instruments, Vernon Hills) as described by EPA guidelines with three replicates (EPA 2007). Water samples were taken in sterilized 600 ml plastic bottles, following APHA and EPA guidelines (APHA 2005; EPA 2007). Green leafy vegetables (lettuce or chard) and soil samples were collected directly into sterilized plastic bags. Samples were kept at 4–6 °C upon transportation to the laboratory of the Instituto de Biología Molecular y Biotecnología (IBMB) and processed on the same day.
Microbiological analysis
Thermotolerant coliform
Thermotolerant coliform is defined as the group of coliform bacteria which produces gas from lactose in 48 h at 44.5 °C (Resolution MEPC 2006). The bacterial density of thermotolerant coliform in water, vegetable and soil samples was evaluated by the multiple tube fermentation technique, using A1-media (Sigma-Aldrich, St. Louis) as described by APHA guidelines (APHA 2005) and reported as MPN/100 ml or MPN/g (in soil and vegetable samples).
Enteropathogenic bacteria detection
Water samples (200 ml) and vegetable elution samples (30 g of vegetables washed in 200 ml of peptone water), were separately filtered using a 0.45 μm pore sized nitrocellulose membrane filter (Millipore-Sigma-Aldrich, St. Louis). Soil samples (3 g) and water and vegetable filters were inoculated into EC Broth at 37 °C, for 18 h.
Diarrheogenic Escherichia coli (DEC)
Overnight bacterial enrichment was streaked on to MacConkey agar plates (Difco Laboratories, Detroit) and incubated for 18 h at 37 °C. From each plate, five lactose positive and five lactose negative colonies were isolated in MacConkey agar and resuspended in tridistilled water and subjected to boiling for DNA extraction. Conventional PCR using specific primers for virulence markers was used to detect among lactose positive bacteria: stx1/stx2 (Enterohemorrhagic E. coli—EHEC), ipaH (Enteroinvasive E. coli/Shigella pathovar—EIEC/Shigella), paa (Enteroaggregative E. coli—EAEC), eae/bfp (Enteropathogenic E. coli—EPEC) and lt/sth/stp (Enterotoxigenic E. coli—ETEC) (Gonzales et al. 2013a; Moon et al. 2005; Rodas et al. 2009). In addition, lactose negative isolates were also tested to detect ipaH (Enteroinvasive E. coli/Shigella pathovar—EIEC/Shigella).
Reference strains that were used as positive controls included: 3b for (stx1/stx2), ATCC-43893 for (ipaH), O42 for (paa), 12b for (eae, bfp) and H17047 for (lt/sth/stp). All positive samples were confirmed twice by independent PCR assays. Negative controls (commensal E. coli ATCC 25922 and tri-distilled water) were included in PCR runs.
Salmonella
Overnight bacterial enrichment was inoculated into semisolid medium Rappaport–Vassiliadis (HiMedia Laboratories, Pennsylvania), and incubated for 48 h at 43 °C. Samples in which a whitish growing halo was observed were used for PCR analysis using primers for virulence marker invA (Rahn et al. 1992). Reference strains Salmonella enterica Serotype Typhimurium DT104 was used as positive control. Negative controls (tri-distilled water) were included in all PCR runs.
Multiple enteropathogen index
The multiple enteropathogenic bacteria (MEB) index was calculated as follows: a/b × c, where a is the aggregate number of positive samples for any enteropathogenic bacteria at each sampling point, b is the number of pathogens evaluated in the study and c is the total number of samples.
Antimicrobial susceptibility testing
Antibiotic susceptibility testing of pathogenic isolates was performed by the Kirby–Bauer disk diffusion method as described by the Clinical and Laboratory Standards Institute guidelines (CLSI 2007), using Mueller–Hinton agar (Difco Laboratories). The antibiotics disks (Oxoid, Hampshire) and their concentration were: ampicillin (AM 10 μg), ampicillin–sulbactam (AB 10 μg/10 μg), cefoxitin (FX 30 μg), cefotaxime (CT 30 μg), ciprofloxacin (CI 5 μg), chloramphenicol (C 30 μg), gentamicin (CN 10 μg), nalidixic acid (NA 30 μg), streptomycin (S 10 μg), tetracycline (TC 30 μg) and trimethoprim–sulfamethoxazole or cotrimoxazole (ST 1.25 μg/23.75 μg). Escherichia coli ATCC 25922 was used as the control strain.
The multiple antibiotic resistance (MAR) index was calculated for each isolate by this formula: a/b, where a is the number of antibiotics to which the isolate is resistant and b is the number of antibiotics evaluated in the study.
Data analysis
All statistical analyses were performed using R software version 2.15.1 at P < 0.05 significance level. Significant differences in water quality parameters, between dry and rainy seasons were tested by Wilcoxon test. Principal Component Analyses (PCA) was used as an exploratory analysis to visualize differences of water parameters between the impacted and the non-impacted sites. Comparison of MEB index between seasons at impacted sites was achieved by Wilcoxon test.
Results
Microbiological and physicochemical parameters
Microbiological and physicochemical parameters (thermotolerant coliforms, pH, temperature, conductivity and redox potential) markedly differed between the un-impacted and impacted sites (Table 1). As shown by Principal Component Analysis based on physicochemical and microbiological parameters, impacted sites 2, 3 and 4 cluster together away from un-impacted site 1. The first two components explained 98.06 % of variance; component 1 included thermotolerant coliforms, conductivity and redox potential, while component 2 pH (Fig. 2).
Table 1.
Mean value and standard deviation for physicochemical and microbiological parameters, along La Paz River basin water, soil and vegetable sampling points
| Site no./samples (N) | pH | Temperature (°C) | Conductivity (μS/cm) | Redox potential (mV) | Thermotolerant coliform density MPNa,b | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Water (N = 12) | ||||||||||
| 1 | 6.84 | 1.10 | 9.46 | 1.64 | 172.83 | 97.28 | −25.15 | 38.87 | 3.73 × 101 | 9.88 × 101 |
| 2 | 7.93 | 0.43 | 12.73 | 1.87 | 1303.50 | 351.07 | −82.62 | 6.62 | 1.42 × 10 6 | 1.90 × 106 |
| 3 | 7.81 | 0.48 | 18.78 | 2.50 | 1216.90 | 341.74 | −76.71 | 11.23 | 3.08 × 10 5 | 1.87 × 105 |
| 4 | 7.64 | 0.65 | ND | ND | 750.48 | 253.04 | −68.45 | 16.81 | 3.05 × 10 5 | 3.77 × 105 |
| 3/soil (N = 12) | NA | NA | NA | NA | NA | NA | NA | NA | 4.61 × 102 | 7.51 × 102 |
| 3/vegetable (N = 12) | NA | NA | NA | NA | NA | NA | NA | NA | 6.85 × 101 | 1.46 × 102 |
MPN values shown in italics are above the standard safe water values, for use in recreational and agricultural activities
NA not applicable
ND not determined
aMPN (most probable number of thermotolerant coliforms)/100 ml in water samples
bMPN (most probable number of thermotolerant coliforms)/g in vegetable and soil samples
Fig. 2.

Principal component analysis (PCA) ordination plot based on physicochemical and microbiological parameters data from river water sampling sites, at La Paz River basin. The percentage of variation explained by each axis is shown
In water samples at all impacted sites, thermotolerant coliforms were continuously detected throughout the sampling year at much higher densities (105–106 MPN/100 ml) than the values found at site 1 (101 MPN/100 ml) (Table 1). The range between the lowest (un-impacted site 1) and the highest (impacted site 2) thermotolerant coliform density, measured during the monitoring year reached 5 orders of magnitude. At impacted sites, the mean density of thermotolerant coliforms at the most contaminated site (site 2) was 1.42 × 106 MPN/100 ml and at the less contaminated site (site 4) 3.05 × 105 MPN/100 ml. Moreover, at all impacted sites, thermotolerant coliforms exceeded by at least one order of magnitude, the standards for either recreational or irrigation water.
Soil and vegetable samples irrigated with river water also presented thermotolerant coliform density ranging from 4.61 × 102 MPN/g (soil) and 6.85 × 101 MPN/g (vegetables) (Table 1).
Regarding seasonality, thermotolerant coliform density significantly differed between rainy and dry seasons at site 2, with higher densities at the latter (P < 0.05) (Table 2). At site 1, differences of mean pH and temperature values were observed between dry and rainy season (Table 2), while no seasonal differences among physicochemical data were found at impacted sites.
Table 2.
Seasonal variation of physicochemical and microbiological parameters, along La Paz River basin water sampling points
| Site no | Season | pH | Temperature (°C) | Conductivity (μS/cm) | Redox potential (mV) | MPN/100 mla |
|---|---|---|---|---|---|---|
| 1 | Dry | 5.95 | 8.43 | 163.23 | −43.70 | 10.23 |
| Rainy | 7.72 | 10.49 | 182.43 | −45.92 | 11.77 | |
| 2 | Dry | 7.67 | 12.13 | 1407.94 | −86.15 | 2.40 × 10 6 |
| Rainy | 8.19 | 13.32 | 1199.06 | −79.09 | 4.35 × 10 5 | |
| 3 | Dry | 7.60 | 17.99 | 1290.44 | −81.13 | 3.55 × 105 |
| Rainy | 8.01 | 19.56 | 1143.39 | −72.28 | 2.62 × 105 | |
| 4 | Dry | 7.38 | 10.95 | 713.47 | −69.98 | 4.81 × 105 |
| Rainy | 7.90 | 17.49 | 781.33 | −66.93 | 1.29 × 105 |
Significant differences in water quality parameters between dry and rainy seasons (P < 0.05), calculated by Wilcoxon, are shown in italics
aMost probable number of thermotolerant coliforms
Enteropathogenic bacteria distribution
Occurrence of enteropathogens clearly differed between un-impacted and impacted sites, since none of the tested pathogens were detected at the former (Table 3). On average 100, 83 and 67 % of polluted water, soil and vegetable samples respectively, harbored any of the tested enteropathogens.
Table 3.
Percentage of enteropathogenic bacteria found at La Paz River basin, water, soil and vegetable sampling points
| Site/sample | ETEC% (N)a | EPEC%d (N)a | EAEC% (N)a | Salmonella% (N)a | EIEC/Shigella%e (N)a | MEBb | Any pathogen%c |
|---|---|---|---|---|---|---|---|
| 1/water | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2/water | 100 (12) | 58 (7) | 67 (8) | 92 (11) | 8 (1) | 0.54 | 100 |
| 3/water | 100 (12) | 50 (6) | 67 (8) | 83 (10) | 0 | 0.50 | |
| 4/water | 83 (10) | 33 (4) | 50 (6) | 92 (11) | 17 (2) | 0.46 | |
| 3/soil | 67 (8) | 25 (3) | 33 (4) | 33 (4) | 33 (4) | 0.32 | 83 |
| 3/vegetable | 67 (8) | 0 | 17 (2) | 33 (4) | 0 | 0.19 | 67 |
EHEC was not detected in any of the samples
a(N) is the number of positive samples detected for each pathogen (i.e. ETEC, EPEC, EAEC, Salmonella and EIEC/Shigella) at each site along the year. A total of 12 samples were collected at each site along the year (1 sample/month)
bMultiple enteropathogenic bacteria index (MEB)
cPercentage of positive samples for at least one enteropathogen detected
dAll EPEC isolates were atypical (bfp negative)
eAll EIEC/Shigella isolates were non-lactose fermenting colonies
Among enteropathogens, ETEC and Salmonella represented the most frequently found isolates followed by EAEC, EPEC and the EIEC/Shigella pathovar, whereas EHEC was not detected in any of the samples (Table 3). As shown in Table 5, ETEC isolated colonies displayed different toxin genes profiles along sites with a higher prevalence of LT/STh (63 %) toxins, while STp was found only in vegetable samples (25 %). All EPEC isolates were atypical (bfp negative) and all colonies assigned to EIEC/Shigella pathovar were non-lactose fermenting.
Table 5.
Antibiotic resistance profile and MAR index of enteropathogenic bacteria isolated (N = 93), at La Paz River basin water, soil and vegetable sampling points
| Enteropathogenic bacteria | Strain codea | Resistance profile | MAR indexb |
|---|---|---|---|
| ETECc | W2-1(LT/STh) | ST | 0.17 |
| W2-2 (LT/STh) | C, TC | ||
| W2-3 (LT/STh) | AM, S, ST | ||
| W2-4 (LT/STh) | AM, NA | ||
| W2-6 (LT/STh) | NA | ||
| W2-7 (STh) | CI, NA | ||
| W2-14 (LT/STh) | AM, AB, S | ||
| W2-22 (STh) | NA | ||
| W2-51 (LT/STh) | AM, AB, C, TC | ||
| W2-61 (LT/STh) | – | ||
| EAEC | W2-72 | ST | 0.18 |
| W2-75 | AM, NA, S, ST, TC | ||
| W2-76 | – | ||
| EPEC | W2-38 | AM, AB, C, S, ST, TC | 0.33 |
| W2-60 | NA, ST, TC | ||
| W2-74 | AM, ST | ||
| Salmonella | W2-9 | NA | 0.22 |
| W2-16 | NA | ||
| W2-26 | AM, C, CN, S, ST, TC | ||
| W2-35 | AM, C, CN, S, ST, TC | ||
| W2-41 | NA | ||
| W2-52 | NA | ||
| W2-57 | – | ||
| W2-90 | AM, C, TC | ||
| ETEC | W3-45 (LT/STh) | AM, AB, NA, S, ST | 0.17 |
| W3-46 (LT/STh) | NA | ||
| W3-48 (LT/STh) | AM, C, TC | ||
| W3-73 (LT) | AM | ||
| W3-77 (LT/STh) | – | ||
| W3-78 (LT/STh) | NA | ||
| W3-79 (LT) | AM, ST | ||
| EAEC | W3-8 | AM, S, TC | 0.24 |
| W3-20 | AM, CN, S, ST, TC | ||
| W3-34 | NA | ||
| W3-47 | AM, ST | ||
| W3-80 | AM, NA | ||
| EPEC | W3-23 | AM, ST | 0.18 |
| Salmonella | W3-10 | NA | 0.23 |
| W3-17 | AM, C, CT, NA, S, ST, TC | ||
| W3-27 | AM, AB, C, CN, FX, NA, S, ST, TC | ||
| W3-42 | NA | ||
| W3-53 | – | ||
| MV58 | – | ||
| W3-65 | TC | ||
| W3-91 | TC | ||
| ETEC | W4-15 (LT) | AM, C, ST, TC | 0.21 |
| W4-33 (LT) | – | ||
| W4-49 (LT) | – | ||
| W4-50 (LT) | AM, CI, CN, NA, S, ST, TC | ||
| W4-62 (LT) | AM, C, ST | ||
| W4-89 (LT/STh) | NA | ||
| W4-88 (LT/STh) | NA | ||
| EAEC | W4-21 | – | 0.23 |
| W4-25 | – | ||
| W4-70 | AM, AB, C, S, ST | ||
| W4-71 | AM, AB, C, S, ST | ||
| Salmonella | W4-12 | – | 0.20 |
| W4-18 | AM, C, S, ST, TC | ||
| W4-30 | AM, AB, C, CN, FX, S, ST, TC | ||
| W4-37 | AM, CI, CN, FX, S, ST, TC | ||
| W4-44 | – | ||
| W4-54 | – | ||
| W4-59 | – | ||
| W4-68 | – | ||
| W4-93 | – | ||
| EIEC/Shigella | W4-69 | AM | 0.09 |
| ETEC | S-5 (LT/STh) | AM, C, ST, TC | 0.16 |
| S-13 (LT/STh) | NA | ||
| S-24 (LT) | – | ||
| S-31 (LT/STh) | NA | ||
| S-84 (LT/STh) | NA | ||
| S-85 (LT/STh) | AM, C, TC | ||
| S-86 (LT) | NA | ||
| S-87 (LT) | AM, ST, TC | ||
| EPEC | S-19 | AM, ST | 0.18 |
| Salmonella | S-29 | AM, AB, C, CI, CN, NA, S, ST, TC | 0.41 |
| S-43 | – | ||
| EIEC/Shigella | S-55 | AM, FX | 0.14 |
| S-56 | AM, FX | ||
| S-66 | – | ||
| S-67 | AM, FX | ||
| ETEC | C-32 (LT/STh) | AM, C, TC | 0.23 |
| L-39 (STp) | AM, AB, C, TC | ||
| L-40 (LT/STh) | AM, C, TC | ||
| L-63 (STp) | AM, NA | ||
| L-64 (LT/STh) | AM, NA | ||
| L-81 (LT/STh) | AM, AB, C, TC | ||
| L-82 (STh) | NA | ||
| L-83 (LT/STh) | NA | ||
| Salmonella | L-11 | – | 0.36 |
| L-28 | AM, C, CI, CN, NA, S, ST, TC | ||
| C-36 | AM, C, CI, CN, NA, S, ST, TC | ||
| L-92 | – |
–, antibiotic sensitive strain
aStrain code was assigned based on the sample type and sampling site, followed by the numerical order of bacterial isolation: water (W), lettuce (L), chard (C) and soil (S) and sites (2, 3 and 4). No pathogens were detected at site 1
bMAR index calculated for each enteropathogenic category
cETEC strains were characterized by presence of one of the toxin genes (LT/STh/STp)
The monthly distribution of the multiple enteropathogenic bacteria (MEB) index at the impacted water sampling sites along the entire year is presented in Fig. 3. During the monitoring, the co-occurrence of different enteropathogenic bacteria was most frequent at the rainy season in contrast to the dry season (P < 0.05) (Fig. 3).
Fig. 3.
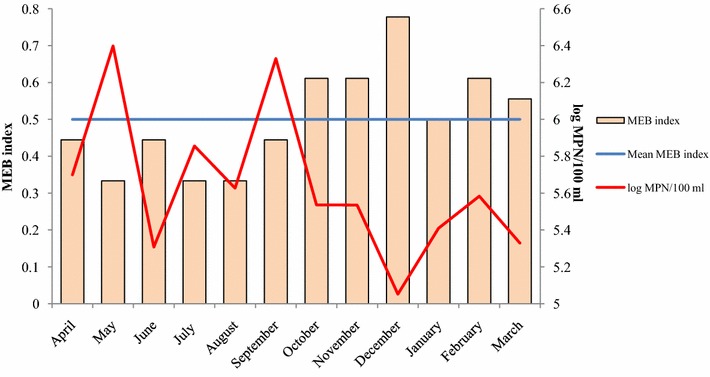
Monthly distribution of MEB index and thermotolerant coliforms, at impacted water samples of La Paz River basin along the study year. Mean MEB index was calculated for each month across sites (sites 2–4). Mean values of MEB index between dry and rainy season, displayed significant differences (Wilcoxon test, P < 0.05)
Enteropathogenic bacteria antibiotic resistance
Among enteric pathogens, a total of 93 colonies (64 % of positive isolates) were tested for antibiotic resistance. As shown in Table 4; 78, 50 and 35 % of the colonies were resistant to at least 1, 2 and 3 antibiotics, respectively. Most of the isolates were commonly resistant to ampicillin, nalidixic acid, trimethoprim–sulfamethoxazole and tetracycline. Although at lower levels, resistance to gentamicin and cefoxitin was also observed, particularly among Salmonella isolates (Table 5).
Table 4.
Percentage of antibiotic resistance enteropathogenic bacteria (N = 93) and MAR index, at impacted La Paz River basin sampling points
| Sample | ≥1 (AR)a | ≥2 (AR)a | ≥3 (AR)a | MARb index |
|---|---|---|---|---|
| Water | 76 | 45 | 36 | 0.21 |
| Soil | 79 | 50 | 29 | 0.20 |
| Vegetable | 85 | 69 | 46 | 0.27 |
| All samples | 78 | 50 | 37 | 0.21 |
aPercentage of enteropathogenic bacteria, resistant to a number of antibiotics
bMultiple antibiotic resistance index. Mean values of MAR Index were calculated for each type of sample (water, soil and vegetables) and across all samples
MAR index values were comparable among water, soil and vegetable samples (Table 4), while in isolated pathogens varied from 0.1 in EIEC/Shigella to 0.4 in Salmonella (Table 5). Despite the fact that Salmonella strains presented the highest MAR values displaying resistance to as many as nine antibiotics, MAR index among pathogens did not show significant differences.
Discussion
The impact of urban contamination, degrading the microbiological quality of La Paz River basin can be measured by the dramatic increase in the number of thermotolerant coliforms in surface water at all impacted sites. According to thermotolerant coliform data, site 1—not impacted by wastewater discharges-, can be classified as a source of water of maximum quality (Estado Plurinacional de Bolivia 1992), opposed to sites 2–4, that largely exceeded the standards for recreational water (i.e. 10,000 MPN/100 ml) (Osmond et al. 1997). Moreover, the average density at the agricultural area—site 3, surpassed in two orders of magnitude the allowed concentration for unrestricted irrigation water, according to WHO guidelines (WHO 1989) (i.e. <1000 MPN/100 ml). In this line, the density of thermotolerant coliforms found in fresh produce also exceeded the satisfactory microbiological hygiene criteria level 1000 MPN/100 g of fresh weight (ICMSF 1996). Overall, these parameters indicate that La Paz River basin at downstream locations aside from point 1, has a very poor microbiological water quality and is severely polluted by urban contaminants throughout the year. It is estimated that around 4.08 × 107 m3/year of wastewater from municipal, industrial and hospital sources, is directly released into the river (Duran et al. 2003).
At impacted sites, the density of thermotolerant coliforms was associated with the presence of enteric pathogens. At least one pathogen was detected at every location in every water sample that contained levels of thermotolerant coliforms exceeding WHO water quality standards. This study demonstrates that collected water, soil and vegetables samples contained cultivable enteric pathogens potentially capable of causing gastrointestinal illness. At the region, farmer communities continuously use river water and sludge to increase food crop production. Therefore, vegetables may be contaminated with enteric pathogens while growing or upon harvesting. The fact that a high percentage of soil (10/12) and vegetables (8/12) samples were polluted with enteric pathogens, suggests that urban river pollution, predominantly from human sources contaminates agricultural fields. However, untreated manure and wild and domestic animals may also contribute to the pathogen load.
Most of the contaminated water and soil samples contained more than one pathogen, particularly ETEC and/or Salmonella which were present in all tested sites. At impacted water samples, the occurrence of multiple enteropathogens measured by the MEB index was higher during rainy season, particularly in the month of December, where at least four of the six tested pathogens were commonly present. Seasonal differences in enteropathogenic bacteria displaying an increased prevalence during the rainy season were also reported by Gonzales et al. (2013a, b) among diarrheal episodes.
Other studies (Salem et al. 2011) have also demonstrated the increased prevalence of Salmonella and ETEC in discharged wastewater compared to other DEC pathogens such as EAEC and EIEC. The marked prevalence of ETEC and Salmonella found in most of the impacted water samples may account for their higher survival rates in the environment. It has been shown that both pathogens can persist for several months in aquatic ecosystems by entering into a viable but non-culturable state (VBNC) (Lothigius et al. 2010; Santo Domingo et al. 2000; Waldner et al. 2012). Moreover soil and vegetables can act as reservoirs for Salmonella and ETEC (Islam et al. 2004; Singh et al. 2010), which attach to fresh produce by different mechanisms (Barak et al. 2007; Berger et al. 2009; Shaw et al. 2011). Both Salmonella and ETEC were identified as source of different foodborne outbreaks associated with the consumption of raw vegetables (Brendan et al. 2013; Yoder et al. 2006). Moreover ETEC is the leading cause of travelers’ diarrhea (Qadri et al. 2005). Overall, our data suggest that ETEC and Salmonella are the most resistant to survive in the environment and the ones that are likely mostly related to food and waterborne gastrointestinal infections in the region among the tested pathogens.
Therefore, the occurrence of multiple enteric bacterial pathogens (ETEC, Salmonella, EAEC and EPEC) at the impacted La Paz River basin area highlights the risk of microbial contamination, particularly with ETEC and Salmonella associated with irrigation water, agricultural soil, produce and vegetables consumption. Moreover, the continuous discharge of waste water run-off, generates concern that river may be one of the vehicles for transporting pathogens to downstream freshwater ecosystems of the Amazon macrobasin. Consequently, this may increase the risk of waterborne diseases among nearby riverine communities and agricultural settlements that rely predominantly on surface water as a primary source for drinking, fishing and swimming activities. Additionally, wild and domestic animals may be exposed to contaminated water upon drinking.
Interestingly, among diarrheal clinical isolates from acute diarrheal episodes in hospitalized children over a four year study (2007–2010) in Bolivia, EAEC was the most frequently detected followed by ETEC and EPEC (Gonzales et al. 2013a). These data support the fact that ETEC may have increased survival in the environment compared to other DEC pathogens. LT and STh were the most commonly found ETEC toxins among clinical strains (Gonzales et al. 2013b), contrary to what was found in this study, where LT/STh genotype were strikingly the most prevalent. Since it is commonly thought that the ETEC distribution in the environment is primarily related to human fecal contamination within the La Paz River, the potential exchange of plasmid carrying toxin genes among strains in the environment may not be excluded. Nevertheless, contamination of zoonotic origin cannot be excluded. Previous studies in Bangladesh reported a distribution of 67 % ST, 24 % LT–ST and 9 % ST among ETEC water isolates (Begum et al. 2007), similar to ETEC found in patients with diarrhea.
Among limitations of this study were the lack of data regarding the pathogen`s host source and the lack of quantitative information of the concentration (CFU/ml) of each tested enteric pathogen. Both data are relevant to better understand the level and origin of microbial pollution at the La Paz River basin area. In addition, pathogen occurrence most likely was underrepresented, since other important enteric pathogens such as Vibrio cholerae, Campylobacter jejuni, Diffuse-adhering E. coli (DAEC) were not tested.
The broad detection of antibiotic resistant enteric pathogens and the numbers of thermotolerant coliforms at La Paz river basin impacted area, raise concern regarding dissemination of multiple antibiotic resistant determinants into the environment. Antibiotic resistance genes are often inserted in integrons, transposons and plasmids, which facilitate their lateral transfer into a wide range of bacterial species (Nikaido 2009). Moreover, these data highlights the risk of acquiring upon exposure, antibiotic resistant bacteria directly from the environment, away from the health care settings.
The emergence and dissemination of antibiotic resistance, particularly multidrug resistance, among E. coli and other bacterial pathogens have become one of the most serious global public health threats (WHO 2015). In this study, enteric pathogens displayed multiresistance to antibiotics typically used in clinical settings. This may be due to the widespread use of several antimicrobial compounds in human therapy. In fact, among antibiotic resistance profiles, resistance to widely used antibiotics for the treatment of local diarrheal and acute respiratory infections (trimethoprim–sulfamethoxazole, nalidixic acid and ciprofloxacin) can be observed (Benguigui 2002). Moreover, resistance patterns are related to clinical DEC isolates found in previous years (Gonzales et al. 2013a; Rodas et al. 2005, 2011), suggesting that environmental and clinical pathogenic isolates may have a common origin. To address this issue, future studies should compare environmental and clinical isolates by molecular typing methods.
By comparing the antibiotic resistance among enteric pathogens with a previous report in the study area (Ohno et al. 1997) where most of the isolates were susceptible to ampicillin, nalidixic acid, tetracycline and chloramphenicol, it can be inferred that antibiotic resistance has increased within pathogenic bacteria isolated from the environment over time.
Overall, the high density of thermotolerant coliforms and presence of multiresistant enteric pathogens at impacted sites pose a risk for the emergence of new multiresistant pathogens.
Conclusions
Within the La Paz River Basin area, river water is highly polluted by untreated wastewater run-off. High levels of thermotolerant coliforms and multiple enteropathogenic bacteria were detected along the sampling year at impacted water sites, irrigated soil and vegetables, with ETEC and Salmonella being the most prevalent. Moreover, 35 % of the total enteropathogens isolated were multiresistant to at least 3 antibiotics. These data highlight the microbial contamination of the La Paz River at the Amazon macrobasin emphasizing health risk of waterborne enteric pathogens transmission associated locally to the production and consumption of vegetables at the cities of La Paz and El Alto and in a larger extent to water intakes by the nearby Amazon riverine communities. Moreover, these results underline the need for improved guidelines and should prompt environmental governmental officials to develop effective prevention and control strategies to prevent and/or minimize the risk of foodborne and waterborne pathogen transmission in the region.
Authors’ contributions
VI conceived and designed the study and supervised laboratory and field work. VP carried out the sampling collection and microbial and physicochemical analysis. VP and VI discussed and interpreted the results and drafted the manuscript. NM participated in the field sampling activities of the study and in the laboratory work. All authors read and approved the final manuscript.
Acknowledgements
The present work was funded by the Swedish International Development Cooperation Agency (SIDA), supporting the UMSA-SIDA project Diarrheal Diseases. The Department of Environmental Quality of the Municipality of La Paz city is acknowledged for logistic support to this study. We gratefully acknowledge Michelle Buelow for proof reading the article. We thank Eduardo Aguirre, Gabriela Lazaro and Fernando Romero for assistance during field sampling and Favio Carvajal for providing the maps of the sampling sites.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- EHEC
Enterohemorrhagic Escherichia coli
- EIEC/Shigella
Enteroinvasive Escherichia coli or Shigella
- EAEC
Enteroaggregative Escherichia coli
- EPEC
EnteropathogenicEscherichia coli
- ETEC
Enterotoxigenic Escherichia coli
- MPN/100 ml
most probable number/100 ml
- MPN/g
most probable number/g
- stx1/stx2
Shiga toxin 1/Shiga toxin 2
- ipaH
invasion plasmid antigen
- paa
plasmid of adherence aggregative
- eae/bfp
enterocyte effacement/bundle forming pili
- lt/sth/stp
heat-labile toxin/heat-stable toxin human/heat-stable toxin porcine
- invA
invasion gene A
- MEB
multiple enteropathogenic bacteria index
- MAR
multiple antibiotic resistance index
- AM
ampicillin
- AB
ampicillin–sulbactam
- FX
cefoxitin
- CT
cefotaxime
- CI
ciprofloxacin
- C
chloramphenicol
- CN
gentamicin
- NA
nalidixic acid
- S
streptomycin
- TC
tetracycline
- ST
trimethoprim–sulfamethoxazole
Contributor Information
Violeta Poma, Email: vio_noemia15@hotmail.com.
Nataniel Mamani, Email: nmamanich@yahoo.es.
Volga Iñiguez, Phone: 591 2-2612815, Email: volgavir@yahoo.com.
References
- American Public Health Association . Standard methods for the examination of water and wastewater analysis. Washington DC: American Water Works Association/Water Environment Federation; 2005. [Google Scholar]
- Barak JD, Jahn CE, Gibson DL, Charkowski AO. The role of cellulose and O-antigen capsule in the colonization of plantas by Salmonella enterica. Mol Plant Microbe Interact. 2007;20:1083–1091. doi: 10.1094/MPMI-20-9-1083. [DOI] [PubMed] [Google Scholar]
- Begum YA, Talukder KA, Nair GB, Khan SI, Svennerholm AM, Sack RB, Qadri F. Comparison of enterotoxigenic Escherichia coli isolated from surface water and diarrhoeal stool samples in Bangladesh. Can J Microbiol. 2007;53:19–26. doi: 10.1139/w06-098. [DOI] [PubMed] [Google Scholar]
- Benguigui Y (2002) Atención Integrada a las Enfermedades Prevalentes de la Infancia. Rev Bol Ped 41:29–35. http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S1024-06752002000100009
- Berger CN, Shaw RK, Brown DJ, Mather H, Clare S, Dougan G, Pallen MJ, Frankel G. Interaction of Salmonella enterica with basil and other salad leaves. ISME J. 2009;3:261–265. doi: 10.1038/ismej.2008.95. [DOI] [PubMed] [Google Scholar]
- Boxall ABA, Hardy A, Beulke S, Boucard T, Burgin L, Falloon PD, Williams RJ. Impacts of climate change on indirect human exposure to pathogens and chemicals from agriculture. Environ Health Perspect. 2009;117:508–514. doi: 10.1289/ehp.0800084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendan RJ, Patricia MG, Dana C, Kelly AW, Shua JC. Outbreak-associated Salmonella enterica serotypes and food commodities, United States, 1998–2008. Emerg Infect Dis. 2013;19:1239. doi: 10.3201/eid1908.121511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz U, Bernard H, Werber D, Böhmer MM, Remschmidt C, Wilking H, Deleré Y, van der Heiden M, Adlhoch C, Dreesman J, Ehlers J, Ethelberg S, Faber M, Frank C, Fricke G, Greiner M, Höhle M, Ivarsson S, Jark U, Kirchner M, Koch J, Krause G, Luber P, Rosner B, Stark K, Kühne M. German outbreak of Escherichia coli O104:H4 associated with sprouts. N Engl J Med. 2011;365:1763–1770. doi: 10.1056/NEJMoa1106482. [DOI] [PubMed] [Google Scholar]
- Buxton N, Escobar M, Purkey D, Lima N (2013) Water scarcity, climate change and Bolivia: planning for climate uncertainties. Stockholm Environment Institute. https://www.sei-international.org/mediamanager/documents/Publications/SEI-DiscussionBrief-Escobar-Spanish-BoliviaWaterClimate.pdf. Accessed 22 Oct 2015
- Centers for Disease Control and Prevention (1993) Surveillance for cholera: Cochabamba Department, Bolivia, January–June 1992. Morb Mortal Wkly Rep 42:636–639. http://www.cdc.gov/mmwr/preview/mmwrhtml/00021431.htm. Accessed 9 Nov 2015 [PubMed]
- Clinically and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing. Pennsylvania: Wayne; 2007. [Google Scholar]
- Confalonieri UEC, Fonseca AFQ (2013) Health security in Amazonia. Report for Global Canopy Programme and International Center for Tropical Agriculture as part of the Amazonia Security Agenda project. http://globalcanopy.org/projects/amazonia-security-agenda. Accessed 17 Dec 2015
- De Paula VS, Diniz-Mendes L, Villar LM, Luz SL, Silva LA, Jesus MS, da Silva NM, Gaspar AM. Hepatitis A virus in environmental water samples from the Amazon Basin. Water Res. 2007;41:1169–1176. doi: 10.1016/j.watres.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Diniz-Mendes L, Paula VS, Luz SL, Niel C. High prevalence of human Torque teno virus in streams crossing the city of Manaus, Brazilian Amazon. J Appl Microbiol. 2008;105:51–58. doi: 10.1111/j.1365-2672.2007.03720.x. [DOI] [PubMed] [Google Scholar]
- Duran A, Moscoso O, Romero A, Huibers F, Agodzo S, Chenini F, van Lier J (2003) Use of wastewater in irrigated agriculture—country studies from Bolivia, Ghana and Tunisia. 1: Bolivia. http://www.ircwash.org/resources/use-wastewater-irrigated-agriculture-country-studies-bolivia-ghana-and-tunisia-vol-1-bolivia. Accessed 12 Oct 2015
- Environmental Protection Agency (2007) EPA guidelines: regulatory monitoring and testing water and wastewater sampling. South Australia. http://www.epa.sa.gov.au/files/8494_guide_wws.pdf. Accessed 16 Nov 2015
- Estado Plurinacional de Bolivia (1992) Ley No. 1333- del 27 de Abril de 1992. Ley del Medio Ambiente. Bolivia
- Gonzales L, Joffre E, Rivera R, Sjoling A, Svennerholm AM, Iniguez V. Prevalence, seasonality and severity of disease caused by pathogenic Escherichia coli in children with diarrhoea in Bolivia. J Med Microbiol. 2013;62:1697–1706. doi: 10.1099/jmm.0.060798-0. [DOI] [PubMed] [Google Scholar]
- Gonzales L, Sanchez S, Zambrana S, Iniguez V, Wiklund G, Svennerholm AM, Sjoling A. Molecular characterization of enterotoxigenic Escherichia coli isolates recovered from children with diarrhea during a 4-year period (2007 to 2010) in Bolivia. J Clin Microbiol. 2013;51:1219–1225. doi: 10.1128/JCM.02971-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M, Barthem RB, Ferreira E. The Smithsonian Atlas of the Amazon. Washington, DC: Smithsonian Books; 2003. [Google Scholar]
- Instituto Nacional de Estadística (2011) Estadísticas en Salud. http://www.sedeslapaz.gob.bo/pdf/snis/ESTADISTICAS%20DE%20LAS%20REDES%201ER%20SEM%202014.pdf. Accessed 29 Mar 2015
- International Commission on Microbiological Specifications for Foods (1996) Food Control 7:99–101. doi:10.1016/S0956-7135(96)90007-9
- Islam M, Morgan J, Doyle M, Phatak C, Millner P, Jiang X. Persistence of Salmonella enterica Serovar Typhimurium on lettuce and parsley and in soil on which they were grown in fields treated with contaminated manure composts on irrigation water. Foodborne Pathog Dis. 2004;1:27–35. doi: 10.1089/153531404772914437. [DOI] [PubMed] [Google Scholar]
- Juliana G, Aaron MW, Arthur W, Barbara J, Paul T, Chad S, Robert TR. Spinach-associated Escherichia coli O157:H7 Outbreak, Utah and New Mexico, 2006. Emerg Infect Dis. 2008;14:1633. doi: 10.3201/eid1410.071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little CL, Gillespie IA. Prepared salads and public health. J Appl Microbiol. 2008;105:1729–1743. doi: 10.1111/j.1365-2672.2008.03801.x. [DOI] [PubMed] [Google Scholar]
- Lothigius A, Sjoling A, Svennerholm AM, Bolin I. Survival and gene expression of enterotoxigenic Escherichia coli during long-term incubation in sea water and freshwater. J Appl Microbiol. 2010;108:1441–1449. doi: 10.1111/j.1365-2672.2009.04548.x. [DOI] [PubMed] [Google Scholar]
- Marcheggiani S, D’Ugo E, Puccinelli C, Giuseppetti R, D’Angelo AM, Gualerzi CO, Spurio R, Medlin LK, Guillebault D, Weigel W, Helmi K, Mancini L. Detection of emerging and re-emerging pathogens in surface waters close to an urban area. Int J Environ Res Public Health. 2015;12:5505–5527. doi: 10.3390/ijerph120505505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miagostovich MP, Ferreira FF, Guimaraes FR, Fumian TM, Diniz-Mendes L, Luz SL, Silva LA, Leite JP. Molecular detection and characterization of gastroenteritis viruses occurring naturally in the stream waters of Manaus, central Amazonia, Brazil. Appl Environ Microb. 2008;74:375–382. doi: 10.1128/AEM.00944-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministerio de Medio Ambiente y Agua (2012) Informe de auditoria sobre el desempeño ambiental respecto a los impactos negativos generados en la cuenca del Río La Paz. http://www.contraloria.gob.bo/portal/Auditor%C3%ADa/Auditor%C3%ADaAmbiental.aspx
- Ministerio de Salud (2000) Colera en Bolivia-Anuario Epidemiológico 2000. http://www.ops.org.bo/textocompleto/nsp16024.pdf. Accessed 22 Mar 2015
- Moon JY, Park JH, Kim YB. Molecular epidemiological characteristics of virulence factors on enteroaggregative E. coli. FEMS Microbiol Lett. 2005;253:215–220. doi: 10.1016/j.femsle.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno A, Marui A, Castro ES, Benitez AA, Elio-Calvo D, Kasitani H, Ishii Y, Yamaguchi K. Enteropathogenic bacteria in the La Paz river of Bolivia. Am J Trop Med Hyg. 1997;57:438–444. doi: 10.4269/ajtmh.1997.57.438. [DOI] [PubMed] [Google Scholar]
- Osmond DL, Cannon RW, Gale JA, Line DE, Knott CB, Phillips KA, Thrner MH, Foster MA, Lehning DE, Coffey SW, Spooner J. WATERSHEDSS: a decision support system for watershed-scale nonpoint source water quality problems. J Am Water Resour As. 1997;33:327–341. doi: 10.1111/j.1752-1688.1997.tb03513.x. [DOI] [Google Scholar]
- Pereira L, Monteiro M, Guimarães D, Matos J, da Costa R. Seasonal effects of wastewater to the water quality of the Caeté river estuary, Brazilian Amazon. An Acad Bras Ciênc. 2010;82:467–478. doi: 10.1590/S0001-37652010000200022. [DOI] [PubMed] [Google Scholar]
- Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005;18:465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn K, De Grandis SA, Clarke RC, McEwen SA, Galán JE, Ginocchio C, Curtiss Iii R, Gyles CL. Amplification of an invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol Cell Probe. 1992;6:271–279. doi: 10.1016/0890-8508(92)90002-F. [DOI] [PubMed] [Google Scholar]
- Rede Interagencial de Informações Para a Saúde (2012) Basic data and indicators, Brazil. http://abnet.datasus.gov.br/cgi/idb2012/matriz.htm. Accessed 3 Sept 2015
- Resolution MEPC (2006) Revised guidelines on implementation of effluent standards and performance test for sewage treatment plants. International Maritime Organization
- Rodas C, Halvorsen K, Iñiguez V (2005) Multiresistencia antimicrobiana asociada a integrones en enteropatógenos de la diarrea infantil y Escherichia coli de la flora normal en niños menores de 5 años en la ciudad de La Paz. Cuad Hosp Clín 50:38–48. http://www.revistasbolivianas.org.bo/scielo.php?pid=S1652-67762005000200006&script=sci_arttext
- Rodas C, Iniguez V, Qadri F, Wiklund G, Svennerholm AM, Sjoling A. Development of multiplex PCR assays for detection of enterotoxigenic Escherichia coli colonization factors and toxins. J Clin Microbiol. 2009;47:1218–1220. doi: 10.1128/JCM.00316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodas C, Mamani R, Blanco J, Blanco J, Wiklund G, Svennerholm A-M, Sjöling Å, Iñiguez V. Enterotoxins, colonization factors, serotypes and antimicrobial resistance of enterotoxigenic Escherichia coli (ETEC) strains isolated from hospitalized children with diarrhea in Bolivia. Braz J Infect Dis. 2011;15:132–137. doi: 10.1016/S1413-8670(11)70158-1. [DOI] [PubMed] [Google Scholar]
- Salem I, Ouardani I, Hassine M, Aouni M. Bacteriological and physico-chemical assessment of wastewater in different region of Tunisia: impact on human health. BMC Res Notes. 2011;4:1–11. doi: 10.1186/1756-0500-4-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo Domingo JW, Harmon S, Bennett J. Survival of Salmonella species in river water. Curr Microbiol. 2000;40:409–417. doi: 10.1007/s002840010079. [DOI] [PubMed] [Google Scholar]
- Shaw RK, Berger CN, Pallen MJ, Sjoling A, Frankel G. Flagella mediate attachment of enterotoxigenic Escherichia coli to fresh salad leaves. Environ Microbiol Rep. 2011;3:112–117. doi: 10.1111/j.1758-2229.2010.00195.x. [DOI] [PubMed] [Google Scholar]
- Singh G, Vajpayee P, Ram S, Shanker R. Environmental reservoirs for Enterotoxigenic Escherichia coli in South Asian gangetic riverine system. Environ Sci Technol. 2010;44:6475–6480. doi: 10.1021/es1004208. [DOI] [PubMed] [Google Scholar]
- Sistema Nacional de Información en Salud (2013) Vigilancia epidemiologica 2013—Enfermedades Diarreicas Agudas, Bolivia. http://snis.minsalud.gob.bo/snis/default.aspx. Accessed 29 Mar 2015
- United Nations Environment Programme . Amazon Basin, GIWA regional assessment. Kalmar: University of Kalmar; 2004. [Google Scholar]
- Waldner L, MacKenzie K, Köster W, White A. From exit to entry: long-term survival and transmission of salmonella. Pathogens. 2012;1:128–155. doi: 10.3390/pathogens1020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (1989) Health guidelines for the use of wastewater in agriculture and aquaculture. Report of WHO Scientific Group. Technical Report Series No 778 [PubMed]
- World Health Organisation (2014) Food safety. http://www.who.int/mediacentre/factsheets/fs399/en/. Accessed 21 Nov 2014
- World Health Organisation (2015) Antimicrobial resistance. http://www.who.int/mediacentre/factsheets/fs194/en/. Accessed 3 Mar 2016
- World Water Assessment Programme . The United Nations world water development report 4: managing water under uncertainty and risk. Paris: UNESCO; 2012. [Google Scholar]
- Yoder JS, Cesario S, Plotkin V, Ma X, Kelly-Shannon K, Dworkin MS. Outbreak of enterotoxigenic Escherichia coli infection with an unusually long duration of illness. Clin Infect Dis. 2006;42:1513–1517. doi: 10.1086/503842. [DOI] [PubMed] [Google Scholar]


