Abstract
AIM: To investigated changes in intestinal Akkermansia muciniphila (A. muciniphila) and explored the mechanism underlying the therapeutic effects of Roux-en-Y gastric bypass (RYGB) surgery on type 2 diabetes in diabetic Goto-Kakizaki (GK) rats.
METHODS: Male diabetic GK rats (n = 12) aged 8 wk were randomly assigned to the surgery group (GK-RYGB) or sham surgery group (GK-Sham) (n = 6 per group), and another 6 male Wistar rats aged 8 wk served as controls (WS-Sham). In the surgery group, RYGB surgery was conducted, and a sham operation was performed in both sham groups. Fasting blood glucose (FBG) levels before and after surgery, fasting levels of serum insulin and serum glucagon-like peptide-1 (GLP-1) and levels 30 min after intragastric injection of glucose, and the amount of A. muciniphila in the stool were determined. Insulin and GLP-1 were measured by enzyme-linked immunosorbent assay, and A. muciniphila were detected by fluorescence-based quantitative polymerase chain reaction.
RESULTS: The FBG was improved, and serum GLP-1 and insulin increased significantly (P < 0.05) in the GK-RYGB group after surgery compared to levels before surgery and to levels in the GK-Sham group. Before surgery, the amounts of A. muciniphila in the GK-RYGB and GK-Sham groups were significantly lower than in the WS-Sham group (P < 0.05). After surgery, the amount of A. muciniphila in the GK-RYGB group increased markedly compared to that before surgery and to that in the GK-Sham and WS-Sham groups (P < 0.05). In addition, the A. muciniphila amount was positively related to GLP-1 (r = 0.86, P < 0.05).
CONCLUSION: Our results demonstrated RYGB surgery may increase GLP-1 secretion, elevate serum insulin after intragastric injection of glucose, and improve insulin resistance in diabetic GK rats, thereby contributing to a significant reduction in blood glucose. The increased amount of A. muciniphila after RYGB surgery may be related to elevated GLP-1 secretion.
Keywords: Roux-en-Y gastric bypass surgery, Type 2 diabetes, Glucagon-like peptide-1, Glucose-dependent insulinotropic peptide, Akkermansia mucinipilia
Core tip: Roux-en-Y gastric bypass (RYGB) surgery can improve blood glucose with definite efficacy in obese patients with type 2 diabetes mellitus and that this effect is also long lasting. But the mechanism of RYGB is not clear. Our study demonstrated RYGB surgery may increase glucagon-like peptide-1 (GLP-1) secretion, elevate serum insulin after intragastric injection of glucose, and improve insulin resistance in diabetic Goto-Kakizaki rats, thereby contributing to a significant reduction in blood glucose. The increased amount of Akkermansia muciniphila after RYGB surgery may be related to elevated GLP-1 secretion.
INTRODUCTION
According to the International Diabetes Federation[1], diabetes mellitus (DM) affected about 370 million people in 2011, and an estimated 550 million people will develop DM. Of DM patients, 90% are diagnosed with type 2 DM (T2DM)[2]. The specific etiology of T2DM is still unclear, but it is widely accepted that T2DM develops as a result of genetic and environmental factors[3]. Intestinal microorganisms have been regarded as environmental factors[4] and are closely related to the occurrence and development of metabolic diseases, including DM[5-8].
In recent years, increasing numbers of studies have reported that Roux-en-Y gastric bypass (RYGB) surgery may alter the intestinal flora. Animal experiments[9,10] and clinical trials[11,12] have revealed that the intestinal flora change significantly after RYGB surgery. However, a majority of studies focused on the bacterial genus, and a specific type of bacterium has never been investigated in depth. Studies in the field of internal medicine typically emphasize Akkermansia muciniphila (A. muciniphila), and findings demonstrate that A. muciniphila is closely related to the occurrence and development of obesity and DM[13-18]. In the present study, RYGB surgery was performed in diabetic Goto-Kakizaki (GK) rats, and blood glucose, glucagon-like peptide-1 (GLP-1), and the amount of A. muciniphila in the stool were measured before and after surgery to evaluate the association of postoperative blood glucose and GLP-1 with the amount of A. muciniphila and to explore the potential mechanisms underlying the therapeutic effects of RYGB surgery on T2DM.
MATERIALS AND METHODS
Animals
GK rats aged 8 wk (specific pathogen free; n = 12) and Wistar rats (n = 6) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. China. GK rats were randomly assigned to 1 of 2 groups (n = 6 per group): The GK-RYGB group and GK-sham group. Wistar rats served as controls (WS-Sham group). This study was approved by the Ethics Committee of the Beijing Tiantan Hospital Affiliated to Capital Medical University, and all the procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Surgical procedures and postoperative treatments
Before surgery, rats were fasted for 12 h and then were intraperitoneally anesthetized with 10% chloral hydrate at 0.35 mL/100 g, followed by RYGB surgery. A 4-cm incision was made in the upper abdomen, and laparotomy was performed: First, the stomach was divided just below the gastroesophageal junction from the greater to the lesser curve, taking care to preserve the vagus nerve in this region. A gastric pouch of approximately 20% of the total stomach volume was preserved. Second, the jejunum was divided 8 cm below the ligament of Treitz, and the distal cut end was anastomosed on the anterior surface of the gastric pouch with 5-0 suture. The anastomosis was 4-6 cm in length. Third, a 1-cm enterotomy was made on the antimesenteric aspect of the jejunum 10 cm distal to the gastrojejunostomy and was anastomosed to the proximal cut end of the jejunum as an end-to-side anastomosis that was 4-6 cm in length.
Sham surgery was performed in the following manner: A midline 4-cm incision was made in the upper abdomen, and laparotomy was performed. The gastrointestinal tract was explored, straightened out, and placed back into the abdominal cavity. Food was withheld for 24 h after surgery, but animals were given ad libitum access to water. Beginning 2 d after surgery, fluid (10% glucose) was administered for 2 d, and normal diet was resumed at 4 d after surgery. None of the animals in the 3 groups died during the experiment.
Fasting blood glucose
Fasting blood glucose (FBG) was measured at 1 wk before surgery and at 1, 2, 3, and 4 wk after surgery. Rats were fasted overnight, blood was collected from the tail vein, and glucose was measured with a glucose meter (Beijing Yicheng Company) at 8:00 AM.
Serum insulin and GLP-1
Serum insulin and GLP-1 were measured 1 wk before surgery and 4 wk after surgery. Rats were fasted overnight, and blood was collected from the tail vein at fasting status and at 30 min after intragastric administration of 1 g/kg glucose. Blood was transferred into an EDTA-pretreated tube and centrifuged at 3000 rpm for 12 min at 4 °C. The serum was collected and stored at -80 °C for further analysis. Rat radioimmunoassay kits (IBL, Germany) were used for the detection of serum insulin and GLP-1 according to the manufacturer’s instructions.
Homeostasis model assessment-insulin resistance
Homeostasis model assessment-insulin resistance (HOMA-IR) was measured before surgery and 4 wk after surgery as follows: HOMA-IR = fasting plasma glucose (mmol/L) × fasting insulin (pmol/L)/22.5. HOMA-IR was used to evaluate the insulin resistance.
A. muciniphila in the stool. Extraction of bacterial DNA took place as follows: Stool was collected 1 wk before and 4 wk after surgery and was stored at -80 °C. The standard MucT (ATCC BAA-835T) was thawed, cultured, and then stored at 4 °C. DNA was extracted with the QIAamp bacterial genomic DNA extraction kit (Qiagen, Germany) according to the manufacturer’s instructions and then was stored at -20 °C for use. Next, primers for polymerase chain reaction (PCR) were designed: On the basis of the V1 and V6 variable regions of 16S RNA of A. muciniphila[17], forward and reverse primers were designed with Primer Premier 5.0 (Premier Biosoft, Palo Alto, CA, United States) and were synthesized as follows:
Forward: 5′ CAGCACGTGAAGGTGGGGAC 3′
Reverse: 5′ CCTTGCGGTTGGCTTCAGAT 3′
Product length: 214 bp.
Routine PCR was conducted as follows: Genomic DNA extracted from the bacteria in rat stool, and standard bacteria served as templates for routine PCR. The purity and integrity of genomic DNA were determined by 1.0% agarose gel electrophoresis. After PCR, the amplified DNA was harvested with a DNA retrieval kit (Company, Country). The retrieved DNA of standard bacteria served as standards for fluorescence-based quantitative PCR, and a standard curve was delineated.
A 10-fold dilution series of standard DNA template and DNA template from stool bacteria were independently prepared and used for real-time quantitative PCR. Product specificity was determined according to the melt curve, and the Ct value and the standard curve were employed to calculate the amount of measured bacteria (copies per g stool).
Statistical analysis
Statistical analysis was performed with SPSS version 19.0 (Chicago, IL, United States), and data were expressed as mean ± standard deviation (x ± s). Comparisons of means among groups were done with one-way analysis of variance. A value of P < 0.05 was considered statistically significant. Correlations between A. muciniphila amount and serum GLP-1 were evaluated with Pearson correlation analyses and univariate regression analyses.
RESULTS
Blood glucose and insulin
Before surgery, FBG in GK rats was significantly higher than that in Wistar rats (P < 0.05). At 1 wk after surgery, FBG was reduced in the 3 groups, but a significant difference was found only in the GK-RYGB group (P < 0.05). FBG increased gradually in the GK-Sham group and the WS-Sham group within 2 wk after surgery. At 2, 3, and 4 wk after surgery, FBG in the GK-RYGB group was lower than that in the GK-Sham group but was still higher than in the WS-Sham group. After surgery, FBG in the GK-RYGB group was significantly different from that in the GK-Sham and WS-Sham groups (P < 0.05) (Table 1).
Table 1.
Fasting blood glucose in different groups before and after surgery (x ± s, mmol/L)
| Time point | GK-RYGB | GK-Sham | WS-Sham |
| 1 wk presurgery | 6.98 ± 0.32a | 7.07 ± 0.57a | 4.57 ± 0.26 |
| 1 wk postsurgery | 6.42 ± 0.25c | 6.82 ± 0.49a | 4.25 ± 0.23 |
| 2 wk postsurgery | 6.01 ± 0.20ace | 6.88 ± 0.52a | 4.37 ± 0.22 |
| 3 wk postsurgery | 5.60 ± 0.26ace | 6.93 ± 0.52a | 4.37 ± 0.23 |
| 4 wk postsurgery | 5.72 ± 0.25ace | 7.03 ± 0.52a | 4.50 ± 0.24 |
P < 0.05 vs WS-Sham group;
P < 0.05 vs GK-Sham group;
P < 0.05 vs presurgery value. GK: Goto-Kakizaki; RYGB: Roux-en-Y gastric bypass; WS: Wistar.
Before surgery, fasting serum insulin (FSI) values in GK rats was significantly lower than those in Wistar rats (P < 0.05). At 4 wk after surgery, the FSI values in the GK-RYGB group and GK-Sham group increased compared to values before surgery (P > 0.05). At 4 wk after surgery, the FSI at 30 min after intragastric administration of glucose in the GK-RYGB group increased significantly compared to that before surgery and was significantly higher than in the GK-Sham group but lower than in the WS-Sham group after surgery (P < 0.05) (Table 2).
Table 2.
Serum insulin in different groups before and after surgery (x ± s, pmol/L)
| Group |
Presurgery |
4 wk postsurgery |
||
| Fasting | 30 min after intragastric glucose | Fasting | 30 min after intragastric glucose | |
| GK-RYGB | 15.20 ± 0.67a | 27.07 ± 1.07a | 16.05 ± 1.41a | 47.57 ± 4.24ac |
| GK-Sham | 15.26 ± 0.76a | 27.49 ± 1.29a | 15.43 ± 0.97a | 27.26 ± 1.26a |
| WS-Sham | 18.54 ± 0.99 | 104.00 ± 4.96 | 19.01 ± 0.42 | 103.68 ± 4.85 |
P < 0.05 vs WS-Sham group;
P < 0.05 vs GK-Sham group. GK: Goto-Kakizaki; RYGB: Roux-en-Y gastric bypass; WS: Wistar.
Before surgery, HOMA-IR in GK rats was significantly higher than that in Wistar rats (P < 0.05), suggesting higher insulin resistance in GK rats before surgery. At 4 wk after surgery, HOMA-IR in the GK-RYGB group was significantly lower than that before surgery and that in the GK-Sham group after surgery (P < 0.05), indicating that RYGB surgery can improve insulin resistance in GK rats (Table 3).
Table 3.
Homeostasis model assessment-insulin resistance in different groups before and after surgery
| Group | Presurgery | 4 wk postsurgery |
| GK-RYGB | 4.72 ± 0.34a | 4.07 ± 0.30ce |
| GK-Sham | 4.80 ± 0.51a | 4.83 ± 0.54 |
| WS-Sham | 3.75 ± 0.07 | 3.74 ± 0.20 |
P < 0.05 vs WS-Sham group;
P < 0.05 vs GK-Sham group;
P < 0.05 vs presurgery. GK: Goto-Kakizaki; RYGB: Roux-en-Y gastric bypass; WS: Wistar.
GLP-1
Before surgery, fasting GLP-1 in GK rats was significantly lower than that in Wistar rats (P < 0.05). At 4 wk after surgery, fasting GLP-1 in the GK-RYGB surgery group was significantly higher than in the GK-Sham group (P < 0.05) but was comparable to that in the WS-Sham group (P > 0.05) (Table 4).
Table 4.
Serum glucagon-like peptide-1 in different groups before and after surgery (x ± s, pmol/L)
| Group | Presurgery | 4 wk postsurgery |
| GK-RYGB | 21.01 ± 0.90a | 34.36 ± 1.46ce |
| GK-Sham | 21.19 ± 0.53a | 24.98 ± 2.63a |
| WS-Sham | 29.31 ± 1.51 | 32.13 ± 1.52 |
P < 0.05 vs WS-Sham group;
P < 0.05 vs GK-Sham group;
P < 0.05 vs presurgery. GK: Goto-Kakizaki; RYGB: Roux-en-Y gastric bypass; WS: Wistar.
A. muciniphila: The extracted bacterial DNA is shown in Figure 1. The marker was a 100-bp ladder DNA marker with clear bands, suggesting that the extracted DNA was legible.
Figure 1.

Electrophoresis of extracted DNA in the Goto-Kakizaki-Roux-en-Y gastric bypass surgery group.
Amount of A. muciniphila before and after surgery in different groups
At 1 wk before surgery, the amount of A. muciniphila in GK rats was significantly lower than that in Wistar rats (P < 0.05). At 4 wk after surgery, the amount of A. muciniphila was 9.34 ± 0.18 copies per g stool in the GK-RYGB surgery group, which was significantly higher than that before surgery and that in the WS-Sham group and GK-Sham group (P < 0.05). This suggests that the amount of A. muciniphila in rat stool increases in GK rats after surgery (Table 5).
Table 5.
Amount of Akkermansia muciniphila in different groups before and after surgery (log10 copies/g)
| Group | Presurgery | 4 wk postsurgery |
| GK-RYGB | 7.85 ± 0.09a | 9.34 ± 0.18ace |
| GK-Sham | 7.79 ± 0.08a | 7.82 ± 0.09a |
| WS-Sham | 8.51 ± 0.08 | 8.82 ± 0.06 |
P < 0.05 vs WS-Sham group;
P < 0.05 vs GK-Sham group;
P < 0.05 vs presurgery. GK: Goto-Kakizaki; RYGB: Roux-en-Y gastric bypass; WS: Wistar.
Correlation between intestinal A. muciniphila and serum GLP-1
The amount of A. muciniphila served as an independent variable and serum GLP-1 content as a dependent variable. A scatterplot (Figure 2) of the results showed a linear relationship between serum GLP-1 and the amount of A. muciniphila, and spots were found mainly within the 95%CI. Correlation analysis showed that r (the Pearson correlation coefficient) was 0.867, suggesting a significant positive correlation between serum GLP-1 and the amount of A. muciniphila. The amount of A. muciniphila was used as an independent variable and the level of serum GLP-1 was used as a dependent variable for univariate analysis, and the results showed that P = 0.00.
Figure 2.
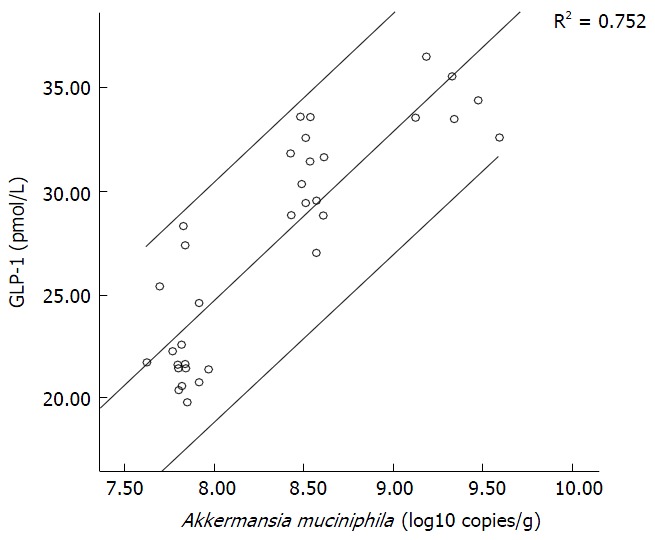
Scatterplot of the amount of Akkermansia muciniphila vs levels of glucagon-like peptide-1. GLP-1: Glucagon-like peptide-1.
DISCUSSION
The effectiveness of surgery in the treatment of T2DM has been widely accepted after 20 years of clinical practice, and surgery has been included in guidelines for the treatment of DM[19,20]. Previous studies have shown that RYGB surgery can improve blood glucose with definite efficacy in obese patients with T2DM[21-24] and that this effect is also long lasting[14]. Moreover, this surgery may be better to mitigate T2DM-related complications compared to pharmacotherapy[11,13,25].
Although surgery has favorable therapeutic efficacy for T2DM, the specific mechanism of action is still unclear and may be related to changes in gastrointestinal hormones[26,27]. One of the widely studied gastrointestinal hormones is GLP-1, which, with its receptor GLP-1R, has been a focus of studies in internal medicine. To date, GLP-1 analogues (e.g., liraglutide) and GLP-1R agonists (e.g., exenatide) have been developed and used in clinical practice with favorable efficacy[28]. Currently, published animal experiments[26] and clinical trials[29] have demonstrated that GLP-1 increases after RYGB surgery. In the present study, our results also showed that GLP-1 secretion increases in GK rats after RYGB surgery. GLP-1 acts mainly to increase glucose-induced insulin secretion, increase glucagon secretion, improve insulin sensitivity, promote regeneration of islet β cells, and reduce their apoptosis[30], leading to reduction in blood glucose. In our study, the results showed that FBG remained unchanged in GK rats after RYGB surgery, but serum insulin increased after intragastric administration of glucose, suggesting that RYGB surgery may elevate glucose-induced insulin secretion via increasing GLP-1 secretion. In addition, our results revealed that HOMA-IR in GK rats after RYGB surgery was significantly lower than that before surgery and that in the GK-Sham group, indicating that RYGB surgery can improve insulin sensitivity via GLP-1 induction in GK rats.
The mechanism whereby RYGB increases GLP-1 secretion is still unclear. Evidence suggests that early contact of the distal small intestine with food after food intake following RYGB surgery may induce the secretion of GLP-1[27], but studies to date have not elucidated the mechanisms whereby food induces GLP-1 secretion. Thus, the role of food in increased GLP-1 secretion still requires clarification. Tolhurst et al[31] speculated that intestinal microorganisms were closely related to GLP-1 and their metabolites (such as short-chain fatty acids) may act on the corresponding receptors on L cells to stimulate GLP-1 secretion. However, whether intestinal microorganisms induce GLP-1 secretion after RYGB surgery and the putative mechanisms of this action are still poorly understood.
Studies[29,32] have confirmed that intestinal microorganisms may affect body weight and blood glucose. A. muciniphila has been a focus in current studies because apparently it can reduce body weight and improve blood glucose as well as insulin resistance[15-18]. However, the role of A. muciniphila in the therapeutic effects of RYGB surgery on T2DM have never been studied. In this study, the results showed that the amount of A. muciniphila in the stool of GK rats was significantly lower than that in Wistar rats before surgery, suggesting that the amount of A. muciniphila in diabetic rats was lower than that in healthy rats. At 4 wk after RYGB surgery, the amount of A. muciniphila increased significantly in GK rats, suggesting that RYGB surgery can increase the intestinal amount of A. muciniphila in GK rats. Hansen et al[33] speculated that 2-oleoylglycerol, a metabolite produced by intestinal A. muciniphila, may stimulate the secretion of gastrointestinal hormones, especially GLP-1, in L cells in the distal small intestine. In the present study, correlation analysis and regression analysis confirmed that the amount of A. muciniphila was positively correlated with that of GLP-1. Thus, we speculate that RYGB surgery not only increases the intestinal amount of A. muciniphila but also elevates GLP-1.
Our findings indicate that GLP-1 increases after RYGB surgery, which then elevates serum insulin after intragastric administration of glucose and improves insulin resistance and effectively reduces blood glucose in GK rats. The increased intestinal amount of A. muciniphila following RYGB surgery may contribute to the elevated secretion of GLP-1 after RYGB surgery. But, the modality wehreby A. muciniphila increases as a results of RYGB still remains undefined, nor is clear how GLP-1 increases, which needs further research in the future.
COMMENTS
Background
Type 2 diabetes mellitus (T2DM) is an endocrine and metabolic disease, which has become a significant worldwide health problem. According to the International Diabetes Federation, T2DM has become the third most common type of non-infectious disease worldwide following cardiovascular disease and cancer. The conventional therapies include diet adjustment, exercise, self-monitoring of blood glucose and drug. But none of these therapies is adequately effective in maintaining long-term glycemic control and preventing complications. Therefore, an effective treatment for T2DM is urgently required. Of the current treatments for T2DM, Roux-en-Y gastric bypass (RYGB) surgery is considered to be an effective long-term treatment. However, the mechanisms that drive these outcomes remain incompletely understood.
Research frontiers
Recently, T2DM is characterized by altered gut microbiota, and Akkermansia muciniphila (A. muciniphila) is one of substantial gut microbiota. A. muciniphila which belongs to the phylum Verrucomicrobia, has been identified as a mucin-degrading bacteria that resides in the mucus layer, and it is the dominant human bacterium that abundantly colonizes this nutrient-rich environment. And its abundance inversely correlates with body weight and T2DM in mice and humans. A. mucinihpila becomes hot gut microbiota in basic research for diabetes.
Innovations and breakthroughs
In this study, the authors used Goto-Kakizaki (GK) rats, a genetic model of T2DM, to investigate whether the RYGB surgery influenced the population of A. muciniphila. The authors’ findings indicate that glucagon-like peptide-1 (GLP-1) increases after RYGB surgery, which then elevates serum insulin after intragastric administration of glucose and improves insulin resistance and effectively reduces blood glucose in GK rats. The increased intestinal amount of A. muciniphila following RYGB surgery may contribute to the elevated secretion of GLP-1 after RYGB surgery.
Applications
The authors’ findings indicate that GLP-1 increases after RYGB surgery, and effectively reduces blood glucose in GK rats. The increased intestinal amount of A. muciniphila following RYGB surgery may contribute to the elevated secretion of GLP-1 after RYGB surgery. The authors’ findings may explain the mechanism that RYGB surgery can improve blood glucose with definite efficacy in obese patients with T2DM.
Peer-review
The authors need to be congratulated for their innovative research study on relevant clinical topic. The experimental work has been scheduled and performed according to the current principles of the experimental project. The manuscript itself is concise, written in an elegant style according to all requirements of original contribution.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Institutional Review Board of Beijing Tiantan Hospital Affiliated to Capital Medical University.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Animal Ethical and Welfare Committee of Beijing Tiantan Hospital Affiliated to Capital Medical University (IACUC protocol number: EN-42532).
Conflict-of-interest statement: The authors declared that they have no conflicts of interest to this work.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 3, 2015
First decision: December 18, 2015
Article in press: January 31, 2016
P- Reviewer: Baglaj MS, Romani A S- Editor: Qi Y L- Editor: A E- Editor: Li D
References
- 1.International Diabetes Federation. Diabetes Atlas. 5th ed. Available from: https://www.idf.org/sites/default/files/21991_diabAtlas_5thEd.pdf.
- 2.Mun EC, Blackburn GL, Matthews JB. Current status of medical and surgical therapy for obesity. Gastroenterology. 2001;120:669–681. doi: 10.1053/gast.2001.22430. [DOI] [PubMed] [Google Scholar]
- 3.Charles MA, Fontbonne A, Thibult N, Warnet JM, Rosselin GE, Eschwege E. Risk factors for NIDDM in white population. Paris prospective study. Diabetes. 1991;40:796–799. doi: 10.2337/diab.40.7.796. [DOI] [PubMed] [Google Scholar]
- 4.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 5.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pozzilli P, Signore A, Williams AJ, Beales PE. NOD mouse colonies around the world--recent facts and figures. Immunol Today. 1993;14:193–196. doi: 10.1016/0167-5699(93)90160-M. [DOI] [PubMed] [Google Scholar]
- 8.Calcinaro F, Dionisi S, Marinaro M, Candeloro P, Bonato V, Marzotti S, Corneli RB, Ferretti E, Gulino A, Grasso F, et al. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia. 2005;48:1565–1575. doi: 10.1007/s00125-005-1831-2. [DOI] [PubMed] [Google Scholar]
- 9.Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW, Bloom SR, Darzi A, Athanasiou T, Marchesi JR, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60:1214–1223. doi: 10.1136/gut.2010.234708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liou AP, Paziuk M, Luevano JM, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Doré J, Henegar C, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bostantjopoulou S, Logothetis J, Katsarou Z, Mentenopoulos G. Clinical observations in early and late onset Parkinson’s disease. Funct Neurol. 1991;6:145–149. [PubMed] [Google Scholar]
- 13.Karlsson CL, Onnerfält J, Xu J, Molin G, Ahrné S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring) 2012;20:2257–2261. doi: 10.1038/oby.2012.110. [DOI] [PubMed] [Google Scholar]
- 14.Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88:894–899. doi: 10.1093/ajcn/88.4.894. [DOI] [PubMed] [Google Scholar]
- 15.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Possemiers S, Van Holle A, François P, de Vos WM, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen CH, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sørensen SJ, Buschard K, Hansen AK. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55:2285–2294. doi: 10.1007/s00125-012-2564-7. [DOI] [PubMed] [Google Scholar]
- 17.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 19.International Diabetes Federation. Bariatric Surgical and Procedural Interventions in the Treatment of Obese Patients with Type 2 Diabetes. A Position Statement from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Available from: https://www.idf.org/webdata/docs/IDF-Position-Statement-Bariatric-Surgery.pdf.
- 20.American Diabetes Association. Standards of medical care in diabetes--2009. Diabetes Care. 2009;32 Suppl 1:S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 22.Pérez G, Devaud N, Escalona A, Downey P. Resolution of early stage diabetic nephropathy in an obese diabetic patient after gastric bypass. Obes Surg. 2006;16:1388–1391. doi: 10.1381/096089206778663733. [DOI] [PubMed] [Google Scholar]
- 23.Müller-Stich BP, Fischer L, Kenngott HG, Gondan M, Senft J, Clemens G, Nickel F, Fleming T, Nawroth PP, Büchler MW. Gastric bypass leads to improvement of diabetic neuropathy independent of glucose normalization--results of a prospective cohort study (DiaSurg 1 study) Ann Surg. 2013;258:760–765; discussion 765-766. doi: 10.1097/SLA.0b013e3182a618b2. [DOI] [PubMed] [Google Scholar]
- 24.Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, MacLean LD. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240:416–423; discussion 423-424. doi: 10.1097/01.sla.0000137343.63376.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banks J, Adams ST, Laughlan K, Allgar V, Miller GV, Jayagopal V, Gale R, Sedman P, Leveson SH. Roux-en-Y gastric bypass could slow progression of retinopathy in type 2 diabetes: a pilot study. Obes Surg. 2015;25:777–781. doi: 10.1007/s11695-014-1476-7. [DOI] [PubMed] [Google Scholar]
- 26.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11. doi: 10.1097/01.sla.0000102989.54824.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–2615. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 28.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 29.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings BP, Strader AD, Stanhope KL, Graham JL, Lee J, Raybould HE, Baskin DG, Havel PJ. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology. 2010;138:2437–2446, 2446.e1. doi: 10.1053/j.gastro.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duncan SH, Louis P, Thomson JM, Flint HJ. The role of pH in determining the species composition of the human colonic microbiota. Environ Microbiol. 2009;11:2112–2122. doi: 10.1111/j.1462-2920.2009.01931.x. [DOI] [PubMed] [Google Scholar]
- 33.Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, Andersen UB, Holst JJ, Hansen HS. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96:1409–1417. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]


