Abstract
The possible hydrolytic activity towards chlorophyll molecules was predicted for DUF538 protein superfamily in plants. It was examined by using computational as well as experimental tools including in vitro chlorophyll degradation, antioxidant compounds production and in vivo real-time gene expression tests. Comparison of the computational data with the experimental results indicated that DUF538 proteins might be chlorophyll hydrolyzing enzyme (most probably carboxyesterase) which degrade chlorophyll molecules (66 % per 12 hrs) to produce new compounds (1.8 fold per 12 hrs) with antioxidant properties. The relevance of DUF538 gene expression level with the chlorophyll contents (2.8 fold increase per chlorophyll content of 50 %) of the drought-stressed leaves showed that chlorophyll degradation by DUF538 is most probably induced in response to stress stimuli. Despite membranous chlorophyll catabolic pathways, DUF538-dependent reactions is predicted to be occurred in the cytosol of the under stressed plants. We addressed as to whether chlorophyll breakdown to antioxidant compounds by DUF538 is a defense mechanism of plants against stress stimuli, in vivo? This question is going to be investigated in our next research project.
Keywords: Celosia cristata, Chlorophyll degradation, DUF538, Esterase, Maltose-binding protein, Stress response
Introduction
DUF538 protein superfamily consists of several plant proteins of unknown functions. They have been distributed in wide ranges of monocotyledonous and dicotyledonous plant species (Gholizadeh 2011; Takahashi et al. 2013). Their molecular weights are about 19–21 kDa, encoding about 170 amino acids. The only significant and recognizable conserved domain that has been reported for this protein superfamily is named as DUF538. Recently, the three dimensional petal-like structure of Arabidopsis thaliana DUF538 protein has been determined by NMR and released to the universal protein databases (PDB ID: D1ydua1). It has been shown to possess a protein structure dominated by ß-strands.
DUF538 domain containing proteins have been mostly identified using genome annotation tools and cloned as induced genes from plants challenged with various environmental stresses such as nutrient deficiency, crown gall, mixed elicitors, and mild drought (Gholizadeh and Baghbankohnehrouz 2010). On the basis of the high phosphorylation potential, DUF538 proteins have been predicted to play important regulatory roles in different stress-challenged plants (Nakagami et al. 2010). Our report has been revealed that the exogenously applied fusion form of a DUF538 protein by using a plant tissue abrading material activates the redox system of the plant cells (Gholizadeh 2011).
In a recent study, DUF538 proteins have been predicted as being potential homologues of BPI in mammalians immune system (Gholizadeh and Baghbankohnehrouz 2013). Thereby, they have been suggested to affect the bacterial growth rates through the binding to the LPS molecules on the outer leaflet of the bacterial membranes somewhat similar to BPI in mammalians innate immune system. Later on, by using the bioinformatic tools, a tertiary structural similarity was predicted between the BPI protein superfamily and the esterase-type hydrolases or lipolytic enzymes of which carboxyesterease type B, acyl-peptide hydrolase, entrochelin esterease, and peroxisomal long chain acyl-coA hydrolase were the best matches (Gholizadeh 2014). All of these hydrolases were found to use lipids or their aromatic derivatives as substrates and hydrolyze ester bonds. More recently, the photoconvertible WSCP1 of Chenopodium album was found to be a member of DUF538 superfamily (Takahashi et al. 2013). It has been generally speculated that WSCP1 are chloroplastic proteins and act as scavengers of free chlorophyll molecules, by transporting it from the thylakoid membrane to the chloroplast envelope, where the membrane bound chlorophyllase enzyme catabolize the chlorophylls (Noguchi et al. 1999; Satoh et al. 2001; Takahashi et al. 2013).
Considering all these information together, we hypothesized that DUF538 members may function as hydrolase enzyme and degrade chlorophyll molecules upon forming complex with them in stress-challenged plants. The relevance of the chlorophyll breakdown and chlorosis to the leaf senescence, fruit ripening and different stress responses has been massively reported in plants. But, whether these processes are only related to the hydrolytic activity of chloroplastic chlorophyllase enzyme is not yet clear. Chlorophyllase (chlorophyll-chlorophyllido hydrolase; EC 3.1.1.14), is the first esterase-type enzyme in the chlorophyll degradation pathway of chloroplasts (Eckardt 2009; Hörtensteiner and Krautler 2011; Chairat et al. 2013). It is a hydrophobic protein localized in the envelope membranes of chloroplasts. However, a cytosolic isoform of chlorophyllase has newly been detected in Arabidopsis, pointing a novel chlorophyll catabolism pathway in vacuoles, different from that in plastids (Schenk et al. 2007). Therefore, the relevance of all types of chlorophyll breakdown processes to the chloroplastic membranous chlorophyllase enzyme may not be necessarily true. Besides this, correspondence of Arabidopsis cytosolic chlorophyllase activity to the abiotic stress responses including pathogen-induced protective responses through the signaling of ROS is still obscure (Kariola et al. 2005; Roberts and Paul 2006).
Keeping these in view, the present results suggest a different type of chlorophyll catabolic reactions correspond to DUF538 protein superfamily and antioxidative system in stress-challenged plants. Despite chloroplastic membranous catabolic reactions, DUF538-dependent pathway is reliably predicted to occur in the cytosol of the stressed plants. We hope the overall results of this study will open a new field in the area of chlorophyll breakdown process in the photosynthetic system of plants.
Materials and methods
Materials
The leaf extract of Celosia cristata plant was considered as test material for chlorophyll assay. The seeds of this plant were taken from our laboratory stock. Bacteria (E. coli strain TB1) harboring MBP-fused DUF538 were taken from already prepared laboratory stock (Gholizadeh 2011). Materials and columns for fusion protein isolation and purification were provided in protein fusion and purification system kit (Cat. No. E8000S; NEW ENGLAND, Bio Lab). Antibody against MBP provided in protein fusion and purification system kit (Cat. No. E8000S; NEW ENGLAND, Bio Lab). TRIzol reagent used for total RNA extraction was purchased from Gibco BRL, USA (Cat. No. 15,596–013). The mRNA purification kit was from QIAGEN, USA (Cat. No.70022). Chemicals used for the cDNA synthesis was provided from Promega cDNA synthesis kit (Cat. No. C4360, USA). All the other chemicals used for this study were of molecular biology grade.
Computational analysis
Ortholog cluster analysis was performed by OC server at www.genome.ad.jp. BLAST and CLASTALW servers at http://www.ncbi.nlm.blast.com/and http://www.genome.ad.jp/were used for the amino acid sequence analysis and alignments, respectively. The online PSIPRED v 2.0 server was used for secondary structure predictions. Protein tertiary structure predictions were done using the internet-based Phyre v 2.0 server. Predictions of the functional similarities and binding sites were performed by using I-TASSER server. The presence of signal peptide was predicted by using SignalP server.
Extraction and purification of fused DUF538
For the extraction of fused DUF538 product from recombinant bacteria, the cells were grown in 500 mL of rich broth medium (supplemented with glucose and ampicillin). The fused product was inductively expressed by the addition of IPTG (0.3 mM final concentration) for an incubation period of 8 h. Then after, the cells were harvested and precipitated by centrifugation at 4000 × g for 10 min, followed by dissolving the pellet in 25 mL of extraction buffer (consisted of 20 mM of Tris-Cl, 200 mM of NaCl, 1 mM of EDTA, 1 mM of sodium azide, and 10 mM of BME), freezing in the same extraction buffer at −20 °C and then sonicating in short pulses of 15 s. The sonicated sample was finally centrifuged at 10,000 × g at 4 °C for 20 min, and the obtained supernatant was used as crude protein extract of fused DUF538 protein. The purification process was carried out by using the single-step affinity column chromatography. For this purpose, columns having the dimensions of 2.5 cm × 45 cm were packed with amylase resins (specific for the maltose-binding protein). The bound MBP-fused DUF538 protein was eluted out from the amylase column by using the specific elution buffer consisted of the crude protein extraction buffer plus 10 mM maltose. The homogeneity of the eluted product was analyzed by separating it on 10 % polyacrylamide gel using SDS-PAGE (Laemmli 1970).
Western blotting
This analysis was carried out on the induced protein extract using anti-body against maltose-binding protein provided. For this, About 10–15 μg of total protein extract was separated on 10 % SDS-PAGE and transferred onto nitrocellulose membrane using blotting buffer containing 0.025 M Tris-Cl pH 8.3, 0.192 M Glycine and 20 % ethanol. The blot was then kept in TBS–BSA buffer (0.02 M Tris-Cl, pH 7.5, 0.5 M NaCl, 1 % BSA) overnight at 4 °C and then incubated for 2 h with specific antiserum (anti-MAL) at 1: 500 ratios in the same buffer at 37 °C for 1 h. After washing with TBS-T (TBS + 0.05 % Tween 20) the membrane was incubated for 1 h with alkaline phosphate conjugated goat-rabbit antibody (GARXAP) diluted 1:20,000 in TBS-BSA buffer. After extensively washes, the signal band was visualized using a substrate solution containing 0.33 mg/ml NBT and 0.165 mg/ml BCIP in 0.1 M Tris-Cl buffer, pH 9.5 containing 0.1 M NaCl and 5 mM MgCl2.
Chlorophyll extraction and estimation
The chlorophyll content of the test leaf extract was detected according to method of Moran (1982) with some modifications. The harvested fresh leaf materials (about 200 mg) were chapped and treated with 4 ml of ethanol and DMF (at the rate 1/100) at 4 °C for 48 h. DMF was then removed from the mixture by using a rotary evaporator. The dried sample was dissolved in 100 % methanol and its Chlorophyll (a, b, pchl and total) contents were quantified according to the following equations:
Where: Ca, Cb, Cp and CT represent the concentrations of a, b, proto and total chlorophylls.
Data on the graph shows the changes of total chlorophyll contents.
Reconstitution of fused DUF538 and chlorophyll degradation assay
The purified fused product (2–12 μg/ml) of DUF538 was dissolved in aqueous buffer (consisted of 20 mM of Tris-Cl, 200 mM of NaCl, 1 mM of EDTA) and then mixed with chlorophyll extracts in final concentration of 20 % methanol. The mixture was incubated under laboratory temperature and light conditions for 12 h. The total chlorophyll content of the test and controls were examined in time-dependent manner at 2 h time intervals. MBP-treated mixture was also considered as a different control to evaluate the possible effect of MBP on chlorophyll. Changes in the chlorophyll contents of test sample were estimated and presented in time-course and concentration dependent experiments.
Total antioxidant assay
The total antioxidant ability of each test sample was determined using FRAP assay (Benzie and Strain 1999). For this assessment, to 1 ml of chlorophyll extract, 0.1 M phosphate buffer (pH 7.0), 3 ml of FRAP reagent (consisting of 10 mM TPTZ, 20 mM FeCl3. 6H2O and 300 mM sodium acetate buffer (pH 3.6) in the ratio of 1: 1: 10) was added and the reaction mixture was incubated at 37 °C for 4 min. The assessment was carried out spectrophotometerically at A593. Antioxidant potential of the samples was determined against the standard curve of ferrous sulphate (Fe, 100–1000 μM). Ascorbic acid (100 μM) served as a positive control and BSA considered as negative control. FRAP values were calculated as follows: FRAP value (μmol 100 mg −1) = A593 of test sample/A593 of standard) × FRAP value (μmol FeII 100 mg−1) of standard. Changes in the FRAP values of the samples were presented as the percentage data on the graph.
Real-time quantitative RT-PCR assay
Total cellular RNA from drought stress-challenged leaf tissues of Celosia cristata plants were separately isolated using Trizol reagent protocol. Poly (A+) RNA was purified from the total cellular RNA using oligo-dT columns according to the kit manufacturer. Single and double-stranded cDNAs were then synthesized according to the protocol of Promega cDNA synthesis kit. The integrities of the total RNA, purified mRNA and synthesized double strand cDNAs were analyzed by electrophoresis using 1 % non-denaturing agarose gel. The quantities of the cDNA in the starting test materials were measured spectrophotometrically for the next experiments. The relative expression levels of DUF538 in the test leaf materials having 3 different scores of chlorosis were analyzed by real-time RT-PCR using iQ SYBR Green supermix (Bio Rad, USA) on Bio Rad Miniopticon Real Time PCR Detection System. All the expression data were normalized by adjusting the expression level of actin gene in test samples and the relative expressions were calculated using the 2-ΔΔCt value (Livak and Schmittgen 2001). The specific primer pair and the PCR conditions have been presented in our previous work (Gholizadeh 2011).
Statistics
Each experiment was carried out with three replications using the same starting materials and experimental conditions. Data points on the graphs represent the mean values ± SD of replicates.
Results and discussion
Distribution of DUF538 in nature
To date, numbers of DUF538 superfamily have been reported in different plant species. Most of these sequences have been reported as unknown putative or hypothetical proteins, while the others characterized as induced proteins in stress-challenged plants such as tomato crown gall-induced protein (GenBank, BG131101), tomato mixed elicitor, BTI-induced protein (GenBank, AW040635), tomato nutrient deficient roots-induced protein (GenBank, BFT415717) and Populous mild-drought stress-induced protein (GenBank, CU232042). Our ortholog cluster analysis results showed that DUF538 superfamily is distributed widely in dicot and monocot plants (appended as appendix). Basal magnoliophyta, ferns and mosses were also found to have got this protein. But, animals, green algae, red algae, fungi, protests and prokaryotes were not found to contain DUF538 proteins.
The distribution pattern of DUF538 in eukaryotic photosynthesizing organisms implies that the functional properties of this protein superfamily might be conserved and it might play an important role in photosynthetic apparatus. Despite this, the nomination of DUF538 protein family as the potential structural and functional homologue of BPI protein in plants, not only provide a basis to study the potent biological functions of this protein family in the innate immune system of mammalians but also it open a new gate to investigate the homologues of DUF538 members in these organisms, too (Gholizadeh and Baghbankohnehrouz 2013).
Structure-function prediction of DUF538
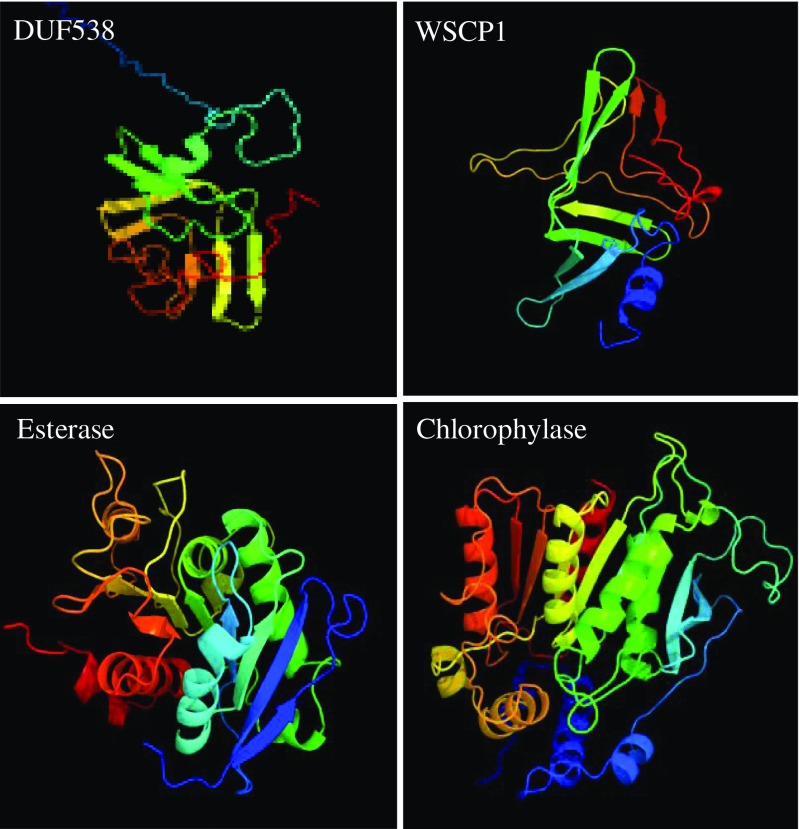
DUF538 proteins are recently known to have the structural and functional signatures of bactericidal permeability increasing proteins in the immune system of mammalians (Gholizadeh and Baghbankohnehrouz 2013) and the water soluble chlorophyll binding proteins 1 in the photosynthetic apparatus of plants (Takahashi et al. 2013). Later on, finding of structural similarity between BPI and esterase type hydrolase enzymes (Gholizadeh 2014) helped us to make this idea that DUF538 protein superfamily may be a member of hydrolases and degrades chlorophyll molecules in stress-challenged plants (considering DUF538 domain containing protein superfamily as stress-related proteins in plants). As an early step towards understanding the possible functions of DUF538 proteins, we tried to identify the potential domains and folds present in their structures using computational tools. The likely tertiary structures of a DUF538 (Acc. no: AJ535713), a WSCP1 (Acc no: K4PX49), a BPI homologue of lipid esterase (Acc. no: P38139), a chlorophyllase (Acc. no: Q9LE89) were predicted and compared by using the online Phyre v 2.0 computational server (Fig. 1). Interestingly, the obtained results showed that they can be the putative matches to each other. It was predicted that the regions dominated by the ß-strands are the homologues parts between DUF538, WSCP1, esterase and chlorophyllase. As it is clear on the figure, the ß-stranded regions considerably exhibit the similar patterns and orientations. It seemed that these proteins contain the hydrolase fold characteristic of enzymes that catalyze ester hydrolysis. Despite the structural similarity, chrorophyllase and WSCP (a signal peptide predicted by SignalP) are chloroplastic proteins, while lipid esterase and DUF538 (no signal peptide predicted by SignalP) are cytoplamic proteins.
Fig. 1.
Computational 3D structure prediction. The three dimensional structures of a DUF538, a WSCP1, a lipid esterase and a chlorophyllase were predicted by using Phyre 2 server and compared
In a different computational analysis, the functional homologues of DUF538 were performed by using the online I-TASSER server. Based on the results obtained, the first and the best functional match for the DUF538 superfamily was predicted to be carboxyesterase (EC 3.1.1.1) family. Predicted binding site for DUF538 by using the same server showed that this protein superfamily like carboxyesterases might act on aromatic compounds. Carboxyesterases or carboxylic-ester hydrolases catalyze carboxylic esters to alcohols and carboxylates. They are widely distributed in eukaryotes, and many of them participate in aromatic toxin or drug catabolism in mammalians. Considering chlorophyll as an aromatic carboxylic ester, the predicted function for DUF538 can be acceptable.
Esterase activity of DUF538 towards chlorophylls
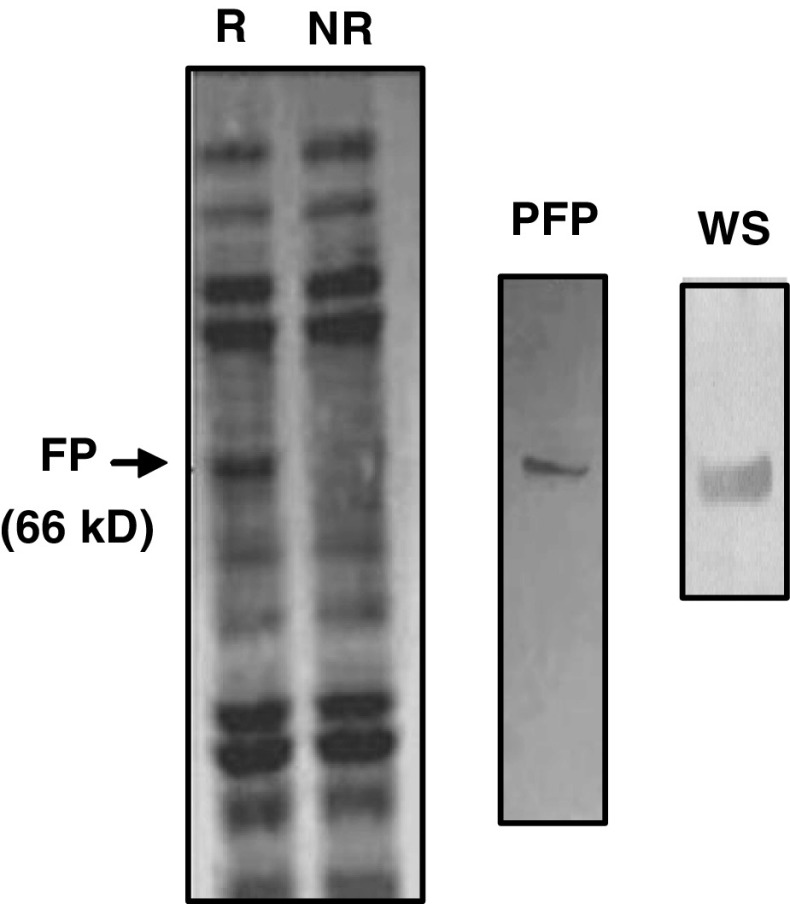
Following the computational-based predictions concerning the structural and functional similarity between DUF538 and esterase-type hydrolase enzymes, we extended our research work by experimental tools to gain a better understanding of the predicted structures. Usually, for experimental studies, the efficient production and purification of correctly folded protein of interest in a heterologous system is the important step. In this study, the already prepared recombinant bacteria harboring MBP-fused DUF538 protein (Gholizadeh 2011) were used as the starting experimental material and processed for the next steps. The selection of MBP tag was due to its high level protein expression and folding enhancement property as compared to other fusion tags. As the first step, transformed recombinant TB1 cells were allowed to grow under the induced condition using 0.3 mM final concentration of IPTG. Total crude proteins were extracted and the purification process was carried out by maltose affinity chromatography. The purified product was subjected to SDS-PAGE analysis and validated by Western blotting using the antibody against MBP (Fig. 2).
Fig. 2.
SDS-PAGE analysis of induced fused DUF538. The crude extract and the purified protein along with western analysis result were presented. FP, fusion protein; R, crude protein from bacteria carrying DUF538 containing recombinant vector; NR, crude protein from bacteria do not carrying recombinant vector; PFP, purified fusion protein; WS, Western signal
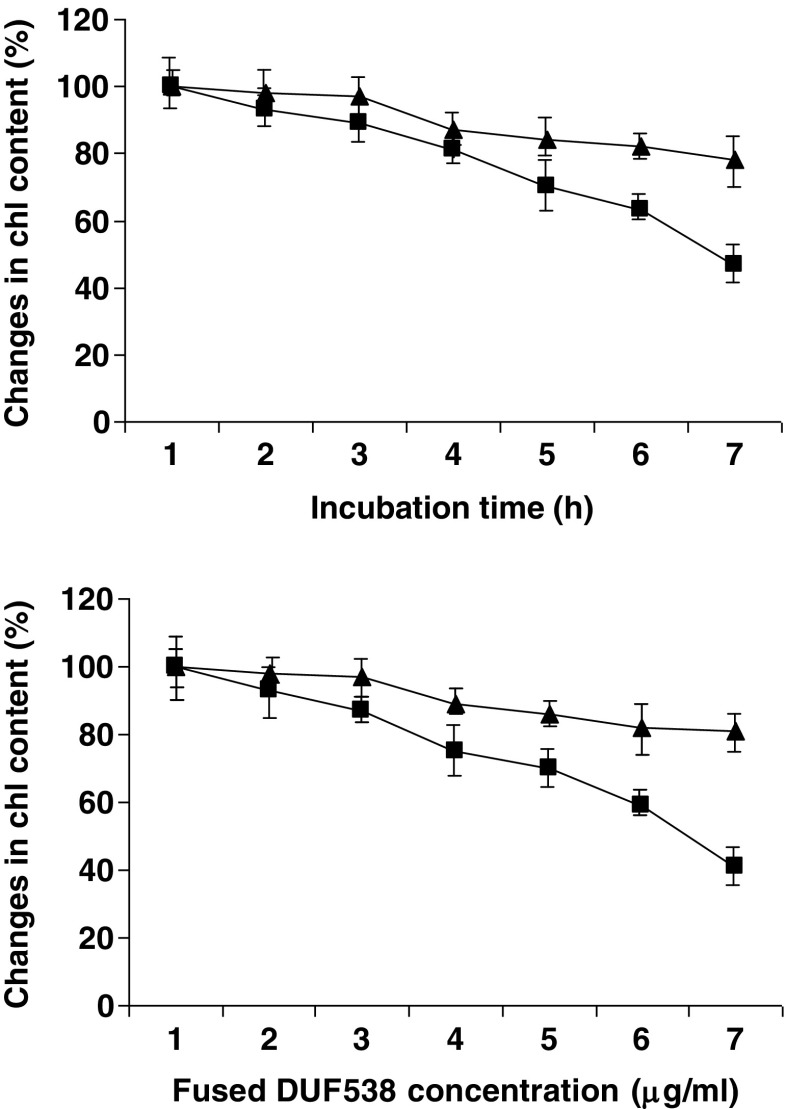
As the second step, the purified fusion form of DUF538 was subjected to chlorophyll degradation test. This was assessed by the addition of a defined amount of homogenously purified fused DUF538 to the solution of chlorophyll extract in 20 % final concentration of methanol. The total chlorophyll content of the test sample containing MBP-fused DUF538 was measured against non-treated control at 2 h time intervals. MBP-treated mixture was also considered as another control sample to evaluate the possible effects of MBP on chlorophyll contents. Time course study of chlorophyll breakdown showed a steady decrease in chlorophyll contents in test and control samples with time (Fig. 3). However, the high values of changes in chlorophyll contents were detected in fusion protein containing test sample. Data showed that the chlorophyll contents of the test and control samples are decreased to 44 % and 83 % after 12 h, respectively. This means that DUF538 doubles the chlorophyll breakdown process in test sample in compare to controls including MBP-treated and non-treated solutions. MBP-containing control sample was showed the same pattern of chlorophyll change as for non-treated control. This revealed that MBP is not involved in the chlorophyll breakdown process (Fig. 3). Parallel to the time course study, the concentration dependent experiment was also performed using different concentrations of purified fused DUF538. Data showed that the chlorophyll content of the test sample is decreased as the concentration of fused protein is increased in the test solution (Fig. 3). This result, together with the time course experiment and the computational data, not only confirms the chlorophyll binding capacity of DUF538 as predicted by Takahashi group (2013), but also it reflects the hydrolase activity of DUF538 towards chlorophyll molecules. Now, the question is this: how do DUF538 bind to the chloroplasts while it is a cytosolic protein.
Fig. 3.
Time course and concentration dependent experiments for chlorophyll degradation. Closed triangle, MBP containing solutions; Closed squires, MBP-fused DUF538 containing samples
The relevance of the chlorophyll breakdown and chlorosis occurrence to the leaf senescence, fruit ripening and stress responses has been massively reported in plants. But, whether this process is only via the hydrolytic activity of chloroplastic chlorophyllase enzyme is not yet clear. Chlorophyllase is generally known as the first enzyme in the chlorophyll degradation pathway (Eckardt 2009; Chairat et al. 2013). But, its relevance to all chlorophyll breakdown processes is unclear. Fang group has reported that the loss of chlorophylls in senescing leaves is not directly related to the activity of Chlorophyllase enzyme (Fang et al. 1998). It has been also shown that plant leaf chlorosis elicited by Diuraphis noxia feeding is different from the chlorophyll degradation via chlorophyllase enzyme (Ni et al. 2001). Similarly, it was concluded that Arabidopsis thaliana chlorophyllases 1 and 2 are not required for senescence-related chlorophyll breakdown in vivo and proposed that genuine chlorophyllase has not yet been molecularly identified (Schenk et al. 2007). Despite these, the oppose reports have indicated the activity of Chlorophyllase correspond with lower chlorophyll levels in senescing leaves, fruit ripening with ethylene (Kura-Hotta et al. 1987; Trebitsh et al. 1993) and unfavourable environmental conditions like drought stress (Majumdar et al. 1991). This controversy shows that the chlorophyll catabolism is still the obscure process in plants and needs to be more identified.
Based on our present bioinformatics and experimental data along with the considering of DUF538 as a common stress-related protein in photosynthesizing organisms, a different type of metabolic reaction or pathway is proposed for the chlorophyll degradation in the cytosol of stress-challenged plants. Firstly, because of the difference in the ortholog cluster data of chlorophyllase and DUF538 and the presence of chlorophyllase (not DUF538) in algae, the proposing a different specific way of chlorophyll catabolism in plants might be acceptable. Secondly, the present computational and experimental data suggest that DUF538 proteins containing esterase-type hydrolase characteristic of enzymes might be function as a hydrolytic enzyme in chlorophyll metabolism, in vivo. Chlorophyllase is a type of ester hydrolase that catabolize the hydrolysis of chlorophyll molecule to phytol and chlorophyllide. But, chlorophyll molecules are bicarboxylic esters consisting of two different ester bounds, the one is related to phytyl group and the other is concerned to methyl group. Therefore, the carboxyesterase activity of cytosolic DUF538 towards methyl ester group of chlorophyll molecules is going to be studied in the future investigations.
Antioxidation is parallel to chlorophyll breakdown
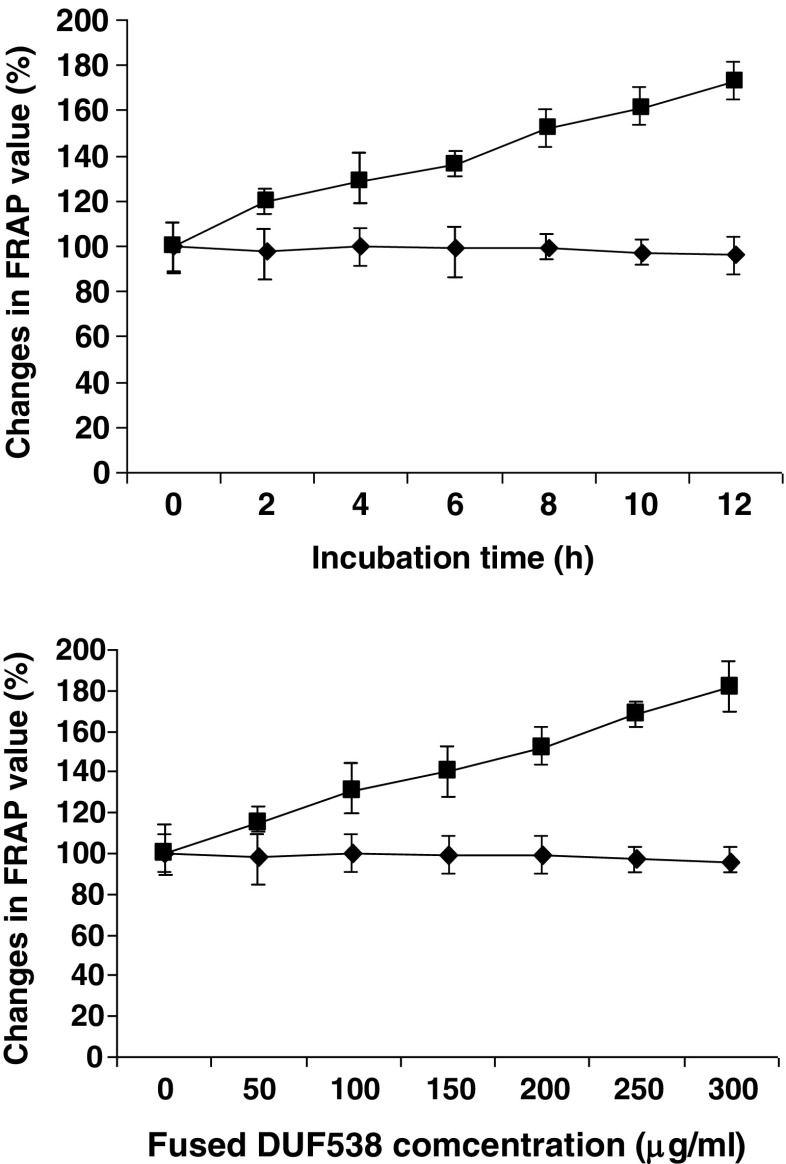
Arabidopsis chlorophyllase enzyme has been reported to play an important role in pathogen-induced chlorophyll breakdown and in prevention of chlorophyll-derived ROS accumulation in damaged tissues (Kariola et al. 2005; Roberts and Paul 2006). However, the exact function of chlorophyllase in this process, and the possible involvement of respective chlorophyll catabolites are still remained unknown. Usually, for protection against ROS, plants develop adaptive antioxidative strategy to reduce oxidative damage resulting from various stress stimuli. In general, they exhibit different types of antioxidative profiling in response to different types of environmental stresses (Larson 1988; Saleh and Plieth 2009). But, no specific profiling of antioxidation system has still been reported with regard to the chlorophyll breakdown in plants. Therefore, parallel to chlorophyll degradation assay, we extended our experiments to assess the total antioxidant abilities of test samples. FRAP method which is known as a simple and reproducible method was used for the assessments. The obtained results showed that the FRAP values of the test sample containing fused DUF538 are considerablely increased with time (Fig. 4). While, The FRAP values are not varied in non-treated or MBP-treated controls. In compare to controls, the test sample shows about 1.8 fold increase in FRAP value after 12 h. Concentration-dependent experiment results also showed the same pattern of antioxidative ability that increases as the concentration of the purified DUF538 product is increased (Fig. 4). This indicates that the chlorophyll loses via DUF538 protein leads to the generation and accumulation of the antioxidant compounds in the test solution. This result might be in agreement with our previous report concerning the induction of antioxidant capacities of test plants applied exogenously with fused DUF538 (Gholizadeh 2011). Since, DUF538 itself has not been detected as an antioxidant compound, therefore the direct role of this protein in antioxidation process is eliminated. Now, this question is raised: whether DUF538 is considered as a component of the antioxidation system in plants or the component of photosynthetic apparatus. This needs to be identified by more detailed researches. Due to the cytosolic localization of DUF538 one may assume that it is a component of antioxidative system in plants.
Fig. 4.
Time course and concentration dependent experiments for antioxidant ability of test solutions. Closed rectangulars, MBP containing solutions; Closed squares, MBP-fused DUF538 containing samples
DUF538 gene expression is relevant to chlorophyll lose
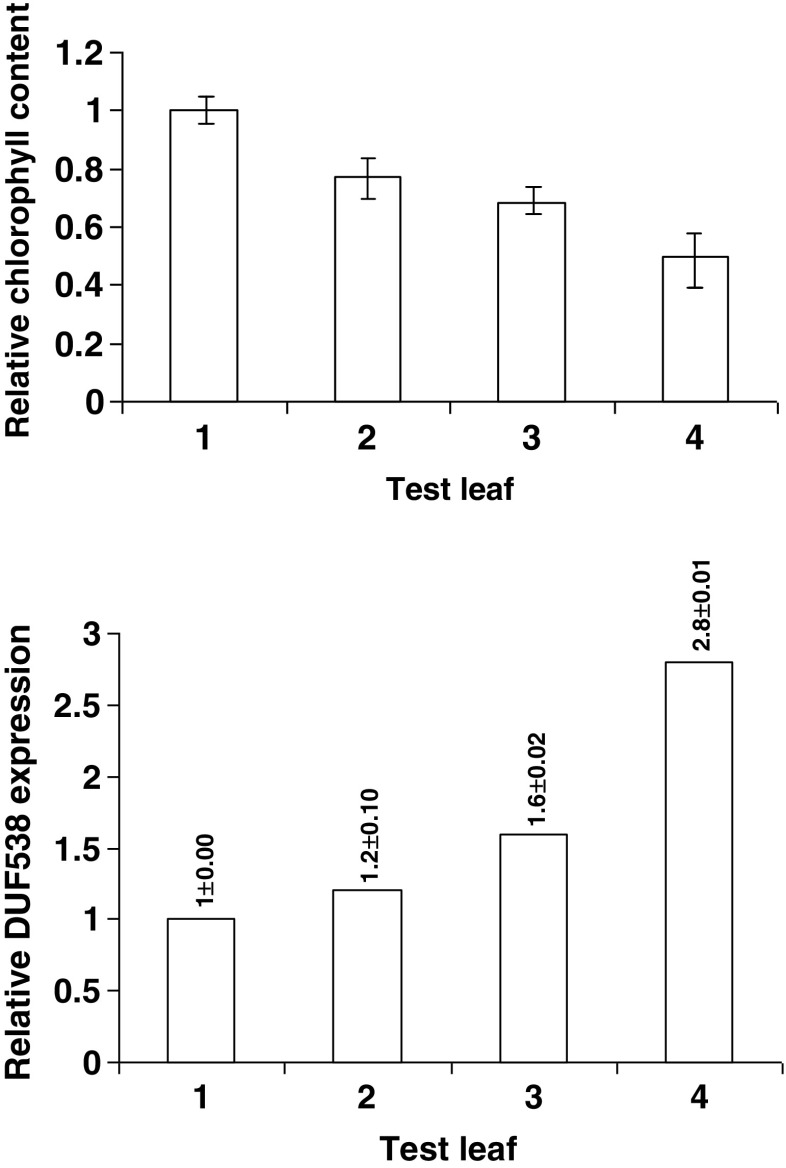
The expression of Celosia leaf DUF5538 gene has been previously examined under drought stress conditions by using basic RT-PCR method through a time-course experiment (Gholizadeh and BaghbanKohnehrouz 2010). In this work, parallel to the chlorophyll assays, the presence and the level of DUF538 gene expression was analyzed in 3 different drought-stressed leaves of Celosia plant using real time gene expression method. All data were normalized by actin expression and related to the MBP-containing control sample using 2-ΔΔCt values. The results showed that parallel to a decrease in chlorophyll content in each test sample, an increase is detectable in the expression level of DUF538 (Fig. 5). The expression levels of DUF538 gene showed 1.2, 1.6 and 2.8 folds increase when the chlorophyll contents of the drought-stressed leaf samples decreased to the levels of 77 %, 68 % and 50 %, respectively. This experiment result suggests that DUF538 protein superfamily may play an important role in chlorophyll breakdown process, in vivo. The relevance of the DUF538 gene expression level to the stress severity and chlorophyll content in test leaf materials propose that DUF538 members may be induced to act as defense mechanism through the degradation of chlorophyll molecules under stress conditions. The present results may be in accord with the previous reports suggesting the possible presence of different chlorophyllases in the cytosol of plant cells (Kariola et al. 2005; Roberts and Paul 2006; Schenk et al. 2007). But how these potent cytosolic chlorophyllases access the chlorophyll molecules remains obscure. We propose that the small chloroplastic chlorophyll catabolites may enter to the cytosol for further hydrolysis by other chlorophyllases. This proposal is recommended to be investigated in the future.
Fig. 5.
Parallel experiments concerning to in vivo chlorophyll breakdown and DUF538 gene expression assays under drought stress conditions. 1, green leaf; 2, leaf material with low score of chlorosis; 3, leaf material showing mild score of chlorosis; 4, leaf material having high score of chlorosis
Conclusion
The present study for the first ever time reports the involvement of DUF538 protein superfamily in the chlorophyll breakdown process, in vitro. Although, their structural similarity to WSCP1 and their chlorophyll binding capacities were predicted recently, but our bioinformatics and experimental data not only confirmed the reconstitution of DUF538 proteins with chlorophyll molecules, but also proposed its esterase-type hydrolytic activity toward chlorophyll molecules. The relevance of the chlorophyll breakdown to the antioxidant ability of the DUF538 protein containing sample and to the DUF538 gene expression under drought stress conditions may suggest that stress-induced DUF538 degrades chlorophyll molecules to the antioxidant compounds to defend cytosol of plants against stress conditions, in vivo, which need to be validated further. The present work will open new avenues for functional studies on DUF538 members with regard to the photosynthetic system of plants.
Acknowledgments
The author of this paper is thankful to the Research Institute for Fundamental Sciences (RIFS), University of Tabriz for the financial support.
Abbreviations
- DUF538
Domain of unknown function
- NMR
nuclear magnetic resonance
- BPI
Bactericidal permeability increasing
- WSCP1
Water soluble chlorophyll binding protein 1
- LPS
Lipopolysaccharide
- ROS
Reactive oxygen species
- MBP
Maltose-binding protein
- IPTG
Isopropyl β-D-thiogalactopyranoside
- EDTA
Ethylenediaminetetracetic
- BME
ß-mercaptoethanol
- BSA
Bovine serum albumin
- NBT
Nitro blue tetrazolium
- BCIP
Bromo chloro indolyl phosphate
- DMF
Dimethyl formamide
- FRAP
Ferric reducing antioxidant power
- TPTZ
Tripyridyl triazine
- RT-PCR
Reverse transcriptase-polymerase chain reaction
Appendix
Figure 6
Fig. 6.
Analysis of DUF538 distribution in nature. Ortholog cluster was analyzed by OC server at www.genome.ad.jp. Organisms sequence include Eudicots, Monocots, Basal Magnoliophyta, Ferns and Mosses
References
- Benzie FF, Strain JJ. The ferric reducing ability of plasma as leisure of antioxidant power, the FRAP assay. Anal Biochem. 1999;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Chairat B, Nutthachai P, Varit S. Effect of UV-C treatment on chlorophyll degradation, antioxidant enzyme activities and senescence in Chinese kale (Brassica oleracea var. alboglabra) Int Food Res J. 2013;20:623–628. [Google Scholar]
- Eckardt NA. A new chlorophyll degradation pathway. Plant Cell. 2009;21:700. doi: 10.1105/tpc.109.210313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Bouwkamp JC, Solomos T. Chlorophyllase activities and chlorophyll degradation during leaf senescence in non-yellowing mutant and wild type of Phaseolus vulgaris L. J Exp Bot. 1998;49:503–510. [Google Scholar]
- Gholizadeh A. Heterologous expression of stress-responsive DUF538 domain containing protein and its morpho-biochemical consequences. Protein J. 2011;30:351–358. doi: 10.1007/s10930-011-9338-9. [DOI] [PubMed] [Google Scholar]
- Gholizadeh A. Prediction of tertiary structure homology between bactericidal/permeability increasing protein of innate immune system and hydrolase enzymes. Int J Biosci. 2014;5:1–6. [Google Scholar]
- Gholizadeh A, Baghbankohnehrouz B (2010) Identification of DUF538 cDNA clone from Celosia cristata expressed sequences of none stressed and stressed leaves. Russ J Plant Physiol 57:247–252
- Gholizadeh A, Baghbankohnehrouz S. DUF538 protein super family is predicted to be the potential homologue of bactericidal/permeability-increasing protein in plant system. Protein J. 2013;32:163–171. doi: 10.1007/s10930-013-9473-6. [DOI] [PubMed] [Google Scholar]
- Hörtensteiner S, Kräutler B. Chlorophyll breakdown in higher plants. Biochim Biophys Acta. 2011;1807:977–988. doi: 10.1016/j.bbabio.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Kariola T, Brader G, Li J, Palva ET. Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell. 2005;17:282–294. doi: 10.1105/tpc.104.025817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kura-Hotta M, Satoh K, Katoh S. Relationship between photosynthesis and Chl content during leaf senescence of rice seedlings. Plant Cell Physiol. 1987;28:1321–1329. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson RA. The antioxidants of higher plants. Phytochem. 1988;27:969–978. doi: 10.1016/0031-9422(88)80254-1. [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Ghosh S, Glick BR, Dumbroff EB (1991) Activities of chlorophyllase, phosphoenolpyruvate carboxylase and ribulose-1,5-bisphosphate carboxylase in the primary leaves of soybean during senescence and drought. Physiol Plant 81:473–480
- Moran R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982;69:1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami H, Sugiyama N, Mochida K, Daudi A, Yoshida Y, Toyoda T, Tomita M, Ishihama Y, Shirasu K. Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. Plant Physiol. 2010;153:1161–1174. doi: 10.1104/pp.110.157347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X, Quisenberry SS, Markwell JP, Heng-Moss P, HigleyLG (2001) In vitro enzymatic chlorophyll catabolism in wheat elicited by cereal aphid feeding. Entomo Exp et Appl 101:159–166
- Noguchi T, Kamimura Y, Inoue Y, Itoh S (1999) Photoconversion of a water-soluble chlorophyll protein from Chenopodium album: resonance Raman and Fourier transform infrared study of protein and pigment structures. Plant Cell Physiol 40:305–310
- Roberts MR, Paul ND. Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defense against pests and pathogens. New Phytol. 2006;170:677–699. doi: 10.1111/j.1469-8137.2006.01707.x. [DOI] [PubMed] [Google Scholar]
- Saleh L, Plieth C. Fingerprinting antioxidative activities in plants. Plant Methods. 2009;5:2. doi: 10.1186/1746-4811-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Uchida A, Nakayama K, Okada M. Water-soluble chlorophyll protein in brassicaceae plants is a stress-induced chlorophyll-binding protein. Plant Cell Physiol. 2001;42:906–991. doi: 10.1093/pcp/pce117. [DOI] [PubMed] [Google Scholar]
- Schenk N, Schelbert S, Kanwischer M, Goldschmidt EE, Dörmann P, Hörtensteiner S (2007) The chlorophyllases AtCLH1 and AtCLH2 are not essential for senescence-related chlorophyll breakdown in Arabidopsis thaliana. FEBS Lett 27:5517–5525 [DOI] [PubMed]
- Takahashi S, Yoshikawa M, Kamada A, Ohtsuki T, Uchida A, Nakayama K, Satoh H (2013) The photoconvertible water-soluble chlorophyll-binding protein of Chenopodium album is a member of DUF538, a superfamily that distributes in embryophyta. J Plant Physiol 170:1549–1552 [DOI] [PubMed]
- Trebitsh T, Goldschmidt EE, Riov J. Ethylene induces de novo synthesis of chlorophyllase, a chlorophyll degrading enzyme, in citrus fruit peel. Proc Natl Acad Sci U S A. 1993;15:9441–9445. doi: 10.1073/pnas.90.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]