Abstract
Purpose
We evaluated whether initial diagnostic parameters could predict the confirmatory biopsy result in patients initiating active surveillance for prostate cancer, to determine whether some men at low risk of reclassification could be spared unnecessary biopsy.
Materials and Methods
The cohort included 392 men with Gleason 6 prostate cancer on initial biopsy undergoing confirmatory biopsy. We used univariate and multivariable logistic regression to assess if high-grade cancer (Gleason ≥ 7) on confirmatory biopsy could be predicted from initial diagnostic parameters (prostate-specific antigen density, magnetic resonance imaging result, percent positive cores, percent cancer in positive cores, and total tumor length).
Results
Median age was 62 years (IQR 56–66) and 47% of patients were found to have a dominant or focal lesion on magnetic resonance imaging. Of the 392 patients, 44 (11%) were found to have high-grade cancer on confirmatory biopsy, among whom 39 had 3+4, 1 had 4+3, 3 had Gleason 8, and 1 patient had Gleason 9 disease. All predictors were significantly associated with high-grade cancer at confirmatory biopsy on univariate analysis. However, in the multivariable model only prostate-specific antigen density and total tumor length were significantly associated (AUC of 0.85). Using this model to select patients for confirmatory biopsy would generally provide a higher net benefit than performing confirmatory biopsy in all patients, across a wide range of threshold probabilities.
Conclusion
If externally validated, a model based on initial diagnostic criteria could be used to avoid confirmatory biopsy in many patients initiating active surveillance.
Keywords: prostatic neoplasms, biopsy, active surveillance
INTRODUCTION
Current protocols for monitoring men on AS involve repeated DRE, PSA and repeat prostate biopsy.1–3 Given that systematic biopsy may miss Gleason pattern 4 disease,4–7 confirmatory biopsy has become a mainstay of AS. There are, however, several reasons why avoiding repeat biopsy would be desirable. Prostate biopsy is an invasive procedure, sometimes poorly tolerated and commonly associated with hematospermia, hematuria and transient worsening of lower urinary tract symptoms.2 Whilst the majority of these complications are benign and self-limiting, the rates of severe sepsis requiring hospitalization have increased over recent years as a result of emerging antimicrobial resistance.8 The risk of infectious complications increases with each additional biopsy.9
Attempts have been made, using clinical and tumor characteristics, to identify those patients at risk of biopsy progression. Men with a higher PSA density,10,11 positive confirmatory biopsies,10 and a higher number of positive cores11 have been shown to carry an increased risk of progression on AS. We sought to determine whether clinical predictors of progression, including imaging in the form of MRI, could predict the results of the confirmatory biopsy with sufficient accuracy to allow some patients to avoid biopsy.
MATERIALS AND METHODS
Patient Population
A review of our institutional database identified 583 patients on AS from December 2007 to December 2013 who received both an MRI and a confirmatory biopsy. These patients came from across the United States, consistent with our role as a dedicated cancer hospital. Inclusion criteria for AS at our institution are clinical stage ≤ T2a, Gleason score ≤ 3+3=6, PSA ≤ 10 ng/mL and three or fewer positive cores with ≤ 50% positivity in a single core. In order to maintain consistency with these criteria, we excluded 3 patients who chose AS despite an initial biopsy score of 4+3, 31 patients with Gleason 3+4, and 35 patients with a baseline PSA above 10 ng/ml. We also excluded 5 patients missing baseline PSA and 93 patients missing complete initial biopsy information. We also excluded 3 patients for whom more than 14 months elapsed between the diagnostic and confirmatory biopsy. Lastly, we excluded 22 patients who received their MRI after their confirmatory biopsy and 4 patients who received their MRI more than a year before their confirmatory biopsy. This left a final cohort of 392 patients.
Magnetic Resonance Imaging Protocol and Analysis
We utilized whole-body MRI units (GE Healthcare, Waukesha, WI) at 1.5 T (62 patients) and 3T (305 patients). An endorectal coil was used in 370 cases. Data on MRI acquisition parameters were not available in 25 patients. MRI parameters varied over time as clinical protocols at our institution evolved with new developments. Twenty studies involved anatomic T2WI alone. Of the multiparametric MRI studies, 281 utilized DWI and dynamic contrast-enhanced imaging in addition to T2WI. DWI and T2WI were used in 75 cases, dynamic contrast-enhanced imaging and T2WI in 11 cases, and the combination of DWI, T2WI, and magnetic resonance spectroscopy in 6 cases. Twenty-nine MRI studies were performed outside our institution. MRIs reporting a dominant prostatic lesion were considered positive studies for the purpose of this project. We defined a dominant lesion on MRI as a nodule demonstrating reduced signal intensity on T2WI, restricted diffusion on DWI, and/or early enhancement or rapid washout compared to adjacent prostate tissue on dynamic contrast-enhanced imaging. In cases where these sequences were unavailable, MRI positivity was determined as a score greater than 3 on a Likert-type scale. Similar to the Prostate Imaging Reporting and Data System (PI-RADS) score, this corresponds to a greater than 50% likelihood of prostate cancer.
Biopsy Protocol
All patients underwent systematic peripheral and transition zone sampling under local anesthetic at the time of confirmatory biopsy. In those cases where the surgeon used the MRI to help target confirmatory biopsies, such targeting was cognitive. MR fusion guidance systems were not used in this cohort.
Statistical Analysis
We assessed whether initial diagnostic parameters could predict confirmatory biopsy results. To that end we used univariate and multivariable logistic regression to determine whether (a) any grade of prostate cancer and (b) high-grade prostate cancer (Gleason ≥ 7) on confirmatory biopsy could be predicted from PSA density (initial PSA in ng/ml divided by MRI prostate volume in cm3), MRI results (presence/absence of dominant lesion), and initial biopsy results (percent positive cores out of all cores, percent cancer in all positive cores, and total tumor length from all positive cores). If two areas within the same core contained cancer then the length of each segment was added, with the exclusion of intervening normal tissue. Biopsy parameters were analyzed as continuous variables. The area under the receiver operating curve (AUC) was used to assess the discrimination of the model. We also performed decision curve analysis12 for the outcome of high-grade cancer to assess whether our model would be clinically useful for deciding whether to perform confirmatory biopsy. We used ten-fold cross-validation to address overfit. The decision curve was assessed up to a threshold probability of 15%, as this was viewed as the highest threshold risk of high-grade cancer for which a physician would forgo a confirmatory biopsy. All statistical analyses were conducted using Stata 13.0 (Stata Corp., College Station, TX).
RESULTS
Patient characteristics are shown in Table 1. Patients had a median age of 62 years (IQR 56–66) and almost half had a positive MRI (47%). Forty-four patients (11%) were found to have high-grade cancer on confirmatory biopsy, although only 4 patients (1%) had a grade higher than 4+3. Confirmatory biopsies were performed at a median of 5 months after the initial biopsy and MRIs were taken at a median of 3 months after the initial biopsy.
Table 1.
Patient characteristics.
| N=392 | |
|---|---|
| Baseline or initial biopsy results | |
| Median (IQR) age (years) | 62 (56–66) |
| No. clinical stage (%) | |
| T1 | 348 (89%) |
| T2 | 44 (11%) |
| Median (IQR) PSA (ng/ml) | 4.5 (3.4–5.9) |
| Median (IQR) PSA density (ng/ml/cm3) | 0.10 (0.07–0.14) |
| Median (IQR) total number of biopsy cores | 12 (12–13) |
| Median (IQR) percent positive cores from all cores (%) | 8.3 (8.3–16.7) |
| Median (IQR) total tumor length from all positive cores (mm) | 1.5 (0.7–3.5) |
| Median (IQR) percent cancer from all positive cores (%) | 10.0 (5.0–20.0) |
| MRI result*, no. (%) | |
| No dominant/focal tumor | 208 (53) |
| Dominant/focal tumor | 184 (47) |
| Confirmatory biopsy result | |
| No. Gleason score (%) | |
| No cancer on biopsy | 135 (34) |
| 6 | 213 (54) |
| 3+4 | 39 (10) |
| 4+3 | 1 (0.3) |
| 8 | 3 (0.8) |
| 9 | 1 (0.3) |
On univariate analysis, all predictors except positive MRI were significantly associated with any grade of cancer at confirmatory biopsy (Table 2). In the multivariable model, only percent positive cores and percent cancer from all positive cores were significantly associated with the outcome of any cancer found on confirmatory biopsy. This model had a ten-fold cross-validated AUC of 0.72. By contrast, MRI positivity was significantly associated with the presence of high-grade (Gleason ≥ 7) cancer on confirmatory biopsy on univariate analysis (Table 3). However, in the multivariable model only total tumor length and PSA density had a significant association with high-grade cancer on confirmatory biopsy. This model had a ten-fold cross-validated AUC of 0.85.
Table 2.
Univariate and multivariable logistic regression models for any grade cancer on confirmatory biopsy.
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Any Grade Cancer | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| MRI result | ||||||
| No focal/dominant lesion | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Focal/dominant lesion | 1.47 | 0.96–2.23 | 0.075 | 1.25 | 0.79–1.98 | 0.3 |
| PSA density (per 1 ng/ml/10 cm3) | 1.69 | 1.15–2.46 | 0.007 | 1.30 | 0.87–1.95 | 0.2 |
| Percent positive cores from all cores (%) | 1.09 | 1.05–1.13 | <0.0001 | 1.08 | 1.03–1.12 | 0.0003 |
| Total tumor length from all positive cores (mm) | 1.36 | 1.20–1.53 | <0.0001 | 0.96 | 0.81–1.13 | 0.6 |
| Percent cancer from all positive cores (%) | 1.07 | 1.04–1.10 | <0.0001 | 1.07 | 1.03–1.11 | 0.0002 |
Table 3.
Univariate and multivariable logistic regression for high-grade cancer found on confirmatory biopsy.
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| High-Grade Cancer | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| MRI result | ||||||
| No focal/dominant lesion | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Focal/dominant lesion | 2.41 | 1.25–4.65 | 0.009 | 1.33 | 0.62–2.89 | 0.5 |
| PSA density (per 1 ng/ml/10 cm3) | 2.36 | 1.55–3.58 | <0.0001 | 2.03 | 1.22–3.37 | 0.006 |
| Percent positive cores from all cores (%) | 1.04 | 1.01–1.06 | 0.003 | 1.00 | 0.97–1.03 | 1 |
| Total tumor length from all positive cores (mm) | 1.48 | 1.33–1.65 | <0.0001 | 1.35 | 1.15–1.58 | 0.0002 |
| Percent cancer from all positive cores (%) | 1.06 | 1.04–1.08 | <0.0001 | 1.02 | 0.99–1.04 | 0.2 |
Although only total tumor length and PSA density were significant, we evaluated the performance of the full pre-specified multivariable model for high-grade cancer using the MRI result, PSA density, percent positive cores, total tumor length and percent cancer. We used decision curve analysis on the ten-fold cross-validated predicted risks from this multivariable model, as shown in Figure 1. Evaluating the decision curve up to a threshold probability of 15%, we found that using our model to determine which patients get a confirmatory biopsy would generally provide a higher net benefit than performing confirmatory biopsy in all patients. The model only ceased being beneficial if a urologist was willing to order a confirmatory biopsy for men with a risk of high-grade disease of 1 or 2%; that is, he or she would be willing to conduct 50–100 confirmatory biopsies to find one case of upgrading. As an illustration of the clinical effects of using the model, if we only wished to perform confirmatory biopsy in patients with a risk of high-grade cancer of 10% or greater, the model would indicate confirmatory biopsies in 240 patients out of 1000 patients, reducing the number of confirmatory biopsies by over 75% (Table 4). We would find 89 (37%) high-grade cancers among these 240 patients, while 23 (3%) of the 760 patients who were not indicated for a confirmatory biopsy would be harboring high-grade disease. Of these 23 patients out of 760, 20 would have 3+4 Gleason on confirmatory biopsy and 3 would have Gleason 4+4 on confirmatory biopsy.
Figure 1.
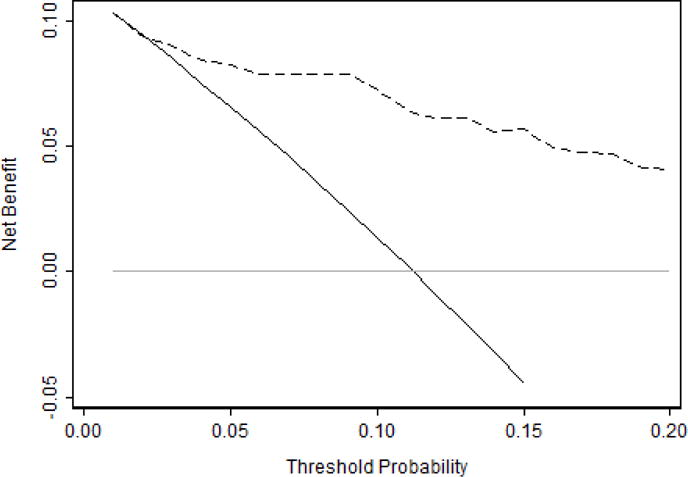
Decision curve for predicting high-grade cancer on confirmatory biopsy. Dashed line is the net benefit of providing a confirmatory biopsy based on PSA density, MRI results, percent positive cores, total tumor length and percent cancer; black line is the net benefit of providing all patients with a confirmatory biopsy; and grey line is the net benefit of providing no patients with a confirmatory biopsy. The curve shows that the net benefit of using the model to choose who receives confirmatory biopsy exceeds the benefit of performing confirmatory biopsy except for urologists with extremely low threshold probabilities less than 2%.
Table 4.
Decision analysis per 1000 patients for high-grade cancer on confirmatory biopsy, using MRI result, PSA density, percent positive cores, total tumor length and percent cancer.
| Threshold Probability | Number of biopsies | High-grade cancers found | Biopsies avoided | High-grade cancers missed |
|---|---|---|---|---|
| 5% | 474 | 102 | 526 | 10 |
| 10% | 240 | 89 | 760 | 23 |
| 15% | 171 | 74 | 829 | 38 |
DISCUSSION
In the present study, 11% of patients meeting initial criteria for AS were found to have high-grade cancer on confirmatory biopsy. We showed that using our model to select patients for confirmatory biopsy would generally provide a higher net benefit than performing confirmatory biopsy in all patients, across a wide range of threshold probabilities. For physicians unwilling to accept even a 5% risk of missing Gleason ≥ 7 disease, our model is still able to avoid confirmatory biopsy for more than half of the patient population.
We found PSA density, along with total tumor length, to be significantly associated with Gleason ≥ 7 cancer at confirmatory biopsy in our multivariable model. In a recent update on the PRIAS Study, Bul et al identified PSA density and the number of positive cores as the strongest predictors for reclassification on confirmatory biopsy.11 Likewise, Cary et al identified an association between lower PSA density and decreased odds of biopsy progression at 3 years in a cohort of 465 patients.10 San Francisco et al found PSA density > 0.08 ng/ml/cm3 and positive family history as significant predictors of progression in a cohort of 135 patients undergoing saturation confirmatory biopsy.13 In another small study, PSA density > 0.15 ng/ml/cm3 was associated with upgrading in 31% of patients undergoing repeat biopsy, compared to only 10% in patients with PSA density < 0.15 ng/ml/cm3.14 Although a previous study from our institution did not associate PSA density with biopsy progression,4 it should be noted that this finding was based on PSA density at the time of confirmatory biopsy, rather than at the time of diagnosis as in the present analysis.
Wang et al recently tested the ability of several existing nomograms — Kattan,15 Steyerberg,16 Nakanishi,17 and Chun18 — to predict indolent disease in a cohort of 273 men.19 These men met Epstein criteria for indolent disease and underwent multiple biopsies and/or delayed radical prostatectomy over a minimum 6-month period. Unsurprisingly, Wang et al found that patients with progression had lower probabilities of indolent disease than those without. The Nakanishi nomogram, which was the only one to include PSA density as a predictor, had the best performance with an AUC of 0.67 for biopsy progression. The authors also performed decision curve analysis, and determined that these nomograms only provided a net benefit when the threshold probability of progression was between 40% and 60%. Since the norm is to offer confirmatory biopsy to everyone, and nobody would withhold confirmatory biopsy until a patient had a 40% chance of non-indolent cancer, the real clinical threshold is obviously lower than 40%. Our model, which incorporates PSA density, MRI results, and initial biopsy results, delivers a reasonable estimation of the risk of Gleason ≥ 7 at confirmatory biopsy, with an AUC of 0.85. It is useful for all thresholds except for those physicians who have extremely low risk tolerance.
MRI has previously shown utility in identifying men at risk of reclassification at confirmatory biopsy.20–25 In line with these findings, a positive MRI in the present study was associated with an OR of 2.41 for detecting Gleason ≥ 7 cancer on univariate analysis (CI 1.25 – 4.65, p = 0.009). This is consistent with previous data from our institution,23 which found that low MRI scores on a Likert-type scale had a high negative predictive value (0.96) for upgrading on confirmatory biopsy. Among the 208 patients with negative MRI studies, 193 (93%) did not have high grade cancer on confirmatory biopsy. Among the 184 patients graded with positive studies, 29 (16%) were not found to have high grade cancer on confirmatory biopsy. We did not, however, find MRI to be as useful when we included it in a multivariable model including other initial diagnostic characteristics.
A Likert-type scale was only used for part of the study period (2009 onwards), and prior to this the presence of a dominant or focal lesion on MRI was interpreted from review of radiology reports. However, when we performed a sensitivity analysis using Likert scores in the multivariable model, our results were unchanged. Only PSA density (OR 1.99 per 1ng/mL/10 cm3, p = 0.032) and total tumor length (OR 1.37 per mm, p = 0.002) were significantly associated with high grade cancer on confirmatory biopsy on multivariable analysis. We also allowed MRIs performed up to 12 months prior to confirmatory biopsy, whereas the prior study only included MRIs and biopsies performed within 6 months of diagnosis. Bearing this in mind, we performed a sensitivity analysis excluding 7 patients who had MRI prior to 2009 and 44 patients whose MRI was more than 6 months prior to their confirmatory biopsy. The result was essentially similar: PSA density (OR 2.7 per 1ng/mL/10 cm3, p = 0.002) and total tumor length (OR 1.4 per mm, p < 0.001) achieved significance in this model, whereas positive MRI did not (OR 1.03, p = 0.9).
We also performed a sensitivity analysis including only those 281 patients who received multiparametric MRI with the combination of T2WI, DWI and dynamic contrast-enhanced imaging. Once again, results were not importantly changed: PSA density (OR 2.52 per 1ng/mL/10 cm3, p = 0.009) and total tumor length (OR 1.43 per mm, p = 0.0003) were the only factors on initial diagnosis that were significantly associated with high grade cancer at confirmatory biopsy in the multivariable model. A validation study, using a prospective cohort with standardized MRI acquisition and reporting protocols, may help clarify whether MRI has a predictive role within the context of our multivariable model.
While our multivariable analysis did not demonstrate MRI to be predictive of results on confirmatory biopsy, MRI may still be useful in this population, both as a baseline study as well as a guide for targeted biopsy. Hu et al21 found that 36.3% of men undergoing multiparametric MRI ultrasound-fusion confirmatory biopsy were reclassified beyond Epstein criteria. By contrast, Vasarainen et al26 did not find a correlation between MRI positivity and subsequent biopsy findings, or discontinuation of AS, in 80 patients enrolled in the Finnish arm of PRIAS, despite identifying an anatomical lesion suggestive of cancer in 50%. Taken together, this variability suggests that MRI in isolation may not adequately predict the risk of cancer upgrading on subsequent biopsy.
The primary limitation of our study is that our model requires external validation. It was built upon retrospective data from a single NCI-designated cancer center, meaning that it may not be representative of the overall patient population and may be optimistic despite ten-fold cross-validation. Calibration may also differ between cohorts. One reason why we may have failed to see a strong effect of MRI was that systematic rather than MR-assisted biopsy was used. Future research might consider testing the model in a cohort of patients undergoing MR-fusion biopsy. If the model stands up to external validation, it shall be refined and made available as a nomogram for day-to-day clinical use.
CONCLUSIONS
In the present study we propose a model, based on initial diagnostic criteria, which could be used to determine the need for confirmatory biopsy in patients initiating AS. The model shows considerable promise, with the potential to avoid many additional biopsies while still detecting the majority of high-grade cancers. Should it stand up to external validation, this model would become a valuable tool in the management of patients on active surveillance.
Acknowledgments
Funding:
Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers and by NIH Cancer Center Support Grant P30 CA008748 to PI: Craig B. Thompson, MD.
ABBREVIATIONS AND ACRONYMS
- AS
active surveillance
- AUC
area under the curve
- DRE
digital rectal examination
- DWI
diffusion-weighted imaging
- IQR
interquartile range
- MRI
magnetic resonance imaging
- OR
odds ratio
- PSA
prostate-specific antigen
- T2WI
T2-weighted images
References
- 1.Mohler JL, Armstrong AJ, Bahson RR, et al. National Comprehensive Cancer Network guidelines: prostate cancer version 1.2015. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Published October 24, 2014. Accessed April 17, 2015.
- 2.Mottet N, Bellmunt J, Briers E, et al. European Association of Urology guidelines on prostate cancer (March 2015 update) http://uroweb.org/guideline/prostate-cancer/. Accessed April 17, 2015.
- 3.Thompson I, Thrasher JB, Aus G, et al. American Urological Association guideline for the management of clinically localized prostate cancer. 2007 doi: 10.1016/j.juro.2007.03.003. https://www.auanet.org/education/guidelines/prostate-cancer.cfm. Accessed April 17, 2015. [DOI] [PubMed]
- 4.Adamy A, Yee DS, Matsushita K, et al. Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J Urol. 2011;185:477. doi: 10.1016/j.juro.2010.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berglund RK, Masterson TA, Vora KC, et al. Pathological upgrading and up staging with immediate repeat biopsy in patients eligible for active surveillance. J Urol. 2008;180:1964. doi: 10.1016/j.juro.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dall’Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112:2664. doi: 10.1002/cncr.23502. [DOI] [PubMed] [Google Scholar]
- 7.Ross AE, Loeb S, Landis P, et al. Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol. 2010;28:2810. doi: 10.1200/JCO.2009.25.7311. [DOI] [PubMed] [Google Scholar]
- 8.Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64:876. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 9.Ehdaie B, Vertosick E, Spaliviero M, et al. The impact of repeat biopsies on infectious complications in men with prostate cancer on active surveillance. J Urol. 2014;191:660. doi: 10.1016/j.juro.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 10.Cary KC, Cowan JE, Sanford M, et al. Predictors of pathologic progression on biopsy among men on active surveillance for localized prostate cancer: the value of the pattern of surveillance biopsies. Eur Urol. 2014;66:337. doi: 10.1016/j.eururo.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 11.Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63:597. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.San Francisco IF, Werner L, Regan MM, et al. Risk stratification and validation of prostate specific antigen density as independent predictor of progression in men with low risk prostate cancer during active surveillance. J Urol. 2011;185:471. doi: 10.1016/j.juro.2010.09.115. [DOI] [PubMed] [Google Scholar]
- 14.Kotb AF, Tanguay S, Luz MA, et al. Relationship between initial PSA density with future PSA kinetics and repeat biopsies in men with prostate cancer on active surveillance. Prostate Cancer Prostatic Dis. 2011;14:53. doi: 10.1038/pcan.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kattan MW, Eastham JA, Wheeler TM, et al. Counseling men with prostate cancer: a nomogram for predicting the presence of small, moderately differentiated, confined tumors. J Urol. 2003;170:1792. doi: 10.1097/01.ju.0000091806.70171.41. [DOI] [PubMed] [Google Scholar]
- 16.Steyerberg EW, Roobol MJ, Kattan MW, et al. Prediction of indolent prostate cancer: validation and updating of a prognostic nomogram. J Urol. 2007;177:107. doi: 10.1016/j.juro.2006.08.068. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi H, Wang X, Ochiai A, et al. A nomogram for predicting low-volume/low-grade prostate cancer. Cancer. 2007;110:2441. doi: 10.1002/cncr.23055. [DOI] [PubMed] [Google Scholar]
- 18.Chun FKH, Haese A, Ahyai SA, et al. Critical assessment of tools to predict clinically insignificant prostate cancer at radical prostatectomy in contemporary men. Cancer. 2008;113:701. doi: 10.1002/cncr.23610. [DOI] [PubMed] [Google Scholar]
- 19.Wang S-Y, Cowan JE, Cary KC, et al. Limited ability of existing nomograms to predict outcomes in men undergoing active surveillance for prostate cancer. BJU Int. 2014;114:E18. doi: 10.1111/bju.12554. [DOI] [PubMed] [Google Scholar]
- 20.Hoeks CMA, Somford DM, Van Oort IM, et al. Value of 3-T multiparametric magnetic resonance imaging and magnetic resonance-guided biopsy for early risk restratification in active surveillance of low-risk prostate cancer: a prospective multicenter cohort study. Invest Radiol. 2014;49:165. doi: 10.1097/RLI.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 21.Hu JC, Chang E, Natarajan S, et al. Targeted prostate biopsy to select men for active surveillance: do the Epstein criteria still apply? J Urol. 2014;192:385. doi: 10.1016/j.juro.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marliere F, Puech P, Benkirane A, et al. The role of MRI-targeted and confirmatory biopsies for cancer upstaging at selection in patients considered for active surveillance for clinically low-risk prostate cancer. World J Urol. 2014;32:951. doi: 10.1007/s00345-014-1314-5. [DOI] [PubMed] [Google Scholar]
- 23.Vargas HA, Akin O, Afaq A, et al. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol. 2012;188:1732. doi: 10.1016/j.juro.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margel D, Yap SA, Lawrentschuk N, et al. Impact of multiparametric endorectal coil prostate magnetic resonance imaging on disease reclassification among active surveillance candidates: a prospective cohort study. J Urol. 2012;187:1247. doi: 10.1016/j.juro.2011.11.112. [DOI] [PubMed] [Google Scholar]
- 25.Fradet V, Kurhanewicz J, Cowan JE, et al. Prostate cancer managed with active surveillance: role of anatomic MR imaging and MR spectroscopic imaging. Radiology. 2010;256:176. doi: 10.1148/radiol.10091147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasarainen H, Lahdensuo K, Savolainen R, et al. Diffusion-weighted magnetic resonance imaging in prostate cancer patients on active surveillance one year after diagnosis and before repeat biopsy. Scand J Urol. 2013;47:456. doi: 10.3109/21681805.2013.765910. [DOI] [PubMed] [Google Scholar]


