Abstract
In vitro propagation methods using seeds and nodal segments of a 21-year old Couroupita guianensis - a medicinally important but threatened tree have been developed. Hundred percent of the seeds germinated on half strength Murashige and Skoog (MS) medium with 2.0 mg l−1 indole-3 butyric acid (IBA). Nodal segments were found most suitable for the establishment of cultures. About 90 % explants responded and 4.1 ± 0.23 shoots per node were induced after five weeks of inoculation on MS medium +4.0 mg l−1 6-benzylaminopurine (BAP). Further shoot multiplication was achieved by repeated transfer of mother explants and subculturing of in vitro produced shoots on fresh medium. Maximum number (8.2 ± 0.17) of shoots were regenerated on MS medium with 1.0 mg l−1 each of BAP and Kinetin (Kin) + 0.5 mg l−1 α-naphthalene acetic acid (NAA) with additives (50 mg l−1 of ascorbic acid and 25 mg l−1 each of adenine sulphate, L-arginine and citric acid). The multiplied shoots rooted (4.3 ± 0.26 roots/shoot) on half strength MS medium with 2.5 mg l−1 IBA. All the shoots were rooted ex vitro when pulse treated with 400 mg l−1 of IBA for five min with an average of 7.3 ± 0.23 roots per shoot. Nearly 86 % of these plantlets were acclimatized within 7–8 weeks and successfully transferred in the field. Biologically significant developmental changes were observed during acclimation particularly in leaf micromorphology in terms of changes in stomata, veins and vein-islets, and trichomes. This study helps in understanding the response by the plants towards outer environmental conditions during acclimatization. This is the first report on micropropagation of C. guianensis, which could be used for the large-scale multiplication, restoration and conservation of germplasm of this threatened and medicinally important tree.
Keywords: Couroupita guianensis, Micropropagation, Ex vitro rooting, Acclimatization, Micromorphology, Conservation
Introduction
India harbors many rare, endemic and endangered medicinal plants. Many of these are overexploited and facing serious threat of extinction due to anthropological and environmental issues. Couroupita guianensis Aubl. (Lecythidaceae, Brazil Nut Family) is a threatened tree worldwide (Mitre 2012; Rai 2014). It is large, deciduous, magnificent evergreen tree, growing to a height of 20 m. This bears a large cluster of flowers in cauliflorous inflorescence with stunning fragrance (Sai et al. 2011). The tree is commonly known as Cannon ball tree due to its cannon ball like fruits. Naglingam, Ayahuma, Kailaspati, Calabasse Colin etc. are other known names of this plant in India (Lim 2012).
The cannon ball tree is native to the rainforests of central and South America and distributed in Amazonian Colombia, northern Venezuela, Guyana, Surinam, French Guiana, Amazonian Ecuador, Amazonian Peru, and eastern and southwestern Amazonian Brazil (Sathe et al. 2013). It is a popular ornamental plant in Caribbean and South-East Asian botanic gardens and listed as a rare tree and flower in India (Shah et al. 2012; Shete et al. 2013). This tree gathers highlights due to unique types of fruits, inflorescence and attractive structure of the flower with pleasant aroma (Santosh 2011; Gousia et al., 2013). It is a keystone species of forest ecosystem which provides food and shelter to many birds and the animals.
C. guianensis gains traditional importance because it cures skin diseases, cold, stomach ache, tooth ache and infections (Umachigi et al. 2007). This plant endowed with many important properties like antimicrobial, antifungal, antiseptic (Khan et al. 2003; Kavitha et al. 2011), antidepressant (Sivakumar et al. 2012), antimalarial, antihelmintic, anticancer (Velliangiri and Subban 2012) and immune modulatory activities (Pradhan et al. 2009).
The whole plant contains several chemical constituents with novel structures and possesses bioactive moieties; including stigmasterol, eugenol, linalool, isatin, indirubin, fernesol, nerol, quercertin, saponins, tryptanthrine, indigo, linoleic acid, α- and β- amirins, carotenoids, sterols, flavonoids, phenolics etc. (Jayashree et al. 2001; Rane et al. 2001; Ahire and Laddha 2002; Desal et al. 2003; Rajamanickam et al. 2009; Mariappan et al. 2012).
The magnificent flowers (known as Nagalingam) have attracted the people religiously in India because the staminal sheath resembles the hood of the King cobra (Naga), a sacred snake, protecting the reduced stigma (Linga of Lord Shiva) and its aroma pushed them to grow it as ornamental tree around the temples (Santosh 2011; Santosh et al. 2013).
Due to extraordinary medicinal properties this plant was over exploited which resulted in dramatic reduction of its natural population. Increasing human and livestock populations and environmental disasters have also affected the status of C. guianensis in the wild. The Government of Puducherry (India) has declared C. guianensis flower (Nagalingam flower) as the Official State Flower to conserve this valuable tree under natural habitats in South India (Deepa 2007).
Natural propagation of C. guianensis is very slow. In nature, the plant is propagated by seeds only and the viability of seeds is very low. Seeds have short life span due to recalcitrant nature which cannot be dried well and cannot withstand low temperatures (Gousia et al. 2013). Muniswamy and Sreenath (2000) were unable to germinate Cannon ball seeds in the soil.
The prime objective of the present study was to conserve this threatened keystone species by micropropagation and ex vitro rooting methods and this is the first report on efficient micropropagation of C. guianensis.
The different environmental conditions during in vitro and in the field greatly affect the success of micropropagation. The altered morphology, physiology and anatomy of micropropagated plants could be achieved by gradual hardening process from the greenhouse to the field. Greenhouse and field environments are stressful to micropropagated plants compared to in vitro conditions (Chirinea et al. 2012). The in vitro heterotrophic conditions are responsible for physiological and structural modifications (Yokota et al. 2007). Recent studies have shown the differences in morphology and anatomy of in vitro regenerated plants and the plants growing under in vivo conditions (Rathore et al. 2013; Lodha et al. 2015). The present research also aimed to investigate the foliar epidermal micromorphological developments occurred in plantlets cultured in vitro and field transferred.
Materials and methods
Collection of plant material
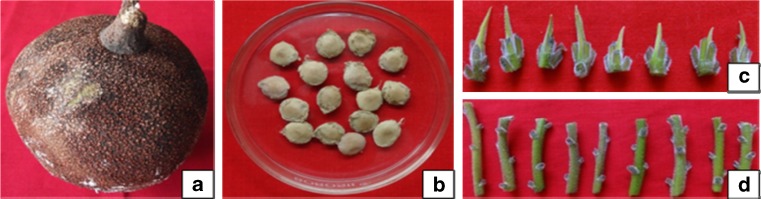
With prior knowledge, field trips were conducted to select the superior and mature trees of C. guianensis. The plant materials were collected during the period of December, 2011 to April, 2014 from the Coromandel Coast (Kanchipuram, Villipuram, Puducherry, Cuddalore, Nagapattinam and Karaikal districts) of the South India. The area lies on the geographical coordinates of 11° 55′ 48″ N, 79° 49′ 48″ E. The mature fruits (Fig. 1a) and fresh shoots were transported to the laboratory. The explanting materials employed in this study were mature seeds, apical shoots and nodal segments (Fig. 1b, c and d).
Fig. 1.
a Mature Cannon ball fruit b Seeds of C. guianensis c and d Apical apices and nodal segments used as explants
In vitro seed germination
The fruits were broken and seeds were separated from the white creamy/sandal pulp. Seed coat hairs were removed with help of soft brush. The seeds were washed 5–7 times with water to remove the unwanted debris. These seeds were treated with 0.1 % (w/v) Bavistin solution (Systemic fungicide, BASF India Ltd.) for 7–8 min and surface sterilized with 0.1 % (w/v) mercuric chloride (Qualigens Fine Chemicals, India) for 5–6 min under laminar air flow cabinet (Technico Pvt. Ltd. Chennai, India). These were washed with autoclaved water for 8–10 times. For germination, the surface sterilized seeds were inoculated on agar gelled (agar 0.8 %) full and half strength MS basal media (Murashige and Skoog 1962) with different types of growth regulators (BAP, Kin, IAA and IBA ranging from 1.0 to 5.0 mg l−1) and incubated under dark field at 25 ± 2 °C temperature.
Preparation of nodal segments and apical shoots as explants
The nodal shoot segments (2–3 cm long) with at least 2–3 nodes per explant and apical shoots were used for culture initiation. The explants were washed with Tween® 20 (Sigma Aldrich, India) detergent solution for 8–10 min and thoroughly washed with sterile water. After that, the explants were treated with Bavistin (0.1 %) for 7–8 min and surface sterilized with 0.1 % HgCl2 for 5–6 min under laminar air flow cabinet and washed with autoclaved double distilled water for 8–10 times. The sterilized explants were inoculated on MS medium supplemented with different concentrations of cytokinins ranging from 1.0 to 5.0 mg l−1.
Bud breaking and multiple shoot induction
The sterilized explants were inoculated in test tubes (size 55 ml) containing 15 ml MS medium + 8.0 g l−1 agar (HiMedia, Mumbai, India) + 30 g l−1 sucrose (Merck, India) and additives (50 mg l−1 of ascorbic acid and 25 mg l−1 each of adenine sulphate, L-arginine and citric acid), supplemented with BAP and/or Kin ranging from 1.0 to 5.0 mg l−1. The cultures were maintained under 45–50 μmol m−2 s−1 Spectral Flux Photon (SFP) light intensity at 25 ± 2 °C temperature.
Multiplication of shoots
The initiated axillary/apical shoots, were multiplied in vitro on agar gelled MS medium by a repeated transfer of mother explants with regenerated shoots, which is followed by subculturing of shoot clumps on MS medium with different concentrations of cytokinins such as BAP and Kin (ranging from 1.0 to 3.0 mg l−1) along with NAA (0.1 to 1.0 mg l−1). Experiments were also conducted to evaluate the effects of Woody Plants (WP) medium (Lloyd and McCown 1980), supplemented with above mentioned growth regulators for multiple shoot induction and further multiplication of cultures.
Induction of roots from the shoots (in vitro)
The healthy, long (3–4 cm) and sturdy shoots were separated from the bunch of multiplied shoots. These were inoculated vertically on full and half strength MS medium incorporated with different concentrations of auxins (IAA, NAA and IBA ranging from 1.0 to 4.0 mg l−1). Activated charcoal (200 mg l−1) was also added to the medium for better root initiation. The entire rooting experiments were kept in dark for one week at 25 ± 2 °C temperature. After 4 weeks of culture, number and length of roots were measured.
Ex vitro rooting and hardening of plantlets
Experiments were also conducted to attain ex vitro rooting from the dissected ends of in vitro raised shoots to achieve rooting and acclimatization simultaneously. Indole-3 butyric acid, IAA and NAA ranging from 100 to 600 mg l−1 was used for pulsing (5 min) to induce roots from the cut ends of the shoots. The pulse treated shoots were transferred to horticultural plastic cups/pots (size 150 ml; Vandana Paper Products, Chennai, India) containing 55 g autoclaved soilrite® (a mixture of perlite, Irish Peat moss and exfoliated vermiculite; KelPerlite, Bangalore, India), moistened with 10 ml aqueous 1/4th MS salts solution by the interval of one week and maintained in the greenhouse for five weeks. The relative humidity (80–85 %) and temperature conditions (25 to 28 °C) were maintained in the greenhouse.
The in vitro rooted plantlets were taken out from the culture vessels after 5 weeks and washed with sterile distilled water to remove nutrient agar from the roots. These plantlets were transplanted immediately to the paper cups containing soilrite® which were moistened with aqueous solution of 1/4th strength of MS basal salts. These were kept in the greenhouse for acclimatization for about 5 weeks. The rooted plantlets were further transferred to nursery/horticultural plastic pots containing garden soil, soilrite® and manure in the ratio of 1:1:1 for hardening.
Foliar micromorphological studies of in vitro grown and field transferred plants
Experiments were conducted to study the micromorphological changes during leaf development like venation pattern, stomata and trichomes in leaves of plants developed in vitro after 4th subculture in multiplication phase and hardened plants in the field after 6th week. Plants were randomly selected for the micromorphological experiments from both the environments. The entire leaves (10 from each stage of plantlets) at third to seventh leaves from the base were excised manually for all the experiments. To observe the changes in structure and functioning of developing stomata, epidermal peels were separated manually by standard traditional method (Johansen 1940) from leaves under in vitro as well as field transferred plants. For venation study, leaves were fixed primarily in Formalin acetic acid solution, FAA (1:1:3) and cleared in 70 % ethanol (v/v) until the chlorophyll was removed (12–24 h), bleached with 5 % (w/v) NaOH for 24–48 h, and rinsed three times in distilled water (Sass 1940). The leaves were then stained with 1 % safranine (Loba chemie, India) aqueous solution for 4–8 min and rinsed carefully in water to remove excess stain and then mounted in distilled water and examined under microscope (Labomed iVu 3100, USA).
Experimental design and statistical analysis
The experiments followed in this study were laid down according to completely randomized block design (RBD) in case of single factor experiments (Compton and Mize 1999), with a minimum of 10 replicates per treatment and were repeated thrice. Data were subjected to analysis of variance and the significance of differences was calculated by Duncan’s Multiple Range Test using SPSS software (version 16.0).
Results and discussion
Explants selection and establishment of culture
The effective selection of explants is the basic requirement for any plant propagation protocol (Jiang et al. 2012). Cultures of C. guianensis were established from the embryos of the seeds, nodal segments and apical shoots. Seeds collected from the mature Cannon ball fruits in the months of November to January recorded maximum (100 %) seed germination under in vitro conditions in the present study. This could overcome the seed viability issue in C. guianensis because all the seeds were germinated (Fig. 2a, b) when these were properly cleaned, washed and treated with HgCl2 and inoculated on half strength MS medium for 10–12 days incubated in the dark environment. John et al. (1997) suggested cold treatment at 15 °C for 72 h for bud breaking in woody trees. However, we observed poor response of seeds that were treated with low temperature as compared to untreated seeds. The seeds were germinated in vitro on half strength MS medium with 2.0 mg l−1 IBA (Fig. 2c, d and e, Table 1); whereas all other growth regulators were less effective to in vitro seeds germination.
Fig. 2.
a, b and c In vitro germination of seeds on MS medium with 2.0 mg l−1 IBA d and e In vitro grown seedlings
Table 1.
Effect of plant growth regulators on seed germination (C. guianensis) on half strength MS medium
| PGRs Conc. (mg l−1) | Response (%) |
|---|---|
| BAP | |
| 1.00 | 40ab |
| 2.00 | 55abc |
| 3.00 | 57bc |
| 4.00 | 67cde |
| 5.00 | 60cd |
| Kin | |
| 1.00 | 37a |
| 2.00 | 50abc |
| 3.00 | 66cde |
| 4.00 | 55abc |
| 5.00 | 40ab |
| IAA | |
| 1.00 | 54abc |
| 2.00 | 63cde |
| 3.00 | 78def |
| 4.00 | 60cd |
| 5.00 | 52abc |
| IBA | |
| 1.00 | 78def |
| 2.00 | 100g |
| 3.00 | 87fg |
| 4.00 | 80ef |
| 5.00 | 67cde |
Mean separation was analyzed by ANOVA using SPSS software (var. 16.0) and significance variation between the concentrations was studied using DMRT at 0.05 % level
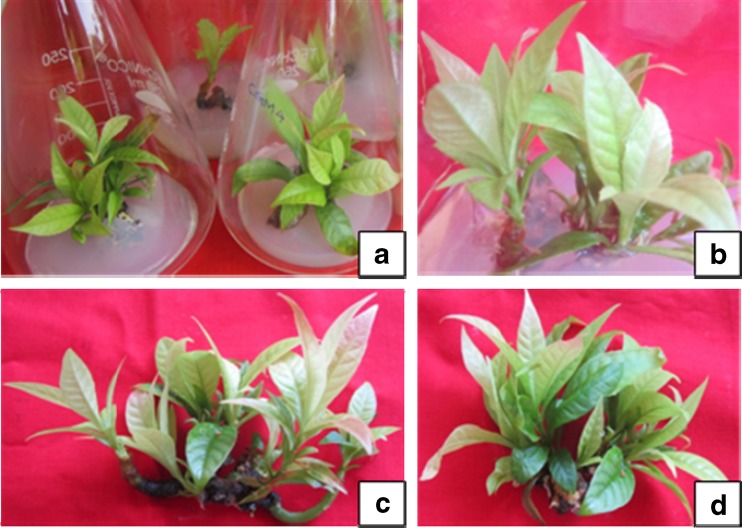
The physiological status of explants plays significant role in the establishment of cultures (Smith 2000). The apical shoots and nodal segment explants were more responsive/regenerative which were collected in the months of June to September. The seasonal effect on culture establishment has also been reported in Azadirachta indica (Arora et al. 2010), Celastrus paniculatus (Phulwaria et al. 2013) and Morinda coreia (Shekhawat et al. 2015a). It was observed that nodal segments with one node died due to the effect of HgCl2 during surface sterilization but the middle nodes were survived even after HgCl2 treatment when the nodal explants were used with 2–3 nodes. The size of explants after removal of upper and lower node was about 0.6 to 0.7 cm long (Fig. 3a). About 90 % bud break was achieved within 2 weeks of inoculation on MS medium supplemented with 4.0 mg l−1 BAP and 4.1 ± 0.23 shoots were regenerated from the node of the explant on this medium combination (Fig. 3b, c and d, Table 2). Apical shoots also responded in similar way but the number of shoots were less (1–2) as compared to the nodal explants (Fig. 3e, f and g). However, MS medium containing both auxins and cytokinin was reported less effective in bud break and induction of multiple shoots from the apical and nodal meristems. The effectiveness of BAP over Kin in bud breaking and shoot proliferation has been well documented in several plant species (Singh and Tiwari 2012; Shekhawat et al. 2015b; Patel et al. 2014).
Fig. 3.
a, c and d Bud breaking and multiple shoots formation from the nodal meristems of explants on MS medium with 4.0 mg l−1 BAP. b Explant with middle node after removal of upper and lower nodes. e, f and g Induction of multiple shoots from the shoot apices on MS medium augmented with 4.0 mg l-1 BAP
Table 2.
Effect of cytokinins (BAP and Kin) concentrations on induction of shoots from the nodal meristems of explants of C. guianensis on MS medium
| Cytokinins Conc. (mg l−1) | Response (%) | Number of shoots (Mean ± SD) |
|---|---|---|
| BAP | ||
| 1.0 | 56 | 1.2 ± 0.67a |
| 2.0 | 73 | 1.9 ± 0.42b |
| 3.0 | 87 | 3.2 ± 0.46e |
| 4.0 | 90 | 4.1 ± 0.23f |
| 5.0 | 82 | 3.0 ± 0.16e |
| Kin | ||
| 1.0 | 41 | 1.1 ± 0.43a |
| 2.0 | 49 | 1.6 ± 0.38b |
| 3.0 | 53 | 2.7 ± 0.13d |
| 4.0 | 61 | 3.2 ± 0.22e |
| 5.0 | 50 | 2.4 ± 0.17a |
| BAP + Kin | ||
| 1.0 | 46 | 1.2 ± 0.29e |
| 2.0 | 49 | 3.3 ± 0.37c |
| 3.0 | 56 | 2.3 ± 0.33a |
| 4.0 | 58 | 1.3 ± 0.28a |
| 5.0 | 52 | 1.0 ± 0.16a |
Mean separation was analyzed by ANOVA using SPSS software (var. 16.0) and significance variation between the concentrations was studied using DMRT at 0.05 % level
The rejuvenation of meristem was achieved in plants by selecting the age of the mother plant, nature of explant, season of explant collection, plant growth regulators and additives (ascorbic and citric acids, adenine sulphate and arginine) used (Arya et al. 2002).
Multiplication of shoots in cultures
The regenerated fresh shoots with mother explants were multiplied by three methods. (i) The in vitro germinated seedlings were used to multiply the shoots, (ii) subculturing of the in vitro produced shoots from nodal and apical meristems, and (iii) repeated transfer of mother explants and shoot clumps on fresh medium.
The young seedlings were cut into small segments and these were placed on MS basal medium with different concentrations of BAP and Kin with or without NAA but very less number of shoots were regenerated. The fresh shoots were excised from the mother explants and transferred to the shoot multiplication medium comprised of MS medium augmented with 1.0 mg l−1 each BAP and Kin +0.5 mg l−1 NAA with additives. The mother explants were also inoculated on this media combination for 3–4 cycles. This media combination significantly enhanced the proliferation of shoots in cultures (Fig. 4a, b, c and d). The maximum number of shoots (8.2 ± 0.17 shoots/culture) were produced when the mother explants were subcultured on fresh medium (Table. 3).
Fig. 4.
a Multiplicaiton of shoots after two weeks on agar gelled medium with 1.0 mg l-1 each of BAP and Kin + 0.5 mg l-1 NAA. b Multiplication of shoots after five weeks in culutre flask. c and d Multiple shoots outside of culture vesels
Table 3.
Effect of cytokinins (BAP and Kin) concentrations on multiplication of shoots on MS Medium containing 0.5 mg l−1 NAA
| BAP + Kin conc. (mg l−1) | Number of shoots (Mean ± SD) | Shoot length (cm) (Mean ± SD) |
|---|---|---|
| 0.0 | 0.0 ± 0.00a | 0.0 ± 0.00a |
| 0.1 | 3.0 ± 0.49b | 5.5 ± 0.75e |
| 0.5 | 5.1 ± 0.22c | 5.5 ± 0.51ef |
| 1.0 | 8.2 ± 0.38e | 5.8 ± 0.42f |
| 1.5 | 6.0 ± 0.61d | 5.2 ± 0.49de |
| 2.0 | 5.7 ± 0.27d | 4.9 ± 0.25cd |
| 2.5 | 4.8 ± 0.24c | 4.6 ± 0.63bc |
| 3.0 | 2.9 ± 0.19b | 4.2 ± 0.24b |
Mean separation was analyzed by ANOVA using SPSS software (var. 16.0) and significance variation between the concentrations was studied using DMRT at 0.05 % level
Shoot multiplication during repeated transfer may be due to inhibition of apical dominance which stimulates basal dormant meristematic cells to produce young shoots (Phulwaria et al. 2013). This approach for shoot multiplication has been used in several plant species (Panwar et al. 2012). Low concentrations of BAP and Kin (1.0 mg l−1 each) promoted shoots multiplication and also minimized the hyperhydricity in cultures.
It was found that the subculturing was necessary within 30 to 35 days to avoid nutritional deficiency in cultures. The cultures were maintained on MS medium supplemented with 0.5 mg l−1 NAA + 1.0 mg l−1 each BAP and Kin at 25 ± 2 °C under 40–50 μmol m−2 s−1 SFP. The present study showed that BAP and Kin were necessary for the regeneration of shoots and the incorporation of NAA enhanced multiple shoot formation. WP medium was also tested with above concentrations of plant growth regulators but it was not found suitable in terms of induction, multiplication and proliferation of shoots in cultures as compared to the MS medium.
Rooting of in vitro regenerated shoots
Half strength MS medium was found superior as compared to full strength medium for rhizogenesis in C. guianensis. Strength of MS medium appeared to be an important factor in influencing the rooting efficiency. Auxins are mainly used in root induction and their effect varies with type and concentration used in different plant species (Swamy et al., 2002; Patel et al. 2014). However, a very low concentration of exogenous auxin is required for better root induction.
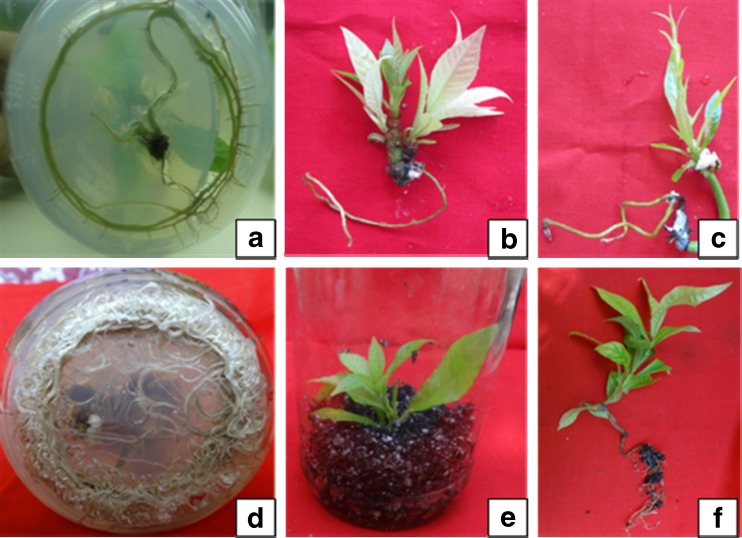
The shoots were rooted on half strength MS medium fortified with 2.5 mg l−1 IBA + activated charcoal (Fig. 5a, b, c, and d). About 97 % of the shoots were rooted on this medium. The root initiation was visible on 15th day of inoculation and 4.3 ± 0.26 roots were observed from the cut ends of each shoot (Table. 4). Whereas, 46 % of the shoots were rooted on half strength MS medium +2.0 mg l−1 IAA containing medium and 3.1 ± 0.22 roots were induced on this media combination. Auxins play an important role in induction of roots from the cut ends of in vitro raised shoots (Misic et al. 2006; Arikat et al. 2004). Incorporation of activated charcoal in medium helps in balancing the pH level as well as improves the nitrogen absorption by the shoots and induces rooting (Thomas 2008). The shoots rooted the best at 25 ± 2 °C temperature under diffused light conditions.
Fig. 5.
a, b, c and d Different stages in in vitro rooting of the shoots with half strength MS medium with 2.5 mg l−1 IBA e and f Ex vitro rooting experiments in the greenhouse (after pulsed with 400 mg l−1 of IBA)
Table 4.
Effect of auxin concentrations on response, number and length of roots induced from the shoots on half strength MS medium
| Auxins Conc. (mg l−1) | Response (%) | Number of roots (Mean ± SD) | Root length (cm) (Mean ± SD) |
|---|---|---|---|
| IAA | |||
| 1.0 | 16 | 2.2 ± 0.91a | 3.2 ± 0.23ab |
| 1.5 | 37 | 2.7 ± 0.38b | 3.8 ± 0.47cde |
| 2.0 | 46 | 3.1 ± 0.22c | 4.1 ± 0.42e |
| 2.5 | 45 | 3.0 ± 0.16cd | 4.0 ± 0.31de |
| 3.0 | 31 | 2.9 ± 0.67c | 3.5 ± 0.28bc |
| 4.0 | 29 | 2.7 ± 0.49c | 3.0 ± 0.24a |
| IBA | |||
| 1.0 | 46 | 2.8 ± 0.57c | 4.2 ± 0.15ef |
| 1.5 | 52 | 3.4 ± 0.49de | 4.8 ± 0.20g |
| 2.0 | 75 | 3.8 ± 0.81fg | 5.3 ± 0.36h |
| 2.5 | 97 | 4.3 ± 0.26g | 7.4 ± 0.27k |
| 3.0 | 72 | 4.0 ± 0.41gh | 6.1 ± 0.35j |
| 4.0 | 61 | 3.5 ± 0.93ef | 5.7 ± 0.29i |
| NAA | |||
| 1.0 | 22 | 2.5 ± 0.23ab | 3.0 ± 0.19ab |
| 1.5 | 31 | 3.1 ± 0.19c | 4.2 ± 0.26ef |
| 2.0 | 48 | 3.5 ± 0.49de | 4.6 ± 0.25fg |
| 2.5 | 40 | 3.9 ± 0.20fg | 4.1 ± 0.38de |
| 3.0 | 35 | 3.4 ± 0.14de | 3.6 ± 0.33bcd |
| 4.0 | 30 | 3.0 ± 0.26cd | 3.2 ± 0.41ab |
Mean separation was analyzed by ANOVA using SPSS software (var. 16.0) and significance variation between the concentrations was studied using DMRT at 0.05 % level
Ex vitro rooting and acclimatization of plantlets
Rooting and acclimatization could be achieved simultaneously using ex vitro rooting method (Baskaran and Van Staden 2013). IBA was reported better auxin for ex vitro root induction as compared to NAA and IAA. About 92 % shoots were rooted (7.3 ± 0.23 roots per shoot) when treated with 400 mg l−1 IBA for 5 min (Fig. 5e, f, Table 5). Generally, ex vitro rooting in plant tissue culture enhance the survival rate of plants during hardening period, which help in the lateral roots formation similar to the natural root system. (Dhavala and Rathore 2010).
Table 5.
Effect of auxins on ex vitro root induction from the cut ends of the micro-shoots in Soilrite
| Auxins Conc. (mg l−1) | Response (%) | Number of roots (Mean ± SD) | Root length (cm) (Mean ± SD) |
|---|---|---|---|
| IAA | |||
| 100 | 20 | 3.4 ± 0.17bcd | 3.5 ± 0.71a |
| 200 | 35 | 3.7 ± 0.24cde | 3.8 ± 0.64b |
| 300 | 45 | 4.0 ± 0.41def | 4.3 ± 0.37c |
| 400 | 57 | 4.2 ± 0.33ef | 5.0 ± 0.44e |
| 500 | 41 | 3.0 ± 0.29ab | 4.6 ± 0.29d |
| 600 | 36 | 2.5 ± 0.49a | 3.7 ± 0.25ab |
| IBA | |||
| 100 | 45 | 4.4 ± 0.46f | 4.2 ± 0.39c |
| 200 | 50 | 5.7 ± 0.28g | 5.6 ± 0.42f |
| 300 | 86 | 6.3 ± 0.12i | 6.1 ± 0.28g |
| 400 | 92 | 7.3 ± 0.23j | 6.5 ± 0.26j |
| 500 | 70 | 6.1 ± 0.31h | 6.4 ± 0.34i |
| 600 | 55 | 5.6 ± 026g | 6.3 ± 0.25h |
| NAA | |||
| 100 | 29 | 3.2 ± 0.18bc | 3.7 ± 0.30ab |
| 200 | 32 | 3.9 ± 0.20def | 4.3 ± 0.41c |
| 300 | 41 | 4.3 ± 0.25ef | 4.9 ± 0.52e |
| 400 | 59 | 4.9 ± 0.38bcd | 5.4 ± 0.28f |
| 500 | 44 | 3.5 ± 0.24bc | 5.0 ± 0.41e |
| 600 | 33 | 3.2 ± 0.47ab | 4.3 ± 0.37c |
Mean separation was analyzed by ANOVA using SPSS software (var. 16.0) and significance variation between the concentrations was studied using DMRT at 0.05 % level
Plantlets rooted under an ex vitro environments are better suited/adapted to the natural conditions and environmental stresses, and therefore easy to harden (Yan et al. 2010). These have more vigor to tolerate stresses experienced during hardening stages. It has been reported that ex vitro rooted plants are better suited to tolerate environmental stresses (Baskaran and Van Staden 2013). Shekhawat et al. (2014, 2015b) have successfully achieved ex vitro root induction in Melothria maderaspatana, Turnera ulmifolia and Morinda citrifolia. It is a cost effective technique which could save labor, time and energy in in vitro plant propagation system (Ranaweeraa et al. 2013).
In vitro rooted plantlets were transferred to the greenhouse for hardening of plantlets and then to the nursery poly-pots after five weeks (Fig. 6a, b). The plantlets were hardened in the greenhouse for about two months. During hardening about 85–90 % of the ex vitro plantlets and 73–75 % of in vitro rooted plantlets survived (Fig. 6c, g) and the hardened plants were finally shifted to the field (Fig. 7a, b).
Fig. 6.
a and b In vitro raised plantlets in paper cups with soilrite c, d, e, f and g. Different stages in hardening of C. guianensis
Fig. 7.
a and b In vitro raised plantlets of C. guianensis transferred to the field
Micromorphological studies
Hardening and field transfer allows the micropropagated plants to attain developmental changes in gradual manner (Kevers et al. 2004; Rathore et al. 2013; Lodha et al. 2015). Morphologically the leaves of in vitro regenerated plants and field transferred plants resemble each other in terms of shape, clustered (Leptocaul form of leaves) with pointed tip and tapering base. The leaves of C. guianensis were amphistomatic and the stomata restricted to the intercostal areas, facing all directions and distributed irregularly.
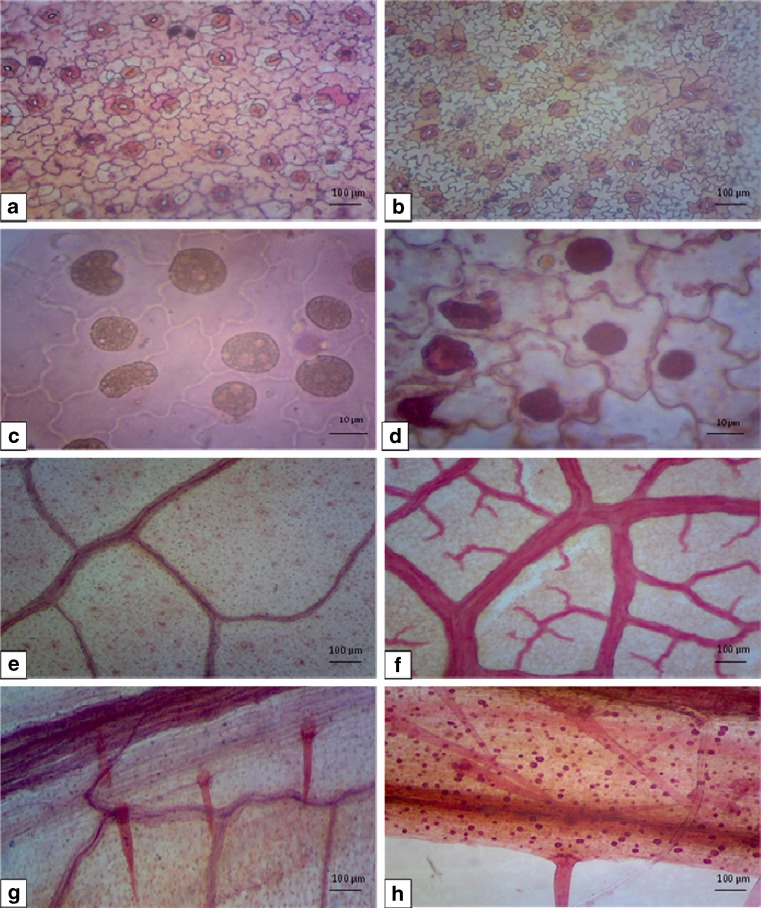
Microscopic evaluation of foliar epidermal study revealed the presence of highly undulated epidermal cells with sinuous anticlinal walls on abaxial epidermis of field transferred plants, but the in vitro cells were comparatively less undulated. Axial parenchyma cells were elongated, rectangular or polygonal in shape. Epidermal cells were tightly packed, highly sinuous, amoeboid and small in size in field transferred plants. Anomocytic stomata were predominant with higher stomatal frequency in in vitro grown leaves than the field transferred plants. Contiguous stomata were frequent but giant stomata rarely observed in in vitro leaves, such abnormalities were rare or completely absent in field transferred plants. Anomocytic and teteracytic stomata were predominant in field transferred plants, whereas anomocytic and anisocytic type of stomata predominant in in vitro leaves. Sathe et al. (2013) reported the presence of anomocytic stomata on upper and lower epidermises in C. guianensis. Regina (2014) observed the presence of anomocytic stomata with indistinct epidermal cells but clear and distinct subsidiary cells were reported in vitro and in field transferred plants in this study.
The stomata of leaves under in vitro conditions were generally opened and comparatively more frequent as compared to field transferred plants (Fig. 8a, b). The increased stomatal frequency on the abaxial surface is probably an adaptation to water loss. Malfunctioning of stomata, poor stomatal functioning and altered morphological and anatomical characteristics of in vitro grown plants resulted in insufficient photosynthetic capacity to achieve positive carbon balance (Pospisilova et al. 2000).
Fig. 8.
Micromorphological studies of C. guianensis. A Stomatal pattern in leaves of in vitro raised and B field transferred plant. C Crystals in epidermal cells in in vitro leaves and D field transferred leaves. E Venation pattern in leaves of in vitro raised shoots and F field transferred plant. G Trichomes in leaves of in vitro raised and H field transferred plant (scale bar 10.0 μm, 100.0 μm)
Presence of calcium oxalate crystal and druses are frequent in C. guianensis cells (Regina 2014; Sathe et al. 2013). Crystals were mostly distributed in axial parenchyma but these were also present in some epidermal cells (Fig. 8c, d). Richter and Dallwitz (2000) also reported presence of one prismatic crystal in each axial parenchyma cell. The vein density and distinct vein-islets were increased during the hardening process of plants. The midrib was fairly prominent and projecting on abaxial surface; veins and vein-islets were fewer in in vitro than the field transferred plants (Fig. 8e, f).
The trichome density increased from in vitro to field environment. These were uniseriate, unicellular, less frequent and underdeveloped in in vitro conditions, became fully developed, unicellular as well as multicellular tufts of trichomes in field transferred plants within 6 weeks (Fig. 8g, h). Distinct and clear oil droplets were observed in the epidermal and the guard cells in the in vitro leaves. These in vitro structures sometimes show affinity between species and define their position in given species (Johansen 1940).
Conclusion
Increasing demand and importance to traditional medicine in the recent years has threatened the survival of some tree species. The traditional conventional plant propagation methods are affected by biotic and variable environmental factors. This report describes the in vitro seed germination methods in C. guianensis where seed viability is very low in nature, and an efficient in vitro propagation protocol using nodal meristems as explants. The shoots were rooted ex vitro could save time, labor and energy in production of plantlets. The results of micromorphological studies could help in understanding the response of plantlets when these were transferred to the field environments. The protocol can be used for conservation strategy to increase the natural population of this threatened medicinal tree.
Acknowledgments
Authors are grateful to the Department of Science, Technology and Environment, Government of Puducherry, India for providing financial support under Grant-In-Aid Scheme [No. 10/DSTE/GIA/RP/JSA-I/2013/213] for Couroupita guianensis Aubl.
Abbreviations
- AC
Activated charcoal
- BAP
6-benzylaminopurine
- IAA
indole-3 acetic acid
- IBA
indole-3 butyric acid
- Kin
Kinetin
- MS
Murashige and Skoog’s (1962) medium
- NAA
α-naphthalene acetic acid
- SPFD
Spectral photon flux density
- PGRs
Plant Growth Regulators
Compliance with ethical standards
Financial disclosures
MSS has received financial support under Grant-In-Aid Scheme of The DST&E, Government of Puducherry, India.
References
- Ahire AE, Laddha KS. Beta amyrin palmitate- isolation from Couroupita guianensis aubl. Leaves. Indian Drugs. 2002;39:216–216. [Google Scholar]
- Arikat NA, Jawad FM, Karam NS, Shibli RA. Micropropagation and accumulation of essential oils in wild sage (Salvia fruticosa mill.) Sci Hortic. 2004;100:193–202. doi: 10.1016/j.scienta.2003.07.006. [DOI] [Google Scholar]
- Arora K, Sharma M, Srivastava J, Ranade SA, Sharma AK. Rapid in vitro cloning of a 40-year-old tree of Azadirachta indica a. Juss. (neem) employing nodal stem segments. Agrofor Syst. 2010;78:53–63. doi: 10.1007/s10457-009-9230-1. [DOI] [Google Scholar]
- Arya V, Singh RP, Shekhawat NS. A micropropagation protocol for mass multiplication and off-site conservation of Celastrus paniculatus- a vulnerable medicinal plant of India. J Sustain For. 2002;14:107–120. doi: 10.1300/J091v14n01_06. [DOI] [Google Scholar]
- Baskaran P, Van Staden J. Rapid in vitro micropropagation of Agapanthus praecox. S Afr J Bot. 2013;86:46–50. doi: 10.1016/j.sajb.2013.01.008. [DOI] [Google Scholar]
- Chirinea CF, Pasqual M, Araujo AG de, Pereira AR, Castro EM de (2012) Acclimatization and leaf anatomy of micropropagated Fig plants. Rev Bras Frutic Jaboticabal – SP 34:1180–1188
- Compton ME, Mize CW (1999) Statistical considerations for in vitro research: I -birth of an idea to collecting data. In Vitro Cell Dev Biol Plant 35:115–121
- Deepa HR (2007) Puducherry comes out with list of state symbols. The Hindu, Sat. 21, Chennnai, TN, India
- Desal T, Golatakar SG, Rane JB, Ambaye RY, Kamath VR. Larvicidal property of Couroupita guianensis aubl. Indian Drugs. 2003;40:484–486. [Google Scholar]
- Dhavala A, Rathore TS (2010) Micropropagation of Embelia ribes burm F. through proliferation of adult plant axillary shoots. In Vitro Cell Dev Biol Plant 46:180–191
- Gousia SK, Ashok KK, Vinay KT, Naveena LL. Biological activities and medicinal properties of Couroupita guianensis. Int J Pharm Pharmaceut Sci Res. 2013;3(4):140–143. [Google Scholar]
- Jayashree BR, Severina JV, Suprabha GG, Ambaye RY, Khadse BG. Chemical examination of the flowers of Couroupita guianensis aubl. Indian J Pharm Sci. 2001;63:72–73. [Google Scholar]
- Jiang Q, Zhang Y, Zhong C, Zeng B, Bogusz D, Franche C. Establishment of an in vitro plant regeneration protocol for Casuarina cunninghamiana Miq. Via indirect organogenesis. New For. 2012;43:143–154. doi: 10.1007/s11056-011-9277-5. [DOI] [Google Scholar]
- Johansen DA. Plant microtechnique. New York: McGraw Hill Co; 1940. [Google Scholar]
- John M, Sanda SI, Amasino AM. Analysis of flowering time in ecotypes of Arabidopsis thaliana. J Hered. 1997;88:69–72. doi: 10.1093/oxfordjournals.jhered.a023061. [DOI] [PubMed] [Google Scholar]
- Kavitha R, Kamalakannan P, Deepa T, Elamathi R, Sridhar S, Suresh KJ. In vitro antimicrobial activity and phytochemical analysis of Indian medicinal plant Couroupita guianensis aubl. J Chem Pharm Res. 2011;3:115–121. [Google Scholar]
- Kevers C, Franck T, Strasser RJ, Dommes J, Gaspar T. Hyperhydricity of micropropagated shoots; a typically stress-induced change of physiological state. Plant Cell Tissue Organ Cult. 2004;77:181–191. doi: 10.1023/B:TICU.0000016825.18930.e4. [DOI] [Google Scholar]
- Khan MR, Kihara M, Omoloso AD. Antibiotic activity of Couroupita guianensis. J Herbs Spices Med Plant. 2003;10:95–108. doi: 10.1300/J044v10n03_10. [DOI] [Google Scholar]
- Lim TK. Couroupita guianensis. Springer Verlag, Berlin Heidelberg: Edible medicinal and nonmedicinal plants fruits; 2012. [Google Scholar]
- Lloyd G, McCown BG. Commercially feasible micropropagation of mountain laurel Kalmia latifolia by use of shoot -tip culture. Int Plant Propagator’s Soc Combin Proc. 1980;30:421–427. [Google Scholar]
- Lodha D, Patel A, Shekhawat NS. A high-frequency in vitro multiplication, micromorphological studies and ex vitro rooting of Cadaba fruticosa (L.) Druce (Bahuguni): a multipurpose endangered medicinal shrub. Physiol Mol Biol Plants. 2015 doi: 10.1007/s12298-015-0310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan P, Srinivasan R, Kandasamy K. Antioxidant and anticancer activities of isatin (1 H-indole-2,3-dione), isolated from the flowers of Couroupita guianensis aubl. Indian J Med Res. 2012;136:822–826. [PMC free article] [PubMed] [Google Scholar]
- Misic D, Grubisic D, Konjevic R. Micropropagation of salvia brachyodon through nodal explants. Biol Plant. 2006;50:473–476. doi: 10.1007/s10535-006-0074-5. [DOI] [Google Scholar]
- Mitre M 2012 Couroupita guianensis. In: IUCN Red List of Threatened Species. Version 2012.2. http://www.iucnredlist.org
- Muniswamy B, Sreenath HL. In-Vitro development of plants from cultured embryos of Cannon Ball Tree (Couroupita guianensis Aubl) Indian. J For. 2000;23:202–204. [Google Scholar]
- Murashige T, Skoog FA. A revised medium for rapid growth and bioassay with tobacco cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Pospisilova J, Synkova H, Rulcova J (2000) Cytokinins and water stress. Biol Plant 43:321–328
- Panwar D, Ram K, Harish SNS. In vitro propagation of Eulophia nuda lindl., an endangered orchid. Sci Hortic. 2012;139:46–52. doi: 10.1016/j.scienta.2012.01.011. [DOI] [Google Scholar]
- Patel AK, Phulwaria M, Rai MK, Gupta AK, Shekhawat S, Shekhawat NS. In vitro propagation and ex vitro rooting of Caralluma edulis (edgew.) benth. & hook. f. An endemic and endangered edible plant species of the Thar desert. Sci Hortic. 2014;165:175–180. doi: 10.1016/j.scienta.2013.10.039. [DOI] [Google Scholar]
- Phulwaria M, Rai MK, Patel AK, Kataria V, Shekhawat NS. A genetically stable Rooting protocol for propagating a threatened medicinal plant-Celastrus paniculatus. Appl Biochem Biotechnol. 2013;170:1163–1173. doi: 10.1007/s12010-013-0266-3. [DOI] [PubMed] [Google Scholar]
- Pradhan D, Panda PK, Tripathy G. Evaluation of the immune modulatory activity of the methanolic extract of Couroupita guianensis aubl. Flowers in rats. Nat Prod Radiance. 2009;8:37–42. [Google Scholar]
- Rai Y. Early seedling growth status of threatened medicinal tree species Couroupita guianensis aubl. In district Meerut, (U.P.) India. Int J Inn Sci Res. 2014;8:252–255. [Google Scholar]
- Rajamanickam V, Rajasekaran A, Darlin quine S, Jesupillai M, Sabitha R. Anthelmintic activity of the flower extract of Couroupita guianensis. Int J Alt Med. 2009;8:107–111. [Google Scholar]
- Rane JB, Vahanwala SJ, Golatkar SG, Ambaye RY, Khadse BG. Chemical examination of the flowers of Couroupita guianensis aubl. Indian J Pharm Sci. 2001;63:72–73. [Google Scholar]
- Ranaweeraa KK, Gunasekaran MTK, Eeswara JP (2013) Ex vitro rooting: A low cost micropropagation technique for Tea (Camellia sinensis (L.) O. Kuntz.) hybrids. Sci Hortic 155:8–14
- Rathore NS, Rathore N, Shekhawat NS. In vitro propagation and micromorphological studies of Cleome gynandra: a C4 model plant closely related to Arabidopsis thaliana. Acta Physiol Plant. 2013 [Google Scholar]
- Regina V. Pharmacognostical studies of various parts of Couroupita guianensis aubl. Int J Curr Res Biosci Plant Biol. 2014;1:17–26. [Google Scholar]
- Richter HG, Dallwitz MJ (2000) Onwards Commercial timbers: descriptions, illustrations, identification, and information retrieval In English, French, German, Portuguese, and Spanish Version: 25th June 2009. http://delta-intkey.com
- Sai KC, Gaddala N, Vanamala S, Naresh V, Elumalai A. A short review on therapeutic uses of Couroupita guianensis aubl. Int Res J Pharm Appl Sci. 2011;1:105–108. [Google Scholar]
- Santosh KS. Cauliflory and cannon ball tree. Sci Report. 2011;48:53–55. [Google Scholar]
- Santosh AS, Gourav NS, Kavita DP, Vaibhavi SP, Samir SW, Suresh GK. Estimation of tannins present in fruit pulp and fruit shell of Couroupita guianensis. J Pharm Res. 2013;2:18–20. [Google Scholar]
- Sass JE. Elements of botanical microtechnique. New York and London: McFraw-Hill Book Co; 1940. [Google Scholar]
- Sathe SB, Jadhav AP, Sonawane AA, More SH, Kadam V. Pharmacognostical evaluation of Couroupita guianensis aubl. Leaf. Indo American J Pharm Res. 2013;3:6119–6124. [Google Scholar]
- Shah GN, Shete SA, Patil VS, Patil KD, Killedar SG. Standardization and anti-bacterial activity of Couroupita guianensis fruit pulp extract. Int J Pharmacog Phytochem Res. 2012;4:85–89. [Google Scholar]
- Shekhawat MS, Kannan N, Manokari M, Ramanujam MP. En efficient micropropagation protocol for high-frequency plantlet regeneration from liquid culture of nodal tissues in a medicinal plant Turnera ulmifolia L. J Sustain For. 2014;33:327–336. doi: 10.1080/10549811.2013.847793. [DOI] [Google Scholar]
- Shekhawat MS, Kannan N, Manokari M. In vitro propagation of traditional medicinal and dye yielding plant Morinda coreia Buch. -Ham. S Afr J Bot. 2015 [Google Scholar]
- Shekhawat MS, Kannan N, Manokari M, Ravindran CP. Enhanced micropropagation protocol of Morinda citrifolia L. through nodal explants. J Appl Res Med Aromat Plant. 2015 [Google Scholar]
- Shete SA, Shah GN, Walke SS, Patil VS, Patil KD, Killedar SG. Standardization and anti bacterial activity of Couroupita guianensis fruit shell extract. Int J Bio. 2013;2:360–364. [Google Scholar]
- Singh J, Tiwari KN. In vitro plant regeneration from decapitated embryonic axes of Clitoria ternatea L.-an important medicinal plant. Ind Crop Prod. 2012;35:224–229. doi: 10.1016/j.indcrop.2011.07.008. [DOI] [Google Scholar]
- Sivakumar T, Shankar T, Vijayabaskar P, Geetha G. Efficacy of Couroupita guianensis against selected human pathogens. Adv Biol Res. 2012;6:59–63. [Google Scholar]
- Smith RH. Plant tissue culture: techniques and experiments. Tokyo: Academic Press; 2000. [Google Scholar]
- Swamy RBV, Himabindu K, Lakshmi sita G. In Vitro micropropagatipon of Elite Rosewood (Dalbergia latifolia Roxb) Plant Cell Rep. 2002;11:126–131. doi: 10.1007/BF00232164. [DOI] [PubMed] [Google Scholar]
- Thomas TD. The role of activated charcoal in plant tissue culture. Biotechnol Adv. 2008;26:618–631. doi: 10.1016/j.biotechadv.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Umachigi SP, Jayaveera KN, Ashok KCK, Kumar GS. Antimicrobial, wound healing, and antioxidant potential of Couroupita guianensis in rats. Pharmacol Online. 2007;3:269–281. [Google Scholar]
- Velliangiri P, Subban R. Quantification of quercetin and stigmasterol of Couroupita guianensis aubl by HPTLC method and in-vitro cytototoxic activity by mtt assay of the methanol extract against Hela, nih 3 t3 and hepg2 cancer cell lines. Int J Pharm Pharmac Sci. 2012;4:126–130. [Google Scholar]
- Yan H, Liang C, Yang L, Li Y. In vitro and ex vitro rooting of siratia grosvenorii, a traditional medicinal plant. Acta Physiol Plant. 2010;32:115–120. doi: 10.1007/s11738-009-0386-0. [DOI] [Google Scholar]
- Yokota S, KArim MZ, Azad MAK, Rahman MM, Eizawa J, Saito Y, Yshiguri F, Iizuka K, Yahara S, Yoshizwan Histological observation of changes in leaf structure during successive micropropagation stages in Aralia elata and Phellodendron amurense. Plant Biol. 2007;24:221–226. [Google Scholar]