Abstract
Eight Saltol quantitative trait locus (QTL) linked simple sequence repeat (SSR) markers of rice (Oryza sativa L.) were used to study the polymorphism of this QTL in 142 diverse rice genotypes that comprised salt tolerant as well as sensitive genotypes. The SSR profiles of the eight markers generated 99 alleles including 20rare alleles and 16 null alleles. RM8094 showed the highest number (13) of alleles followed by RM3412 (12), RM562 (11), RM493 (9) and RM1287 (8) while as, RM10764 and RM10745 showed the lowest number (6) of alleles. Based on the highest number of alleles and PIC value (0.991), we identified RM8094 as suitable marker for discerning salt tolerant genotypes from the sensitive ones. Based upon the haplotype analysis using FL478 as a reference (salt tolerant genotypes containing Saltol QTL), we short listed 68 rice genotypes that may have at least one allele of FL478 haplotype. Further study may confirm that some of these genotypes might have Saltol QTL and can be used as alternative donors in salt tolerant rice breeding programmes.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-016-0342-6) contains supplementary material, which is available to authorized users.
Keywords: Genetic diversity, Microsatellites, Oryza sativa L., Salinity stress, Saltol QTL
Introduction
Rice is an important food crop of India. Among the several constrains which limit rice cultivation, salinity is the one that has profound effect in curbing the rice productivity globally. Salinity stress, in mild form, reduces overall plant growth; and an excess level leads to the enhancement of cell death (Ayala-Astorga and Alcaraz-Melendez 2010), which contributes 5 % of the crop loss (Tabatabaei 2006). Among the two types of salinity, inland salinity is due to irrigation practices with sub-standard quality water (Chinnusamy et al. 2005), whereas coastal salinity occurs due to inflow of ocean water in the coastal region. To counteract environmental stresses, plants utilize number of different mechanisms including phyto-hormone based pathways to combat against the adverse response of stress, ultimately leading to stress tolerance (Bostock 2005; Chehab et al. 2007). Rice cultivation in India and its neighbouring countries such as Bangladesh, SriLanka and Pakistan is affected by salinity as it is grown in vast coastal regions which are liable to sea water ingress during high tidal periods and also in rain-fed areas in main land where problem of increasing salinity prevails in the root zone due to a rising water table.
With the advancement of various genomic resource, significant achievements have been made in developing salt tolerant rice cultivars through various approaches such as conventional breeding, marker assisted breeding (Xu et al. 1996; Lang et al. 2008; Singh et al. 2011). However, identifying the naturally salt tolerant genotypes is important to enrich and broaden the genetic base which serves as a platform to search for new cultivars that can be used as novel donors of genes or QTLs for improving a particular trait. Thus, it is a great challenge for the plant breeders to find out either new genomic resources or new genetic resources (Mondal et al. 2015). Crop improvement programs with respect to salinity stress most importantly require the existence of genetic variability for salt tolerance within species. Therefore, choice of germplasm is of paramount importance towards such improvements as the success depends on it. Broad germplasm collection provides a useful source of genetic diversity for any trait to be studied.
Due to the complex and quantitative nature of salinity tolerance (Yeo and Flowers 1986), it has been difficult to develop an accurate, rapid and reliable screening technique. Microsatellite markers or Simple Sequence Repeats (SSRs) are the markers of choice because of their advantages over other markers. (Garland et al. 1999; Ganie and Mondal 2015). The SSR markers are particularly suitable for evaluating genetic diversity and relationships among plant species, populations, or individuals (Ganie and Mondal 2015; Kuttubuddin et al. 2015); studying rice germplasm for either conservation or utilization (Sharma et al. 2007) and marker-assisted selection breeding (Rani and Adilakshmi 2011). SSR markers have been exploited commendably to map QTLs associated with salt tolerance in rice (Lang et al. 2001; Singh et al. 2007). After extensive studies, a major salinity responsive QTL, Saltol has been mapped on chromosome 1 and subsequently markers linked to this QTL have also been reported and widely used to screen the rice genetic resources for Saltol QTL (Gregorio et al. 1997; Islam et al. 2012). It has been found that Saltol QTL contributes profoundly to salinity stress tolerance at seedling stage by maintaining the Na+/K+ homeostasis (Gregorio et al. 1997; Mohammadi-Nejad et al. 2008).
Therefore, in the present study we have done a genetic diversity study of Saltol QTL with eight polymorphic Saltol linked SSRs (Mohammadi-Nejad et al. 2008; Islam et al. 2012) in 142 rice genotypes and identified 68 genotypes that have at least one allele of Saltol QTL which might act as alternative donors of this QTL in the salinity tolerance breeding of rice.
Materials and methods
Plant material and genomic DNA extraction
One hundred and forty-two genotypes, comprising 129 salt tolerant and 13 salt sensitive genotypes, were used in the present study (Supplementary Table 1). Genotypes comprised of improved cultivars and landraces traditionally grown in Sundarban, coastal region of South Bengal, India. Total genomic DNA was isolated and purified as per the protocol described by Ganie et al. (2015).
Markers used for genotyping
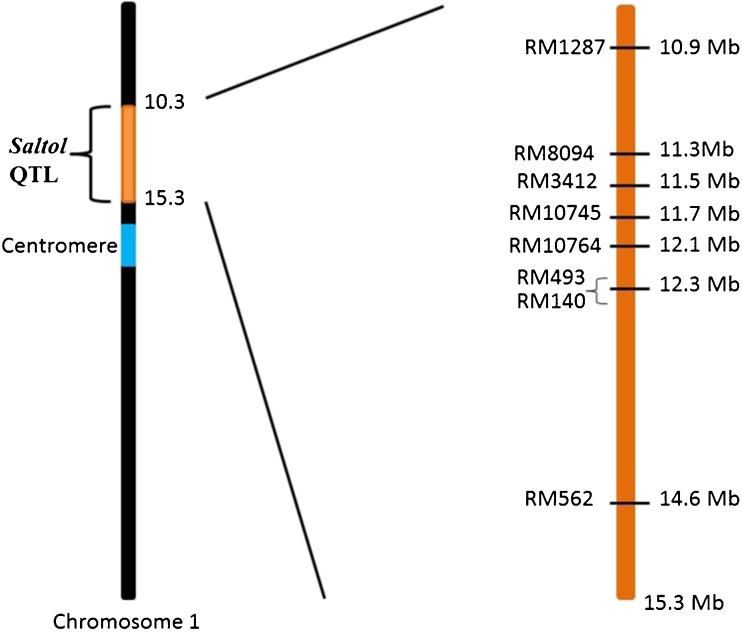
Based on published literature (Gregorio et al. 1997; Mohammadi-Nejad et al. 2008; Islam et al. 2012; Mondal and Ganie 2014) as well as preliminary testing, we selected eight highly polymorphic markers that are tightly linked with Saltol QTL for using in the present study. The details of the primers, retrieved from Gramene (Jaiswal et al. 2006), are given in Table 1. The position of these markers ranged from 10.9 Mb to 14.6 Mb (Pertea et al. 2003) of Saltol QTL on chromosome 1 as depicted in Fig. 1.
Table 1.
SSR markers on chromosome 1 used in the present study
| SSR markers | Repeat motif | Forward primer sequence | Reverse primer sequence |
|---|---|---|---|
| RM1287 | (AG)17 | 5-GGAAGCATCATGCAATAGCC-3′ | 5-GGCCGTAGTTTTGCTACTGC-3′ |
| RM8094 | (AT)31 | 5-AAGTTTGTACACATCGTATACA-3′ | 5-CGCGACCAGTACTACTACTA-3′ |
| RM10745 | (TATG)9 | 5-TGACGAATTGACACACCGAGTACG-3′ | 5-ACTTCACCGTCGGCAACATGG-3′ |
| RM10764 | (AT)28 | 5-AGATGTCGCCTGATCTTGCATCG-3′ | 5-GATCGACCAGGTTGCATTAACAG-3′ |
| RM562 | (AAG)13 | 5′-CACAACCCACAAACAGCAAG-3′ | 5′-CTTCCCCCAAAGTTTTAGCC-3′ |
| RM140 | (CT)12 | 5-TGCCTCTTCCCTGGCTCCCCTG-3′ | 5-GGCATGCCGAATGAAATGCATG-3′ |
| RM3412 | (CT)17 | 5-AAAGCAGGTTTTCCTCCTCC-3′ | 5-CCCATGTGCAATGTGTCTTC-3′ |
| RM493 | (CTT)9 | 5-TAGCTCCAACAGGATCGACC-3′ | 5-GTACGTAAACGCGGAAGGTG-3′ |
Fig. 1.
Physical location of the eight SSR markers on Saltol QTL on chromosome 1 of rice
PCR amplification and data analysis
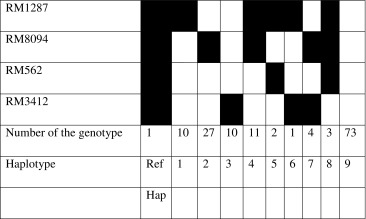
PCR amplification and the data analysis were performed according to Mondal and Ganie (2014). Haplotype analysis was conducted according to Liu and Anderson (2003) with four most polymorphic SSR markers, i.e., RM8094, RM3412, RM562 and RM1287 using FL478 genotype as a reference.
Results
Major alleles, rare alleles, null alleles and allelic heterozygosity
PCR amplification of the genomic DNA of the 142 genotypes with the eight SSR markers generated a total of 99 alleles, amongst which 20 were rare and 16 null alleles. The differences in molecular size between the smallest and the largest allele for a given SSR locus varied from 30 bp (RM10764) to 150 bp (RM8094). The lowest (90 bp) and the highest (320 bp) amplicon sizes were produced by RM8094 and RM562 respectively. Supplementary Fig. 1 shows the amplification of genomic DNA of 142 diverse genotypes with RM562. The number of alleles per locus varied with an average of 12.37. It thus reflected the diversity among the tested genotypes. Highest number of alleles (13) was produced by RM8094 followed by RM3412 (12), RM562 (11), RM493 (9) and RM1287 (8), whereas, the lowest number was produced by RM10764 and RM10745 (6). The highest PIC value was exhibited by RM8094 (0.991) whereas, the lowest PIC was recorded with RM10745 (0.962).
A total of 20 rare alleles were identified from the eight polymorphic SSRs. The highest number of rare alleles (5) was generated by the marker RM3412 and RM562 followed by RM1287 and RM493 (3 each) whereas, the lowest number of rare alleles (1 each) was shown by RM140,RM10745, RM10764 and RM8094. Similarly, 16 null alleles were detected from five out of the eight polymorphic loci. The highest number of null alleles (9) was generated by the marker RM10764, followed by RM8094 (3), RM493 (2) whileas, RM140 and RM 10745 generated the lowest (1) null allele number. Markers RM562, RM1287 and RM3412 did not put any null allele on display. Fifteen major alleles for all the eight SSR loci were detected in the present study. The allele (size 103 bp) of RM8094 locus was a major one and appeared in a maximum number (70) of genotypes followed by the major allele (size 173 bp) of RM1287 that occurred in 63genotypes. Another major allele (size 165 bp) was observed at RM10764locus and appeared in 55genotypes. It was observed that all the eight SSR primers amplified multiple heterozygous bands. Among the studied markers, RM8094 was the most heterozygous (97.2) while RM562 was the least. Table 2 details the data generated by eight SSR markers for 142 genotypes.
Table 2.
Data generated by eight SSR markers used in the present study
| Marker | No. of alleles | Locus heterozygosity | Av. frequency of major alleles | Av. frequency of rare alleles | Frequency of null alleles | PIC Value | Amplicon size (bp) |
|---|---|---|---|---|---|---|---|
| RM1287 | 8 | 9.79 | 50.00 | 2.10 | 0 | 0.981 | 150–220 |
| RM8094 | 13 | 97.20 | 50 | 2.8 | 2.09 | 0.991 | 90–240 |
| RM10745 | 6 | 14.68 | 36.9 | 2.8 | 0.69 | 0.962 | 170–220 |
| RM10764 | 6 | 13.28 | 39 | 0.7 | 6.29 | 0.967 | 150–180 |
| RM562 | 11 | 6.29 | 25.3 | 3.50 | 0 | 0.990 | 200–320 |
| RM140 | 8 | 13.28 | 28.8 | 4.2 | 0.69 | 0.980 | 250–310 |
| RM3412 | 12 | 28.67 | 37.5 | 2.5 | 0 | 0.988 | 160–290 |
| RM493 | 9 | 16.08 | 25 | 3.0 | 1.39 | 0.984 | 190–280 |
Haplotype diversity analysis
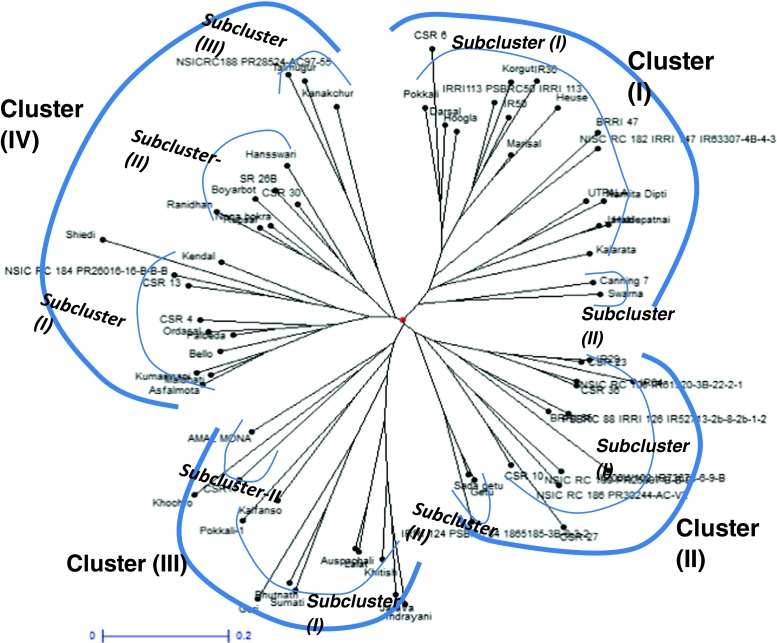
From eight polymorphic SSRs, four tightly linked to Saltol QTL were selected for haplotyping. Including the reference haplotype, 10 haplotypes were detected in the entire germplasm of 142 rice genotypes studied (Table 3). The nine haplotypes were represented by a minimum of one genotype viz., PSBRC50 in haplotype-6 to a maximum of 73 genotypes in haplotype-9. Among the 142 genotypes, none produced identical haplotype as that of reference genotype FL478 for all the four markers used for haplotyping. Sixty-eight genotypes had different combinations of FL478 reference alleles while, 73 genotypes did not produce any common alleles with the FL478 haplotype (haplotype- 9). Forty-seven genotypes had only one of the allele common with the FL478 haplotype (haplotypes 1, 2 and 3). Also haplotype-2, formed only by RM8094, has the highest number of salt tolerant genotypes (27). This marker seems to have a close association with salt tolerance which may find application in breeding for salt tolerance through marker-assisted selection. Figure 2 depicts the genetic relationship among the 68 rice genotypes that have the similar haplotypes as that of FL478. The UPGMA-based dendrogram obtained from the binary SSR data deduced from the corresponding DNA profiles of the 68 genotypes divided the genotypes into four groups/clusters. Cluster-I, II and III had 19, 15 and 13genotypes respectively and were divided into 2 subclusters each. Cluster-IV had 21 genotypes and divided into 3 sub-clusters. Cluster-III comprised exclusively of salt tolerant genotypes whereas, all the other 3 clusters possessed salt tolerant genotypes predominantly along with few salt sensitive genotypes e.g. Kalarata and Swarna in subcluster-1 and 2 respectively of the cluster-I, IR29 in the subcluster-1 of cluster-II and Khitish in the subcluster-1 of cluster-III. Although the presence of sensitive genotypes in the dendrogram is not clear, however, this might be due to the fact that the analysis is based only on 4 SSR markers which do not span across all the salinity tolerant allele in Saltol, a phenomenon well reported in the literature about the similar studies (Krishnamurthy et al. 2014).
Table 3.
Ten haplotypes produced by eight SSR markers located in the Saltol QTL region on chromosome 1 with reference to FL478 genotype (Ref Hap)
(Genotypes that composed different haplotypes are: Reference Haplotype = FL478; 1 = Jarava, Indrayani, Bhutnath, Sumati, CSR10, CSR27, Ordasal, IR36, Swarna,; 2 = Kalarata, Boyarbot, Hansswari, Rupsail, Ranidhan, Kanakchur, NamitaDipti, Amal Mona, Nona bokra, Pokkali-1; CSR30, Shiedi, Kumarmani, Kendal, SALINAS 3, Malabati, Asfalmota, SR 26B, Bello, Utpala, PSBRc 84, NSIC RC 190, Kalfanso, CSR11, Talmugur, SALINAS 4, Khitish; 3 = CSR 6, Korgut, Heuse, Hoogla, Canning 7, NISC RC 182, Khochro, Geri, Haldepatnai, Jamai; 4 = Sadagetu, SALINAS 2, BRRI-55, Auspachali, CSR4, PSBRC 88, IR50, Getu, Lalat, CSR13, Palbeda; 5 = IR64, CSR23; 6 = PSBRC50; 7 = Pokkali 3, BRRI-47, Marisal, Darsal; 8 = IR29, CSR36, SUMILAO; 9 = Khejurchhodi, PSBRc 86, Kumrogoor, Benimadhav, Niko, PSBRC 48, Sadaketu, Cheriviruppu, Patnai-23, Annapoorna, Kalimekri, Matla, ADT-38, PNR-519, Mohan-9, CARI Dhan-5, CARI Dhan-4, Pokkali 2, Pokkali 4, Pokkali 9, Pokkali 5, B-20, Pokkali 8, Pokkali 10, Auspachali, Sadamota, Lunishree, Pokkali 12, Jarava, PNL-1, PNL-3, Bura Rata, Payjam, IT-5656, Dharitri, A1, Bengal, Kalanunia, Pokkali 11, Pokkali 6, Pokkali 13, PNL-2, Mala raja, Katrangi, Kanakchur, Dudheswar, Haldiguri, BRRI-53, Damgo, Pala Bhir, Jyothi, Shyamli, Rajlaxmi, Nilanjana, Uchapatnai, Assgo, Khochro, VTL-6, Pokkali 7, Pokkali 14 Durga, Muno, Kalonovan, CSR43, 1280, Patnai 23, Dudheshwarmota, Natopatnai, Kamini, LathiPatnai, Lalkamini, GobindaBhog, Kalamota)
Fig 2.
Dendrogram showing the relationship among 68 rice genotypes that may have Saltol QTL. Dendrogram constructed using unweighted neighbour-joining of simple matching coefficients based on SSR segregation data
The correlation between the groups of dendrogram and the different haplotypes was evaluated. It was found that majority of the genotypes from a particular haplotype belonged to a specific cluster in the dendrogram e.g. 15 of the 21 genotypes in the cluster-IV belonged to the haplotype-1. Almost 33 % of the genotypes from haplotype-1 e.g. Jarava, Indrayani, Bhutnath and Sumati lied in the cluster-III. Haplotype-7 and 8 housed predominantly in the cluster-II whereas, haplotype-5 and 6 lied exclusively in the cluster-II and I respectively. This type of analysis indicated a broad correlation between the haplotypes and genetic relationship among the genotypes.
Discussion
A small percentage of the world rice germplasm collections has been employed in the breeding programs despite the abundance of genetic resources. Consequently a high degree of genetic similarity is found among the commercial rice varieties around the world. For designing the effective breeding programs, awareness about the amount of genetic variation in germplasm collections and genetic relatedness between genotypes are important considerations. It is very important to broaden the genetic base of the cultivated rice varieties so as to reduce their vulnerability to adverse environments and biotic challenges. Besides, genetic improvement depends mainly on the extent of genetic variability present in the population. For assessing these genetic variations and resolve cultivar identities, the molecular marker is a useful tool. Among the different molecular markers, SSRs are more robust in differentiating individual germplasm accessions (Ganie and Mondal 2015). Additionally, SSRs have several benefits over other molecular markers, e.g. abundance in genome, higher polymorphism, co-dominance and cost effectiveness (Ni et al. 2002).
Though, more than 40,000 varieties of rice have been reported globally, yet only a few of them are popular and grown widely (Vanniarajan et al. 2012). Therefore, to develop new rice cultivars, it is important to know the genetic makeup of underutilized or unexplored rice germplasm. For example, it has been found that Pokkali or its descendent FL478 remain dominant donors for Saltol QTL to develop the salt tolerant rice cultivars. These two genotypes are widely used in back crossing programs to introgress this QTL into locally adopted sensitive cultivars. Therefore, there is a need to find out the alternative donors of this QTL to broaden the genetic bases. Although there are morphological markers and physiological techniques available to identify the salt tolerant cultivars, yet they cannot identify the QTLs precisely so that an alternative donor can be identified. However, the prerequisite for identification of donors of a particular salt tolerant QTL is to study the genetic diversity of that QTL by its linked markers.
In the present report, we studied genetic diversity of the Saltol QTL on chromosome 1 with its linked SSRs among a diverse collection of salt tolerant as well as sensitive rice genotypes. It also demonstrated well the usefulness of SSRs in detecting the possible presence/absence of the Saltol QTL in rice genotypes. Further, it elucidated the genetic distances among the 68 rice genotypes likely to have one of the alleles of reference haplotype through analysis of the profiles generated by the four out of eight SSR markers.
All the eight SSR markers amplified polymorphic bands in the 142 genotypes. Several workers also reported that these markers are highly polymorphic (Mohammadi-nejad et al. 2008; Karmakar et al. 2012; Chattopadhyay et al. 2014; Ganie et al. 2014). The PCR profile obtained from the SSR markers generated a total of 99 alleles including 20 rare alleles and 16 null alleles, accounting an average of 12.37 alleles/locus which is higher than the numbers reported by other groups using the SSR markers (Mohammadi-Nejad et al. 2008; Islam et al. 2012; Karmakar et al. 2012; Das et al. 2013). Based upon the PIC value, amplicon size range and the number of alleles, RM8094 is advocated to be the most useful and informative marker than others for studying the diversity of Saltol QTL. It was immediately followed by RM3412, RM562 and RM1287. In addition to this, our findings are in accordance with some previous reports (Thomson et al. 2007; Mohammadi-nejad et al. 2008; Islam et al. 2012; Islam and Gregorio 2013; Babu et al. 2014) who also reported RM8094, RM3412 and RM1287 as the useful markers either for marker assisted selection of Saltol QTL or to study the genetic diversity of this QTL. However, the current study differs from these previous studies in several aspects such as name of the genotypes, number and name of the SSRs and most importantly use of reference genotype for haplotype analysis. For example Babu et al. (2014) used Pokkali as reference. Since there are several Pokkali genotypes, thus to ensure the presence of Saltol QTL, we used FL478 as reference.
The entire germplasm was represented by10 different haplotypes (including the reference haplotype) (Table 3). The genotypes possessing at least one of the FL478 allele for the locus RM8094 may have Saltol QTL which may render these genotypes salinity tolerant. So, the genotypes under the haplotypes 2, 4, 7 and 8 might have Saltol QTL as all of them have at least one allele of RM8094 marker. Salt tolerance of these genotypes has already been reported by various researchers (Supplementary Table 1) including our previous study (Pani et al. 2013). Some of such salt tolerant genotypes that contain RM8094 and/or RM1287 and RM3412 marker allele are Kalarata, Boyarbot, Hansswari, Rupsail, Ranidhan, Kanakchur, Namita Dipti, Amal Mona, Nona bokra, Pokkali-1; CSR30, Shiedi, Kumarmani, Kendal, SALINAS 3, Malabati, Asfalmota, SR 26B, Bello, Utpala, PSBRc 84, NSIC RC 190, CSR11, Talmugur, SALINAS 4, Sadagetu, SALINAS 2, BRRI-55, Auspachali, CSR4, PSBRC 88, IR50, Getu, Lalat, CSR13, Palbeda, Pokkali 3, BRRI-47, Marisal, Darsal, CSR36, SUMILAO. The haplotype-2, which has been formed exclusively by the RM8094 allele, contains the highest number of salt tolerant genotypes (27). Thus, all the genotypes of the haplotype-2 possess the salt tolerant FL478 type allele and hence more likely their salinity tolerance are due to the presence of Saltol QTL. Therefore, this marker (RM8094) can be recommended for using in marker assisted breeding to introgress this QTL for salt tolerance. In fact, salt tolerance is a quantitative trait and is regulated by multiple genes. Therefore, it can be said that the 68 genotypes that possess one of the marker alleles for FL478 haplotype might have this QTL (Saltol) contributing towards the salinity tolerance, however, presence of other QTLs rendering them salinity tolerant cannot be ruled out. However, further research is needed to be carried out along with the phenotypic and physiological screening in order to prove that tolerance in the sixty-nine genotypes is due to the presence of the Saltol QTL.
As a general indicator of the amount of genetic variability with respect to a particular trait, the determination of the amount of heterozygosity across loci can be a good choice. Locus heterozygosity can be very well employed to measure the genetic variation (Allendorf 1986). All the studied SSR primers amplified multiple heterozygous bands. Among the studied markers, the heterozygosity for RM8094 locus was the highest (97.20) whereas the locus RM562 was the least heterozygous. Thus, locus heterozygosity also indicated RM8094 as a superior marker for genetic diversity analysis with respect to salinity tolerance trait.
For identifying populations that need special management and deserve attention, allelic richness, in terms of rare and private alleles, is useful. Allelic richness of populations of an endangered tree species was compared to identify genetically diverse populations so that these populations could be protected (Petit et al. 1998). The number of rare alleles in a population (private allelic richness) is a simple measure of genetic distinctiveness (Kalinowski 2004). A total of 20 rare alleles were identified from the eight polymorphic loci. The highest number of rare alleles (5) was generated by the marker RM3412 and RM562 followed by and RM1287 and RM493 (3 rare alleles each), while the lowest number (1 each) of rare alleles was shown by RM140, RM10745, RM10764 and RM8094. Hence, from the perspective of number of rare alleles also, these 5 markers can be regarded as the superior markers because higher the number of rare/rare alleles for a particular marker the most informative the marker is.
Five out of the eight polymorphic loci showed 16 null alleles. Null alleles are produced most probably due to mutations in the binding region of one or both of the SSR primers (Callen et al. 1993), thereby inhibiting primer annealing and subsequently leading to no amplification at that locus for a particular genotype. Fifteen major alleles for all the eight SSR loci were shown by the studied germplasm.
Conclusion
In conclusion, this is a preliminary study with Saltol QTL linked SSR markers to study the genetic diversity of Saltol QTL in 142 genotypes. On the basis of genetic diversity, we concluded that 68 genotypes may have putative Saltol QTL region, although there may be other QTLs that may provide salinity tolerance. It was shown that RM8094 followed by RM3412, RM562 and RM1287 were useful markers that produced polymorphism of this region and are suitable for marker assisted breeding to introgress this QTL. These markers can also be used in preliminary screening to select the presence of Saltol QTL allele. Based on the present analysis, we also concluded that the salt tolerance of rice genotypes that did not show any similarity with FL478 haplotype, could be due to some other salt responsive alleles (other than Saltol QTL) with different mechanisms for providing salt tolerance.
Electronic supplementary material
(DOCX 354 kb)
(DOCX 35 kb)
Acknowledgements
The authors are thankful to Dr. K. V. Bhat, Head, Division of Genomic Resources, ICARNBPGRfor his support and advice to carry out this work. Showkat Ahmad Ganie is also grateful to the Department of Biotechnology, Govt of India for the fellowship. Authors are also grateful to Director, Prof K.C. Bansal, ICAR-NBPGR for funding this work through in-house project (code:IXX10476).
References
- Allendorf FW. Genetic drift and the loss of alleles versus heterozygosity. Zoo Biol. 1986;5:181–190. doi: 10.1002/zoo.1430050212. [DOI] [Google Scholar]
- Ayala-Astorga GI, Alcaraz-Melendez L. Salinity effects on protein content, lipid peroxidation, pigments, and proline in Paulownia imperialis (siebold & zuccarini) and paulownia fortunei (seemann & hemsley) grown in vitro. Electron J Biotechnol. 2010;13:13–14. doi: 10.2225/vol13-issue5-fulltext-13. [DOI] [Google Scholar]
- Babu NN, Vinod KK, Krishnan SG, Bhowmick PK, Vanaja T, Krishnamurthy SL, Nagarajan M, Singh NK, Prabhu KV, Singh AK. Marker based haplotype diversity of saltol QTL in relation to seedling stage salinity tolerance in selected genotypes of rice. Indian J Genet. 2014;74:16–25. [Google Scholar]
- Bostock RM. Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu Rev Phytopathol. 2005;43:545–580. doi: 10.1146/annurev.phyto.41.052002.095505. [DOI] [PubMed] [Google Scholar]
- Callen DF, Thompson AD, Shen Y, Phillips HA, Richards RI, Mulley JC, Sutherland GR. Incidence and origin of “null” alleles in the (AC)n microsatellite markers. Am J Hum Genet. 1993;52:922–927. [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay K, Nath D, Mohanta RL, Bhattacharyya S, Marndi BC, Nayak AK, Singh DP, Sarkar RK, Singh ON. Diversity and validation of microsatellite markers in saltol-QTL region in contrasting rice genotypes for salt tolerance at the early vegetative stage. Aust J Crop Sci. 2014;8:356–362. [Google Scholar]
- Chehab EW, Perea JV, Gopalan B, Theg S, Dehesh K. Oxylipin pathway in rice andarabidopsis. J Integr Plant Biol. 2007;49:43–51. doi: 10.1111/j.1744-7909.2006.00405.x. [DOI] [Google Scholar]
- Chinnusamy V, Jagendorf A, Zhu JK. Understanding and improving salt tolerance in plants. Crop Sci. 2005;45:437–448. doi: 10.2135/cropsci2005.0437. [DOI] [Google Scholar]
- Das B, Sengupta S, Parida SK, Roy B, Ghosh M, Prasad M, Ghose TK. Genetic diversity and population structure of rice landraces from eastern and North Eastern states of India. BMC Genet. 2013;14:71. doi: 10.1186/1471-2156-14-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganie SA, Mondal TK. Genome-wide development of novel miRNA-based microsatellite markers of rice (Oryza sativa) for genotyping applications. Mol Breed. 2015;35:1–12. doi: 10.1007/s11032-015-0207-7. [DOI] [Google Scholar]
- Ganie SA, Karmakar J, Roychowdhury R, Mondal TK, Dey N. Assessment of genetic diversity in salt-tolerant rice and its wild relatives for ten SSR loci and one allele mining primer of salT gene located on 1st chromosome. Plant Syst Evol. 2014;300:1741–1747. doi: 10.1007/s00606-014-0999-7. [DOI] [Google Scholar]
- Ganie SA, Dey N, Mondal TK. Differential promoter methylation of salt tolerant and susceptible rice genotypes under salinity stress. Funct Integr Genomics. 2015;8:1–11. doi: 10.1007/s10142-015-0460-1. [DOI] [PubMed] [Google Scholar]
- Garland SH, Lewin L, Abedinia M, Henry R, Blakeney A. The use of microsatellite polymorphisms for the identification of Australian breeding lines of rice (Oryza sativa. L) Euphytica. 1999;108:53–63. doi: 10.1023/A:1003688612179. [DOI] [Google Scholar]
- Gregorio GB, Senadhira D, Mendoza RD (1997) Screening rice for salinity tolerance. IRRI Discussion Paper Series No. 22. International Rice Research Institue. Los Banos Laguna, Philippines
- Islam MR, Gregorio GB. Progress of salinity tolerant rice variety development in Bangladesh. SABRAO J Breed Genet. 2013;45:21–30. [Google Scholar]
- Islam MR, Gregorio GB, Salam MA, Collard BCY, Singh RK, Hassan L. Validation of SalTol linked markers and haplotype diversity on chromosome 1 of rice. Mol Plant Breed. 2012;3:103–114. [Google Scholar]
- Jaiswal P, Ni J, Yap I, Ware D, Spooner W, Youens-Clark K, Ren L, Liang C, Zhao W, Ratnapu K, Faga B, Canaran P, Fogleman M, Hebbard C, Avraham S, Schmidt S, Casstevens TM, Buckler ES, Stein L, McCouch S. Gramene: a bird's eye view of cereal genomes. Nucleic Acids Res. 2006;34:717–723. doi: 10.1093/nar/gkj154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinowski ST. Counting alleles with rarefaction: private alleles and hierarchical sampling designs. Conserv Genet. 2004;5:539–543. doi: 10.1023/B:COGE.0000041021.91777.1a. [DOI] [Google Scholar]
- Karmakar J, Roychowdhury R, Kar RK, Deb D, Dey N. Profiling of selected indigenous rice (Oryza sativa L.) landraces of rarh Bengal in relation to osmotic stress tolerance. Physiol Mol Biol Plants. 2012;18:125–132. doi: 10.1007/s12298-012-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy SL, Sharma SK, Kumar V, Tiwari S, Batra V, Singh NK. Assessment of genetic diversity in rice genotypes for salinity tolerance using saltol markers of chromosome 1. Indian J Genet Plant Breed. 2014;74:243–247. doi: 10.5958/0975-6906.2014.00167.9. [DOI] [Google Scholar]
- Kuttubuddin AM, Debnath AB, Ganie SA, Mondal TK. Identification and analysis of novel salt responsive candidate gene based SSRs (cgSSRs) from rice (Oryza sativa L.) BMC Plant Biol. 2015;15:122. doi: 10.1186/s12870-015-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang NT, Li ZK, Bui CB. Microsatellite markers linked to salt tolerance in rice. Omonrice. 2001;9:9–21. [Google Scholar]
- Lang NT, Buu BC, Ismail AM. Molecular mapping and marker assisted selection for salt tolerance in rice (Oryza sativa L.) Omonrice. 2008;16:50–56. [Google Scholar]
- Liu S, Anderson JA. Targeted molecular mapping of a major wheat QTL for fusarium head blight resistance using wheat ESTs and synteny with rice. Genome. 2003;46:817–823. doi: 10.1139/g03-066. [DOI] [PubMed] [Google Scholar]
- Mohammadi-Nejad G, Arzani A, Rezai AM, Singh RK, Gregorio GB. Assessment of rice genotypes for salt tolerance using microsatellite markers associated with the saltol QTL. Afr J Biotechnol. 2008;7:730–736. [Google Scholar]
- Mondal TK, Ganie SA. Identification and characterization of salt responsive miRNA-SSR markers in rice (Oryza sativa) Gene. 2014;535:204–209. doi: 10.1016/j.gene.2013.11.033. [DOI] [PubMed] [Google Scholar]
- Mondal TK, Ganie SA, Debnath AB. Identification of novel and conserved microRNAs related to salinity stress of halophyte, Oryza coarctata, a wild relative of rice. PLoS One. 2015;10(10):e0140675. doi: 10.1371/journal.pone.0140675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Colowit PM, Mand DJ, Mackill (2002) Evaluation of genetic diversity in rice subspecies using microsatellite markers. Crop Sci 42: 601–607.
- Pani DR, Sarangi SK, Subudhi HN, Misra RC, Bhandari DC. Exploration, evaluation and conservation of salt tolerant rice genetic resources from Sundarbans region of West Bengal. J Indian Soc Coastal Agric Res. 2013;30:45–53. [Google Scholar]
- Pertea G, Huang X, Liang F, Antonescu V, Sultana R, Karamycheva S, Lee Y, White J, Cheung F, Parvizi B, Tsai J, Quackenbush J. TIGR gene indices clustering tools (TGICL): a software system for fast clustering of large EST datasets. Bioinformatics. 2003;19:651–652. doi: 10.1093/bioinformatics/btg034. [DOI] [PubMed] [Google Scholar]
- Petit R, El Mousadik A, Pons O. Identifying populations for conservation on the basis of genetic markers. Conserv Biol. 1998;12:844–855. doi: 10.1046/j.1523-1739.1998.96489.x. [DOI] [Google Scholar]
- Rani MG, Adilakshmi D. Genetic analysis of blast resistance in rice with simple sequence repeats (SSR) J Crop Improv. 2011;25:232–238. doi: 10.1080/15427528.2011.555834. [DOI] [Google Scholar]
- Sharma RC, Chaudhary NK, Ojha B, Yadav L, Pandey MP, Shrestha SM. Variation in rice landraces adapted to the lowlands and hills in Nepal. Plant Genet Res. 2007;5:120–127. doi: 10.1017/S1479262107837828. [DOI] [Google Scholar]
- Singh RK, Gregorio GB, Jain RK. QTL mapping for salinity tolerance in rice. Physiol Mol Biol Plant. 2007;13:87–99. [Google Scholar]
- Singh D, Kumar A, Chauhan P, Kumar V, Kumar N, Singh A, Mahajan N, Sirohi P, Chand S, Ramesh B, Singh J, Kumar P, Kumar R, Yadav RB, Nares RK. Marker assisted selection and crop management for salt tolerance: a review. Afr J Biotechnol. 2011;10:14694–14698. [Google Scholar]
- Tabatabaei SJ. Effects of salinity and N on the growth, photosynthesis and N status of olive (Olea europaea L.) trees. Sci Hortic. 2006;8:432–438. doi: 10.1016/j.scienta.2006.02.016. [DOI] [Google Scholar]
- Thomson M, de Ocampo M, Egdane J, Katimbang M, MA R, RK S, GB G, AM I. QTL mapping and marker assisted backcrossing for improved salinity tolerance in rice. Supplementary papers. BioAsia. In: The 1st International Trade Exhibition and Conference for Biotechnology, held during 5-9 November, 2007. Bangkok: Queen Sirikit National Convention Center; 2007. pp. 6–12. [Google Scholar]
- Vanniarajan C, Vinod KK, Pereira A. Molecular evaluation of genetic diversity and association studies in rice (Oryza sativa L.) J Genet. 2012;91:9–19. doi: 10.1007/s12041-012-0146-6. [DOI] [PubMed] [Google Scholar]
- Xu D, Duan X, Wang B, et al. Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol. 1996;110:249–257. doi: 10.1104/pp.110.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo AR, Flowers TJ. The physiology of salinity tolerance in rice (Oryza sativa) and a pyramiding approach to breeding varieties for saline soils. Aust J Plant Physiol. 1986;13:75–91. doi: 10.1071/PP9860161. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 354 kb)
(DOCX 35 kb)