Abstract
Prenatal stress and overexposure to glucocorticoids (GC) during development may be associated with an increased susceptibility to a number of diseases in adulthood including neuropsychiatric disorders, such as depression and anxiety. In animal models, prenatal overexposure to GC results in hyper-responsiveness to stress in adulthood, and females appear to be more susceptible than males. Here, we tested the hypothesis that overexposure to GC during fetal development has sex-specific programming effects on the brain, resulting in altered behaviors in adulthood. We examined the effects of dexamethasone (DEX; a synthetic GC) during prenatal life on stress-related behaviors in adulthood and on the tryptophan hydroxylase-2 (TpH2) gene expression in the adult dorsal raphe nucleus (DRN). TpH2 is the rate-limiting enzyme for serotonin (5-HT) synthesis and has been implicated in the etiology of human affective disorders. Timed-pregnant rats were treated with DEX from gestational days 18–22. Male and female offspring were sacrificed on the day of birth (postnatal day 0; P0), P7, and in adulthood (P80-84) and brains were examined for changes in TpH2 mRNA expression. Adult animals were also tested for anxiety- and depressive- like behaviors. In adulthood, prenatal DEX increased anxiety- and depressive- like behaviors selectively in females, as measured by decreased time spent in the center of the open field and increased time spent immobile in the forced swim test, respectively. Prenatal DEX increased TpH2 mRNA selectively in the female caudal DRN at P7, whereas it decreased TpH2 mRNA selectively in the female caudal DRN in adulthood. In animals challenged with restraint stress in adulthood, TpH2 mRNA was significantly lower in rostral DRN of prenatal DEX treated females compared to vehicle treated females. These data demonstrated that prenatal overexposure to GC alters the development of TpH2 gene expression and these alterations correlated with lasting behavioral changes found in adult female offspring.
Keywords: Dexamethasone, tryptophan hydroxylase-2, anxiety, depression, dorsal raphe nucleus, rat
1. Introduction
Anxiety and affective disorders, such as depression, are prevalent worldwide. Women are diagnosed with these disorders about two times as frequently as men (Palanza, 2001, Pigott, 2003, Steiner et al., 2003); however, the underlying mechanisms for this sex difference in risk remain elusive. Clinical and epidemiological studies provide evidence that early adverse life experiences are associated with increased adult susceptibility to a number of psychiatric disorders, including anxiety and depressive disorders (Brown and Harris, 1993, Brown et al., 1996, Watson et al., 1999, Gutman and Nemeroff, 2003). Animal studies demonstrate that following prenatal stress, adult animals are hyper-responsive to stress and females are more vulnerable to this insult than males (Weinstock et al., 1992, McCormick et al., 1995, Richardson et al., 2006). Adult females develop anxiety- and depressive- like behaviors following maternal stress (Fride and Weinstock, 1988, Alonso et al., 1991, Keshet and Weinstock, 1995, Vallee et al., 1997, Frye and Wawrzycki, 2003, Richardson et al., 2006, Zagron and Weinstock, 2006) or overexposure to glucocorticoids (GC) (Welberg et al., 2001, Oliveira et al., 2006, Zagron and Weinstock, 2006, Nagano et al., 2008). Prenatal stress and overexposure to GC also have been shown to have a variety of sex-specific impacts on brain regions thought to be involved in stress regulation, such as the hypothalamus, amygdala, and frontal cortex (Tobe et al., 2005, Murmu et al., 2006, Zuloaga et al., 2011, Carbone et al., 2012, Zuloaga et al., 2012). These data, if applicable to the human condition, imply that developmental overexposure to GC alters brain programming in a sex selective manner, resulting in increased risk in females for developing anxiety or depressive disorder in adulthood.
The brain serotoninergic system, a neurotransmitter system implicated in stress regulation and etiology of affective disorders (Lucki, 1998, Ressler and Nemeroff, 2000, Nestler et al., 2002), is a potential target for fetal GC exposure (Slotkin et al., 1996, McGrath et al., 1997, Muneoka et al., 1997, Slotkin et al., 2006, Mueller and Bale, 2008). Prenatal exposure to dexamethasone (DEX), a synthetic GC, alters forebrain serotonin (5-HT) turnover and binding of 5-HT receptors and the 5-HT transporter in a sex specific fashion in adulthood (Slotkin et al., 2006, Slotkin and Seidler, 2010). Changes in forebrain 5-HT activity may result from fundamental alterations in the dorsal raphe nucleus (DRN), a major source of the serotonergic innervation in the forebrain. The DRN consists of multiple subregions with distinct responses to diverse stimuli (Kirby et al., 1995, Adell et al., 1997, Kirby et al., 1997, Kirby and Lucki, 1998). In particular, tryptophan hydroxylase-2, (TpH2), the brain specific isoform of the rate-limiting enzyme for 5-HT biosynthesis is expressed at high levels in the DRN and polymorphisms of the TpH2 have been implicated in affective disorders and suicide (Sun et al., 2004, Nash et al., 2005, You et al., 2005, Zhang et al., 2005). Previous studies have examined the effects of prenatal stress on TpH2 mRNA in the DRN of mice (Mueller and Bale, 2008); however, no study to date has evaluated the sex-specific effects of prenatal GC overexposure on TpH2 in developing and adult rats. Further, as DRN TpH2 has been demonstrated to have region specific effects on anxiety-like behaviors (Hiroi et al., 2006a, Hiroi et al., 2011), unraveling the possible link between prenatal GC overexposure and TpH2 in regulating these behaviors may have important clinical implications.
Therefore, in the current study, we examined the effects of prenatal DEX treatment on the development of TpH2 mRNA in the DRN, focusing on the subregions, and on adult anxiety- and depressive- like behaviors. As prenatal stress has a profound impact on adult stress responsivity, we also examined the sex-specific effects of prenatal DEX treatment on TpH2 mRNA in the subregions of the DRN of adults challenged with restraint stress.
2. Experimental Procedures
2.1 Animals
Thirty-six timed-pregnant Sprague-Dawley rat dams (Charles-River Laboratories, Wilmington, MA) arrived at the laboratory animal facility at Arizona State University at gestational day (GD) 7 and were allowed to habituate for one week in a temperature- and humidity-controlled environment. Timeline of the experimental procedures is illustrated in Figure 1. Animals were maintained on a standard 12:12 light/dark cycle with lights on from 0700 to 1900. Beginning on GD14, dams were handled for 5 min daily to habituate the animals to subsequent experimental manipulations and thereby alleviate some stress due to the experimenter. Once daily administration of DEX (0.4mg/kg, s.c.) or safflower oil vehicle commenced at GD 18 until parturition at GD 22, also termed postnatal day 0, (P0). Litters were thinned to 10 per litter (mixed sex) at birth to ensure equal nutritional status. On the day of birth, all offspring were weighed and randomly assigned to a litter composed of five males and five females as described previously (Carbone et al., 2012). This assignment was designed to minimize any litter effect that may influence the study. Vehicle- or DEX-exposed offspring were always assigned to a dam that had received that respective injection. To further minimize litter specific effects, only 2 males and 2 females were taken from each litter for inclusion in each treatment group.
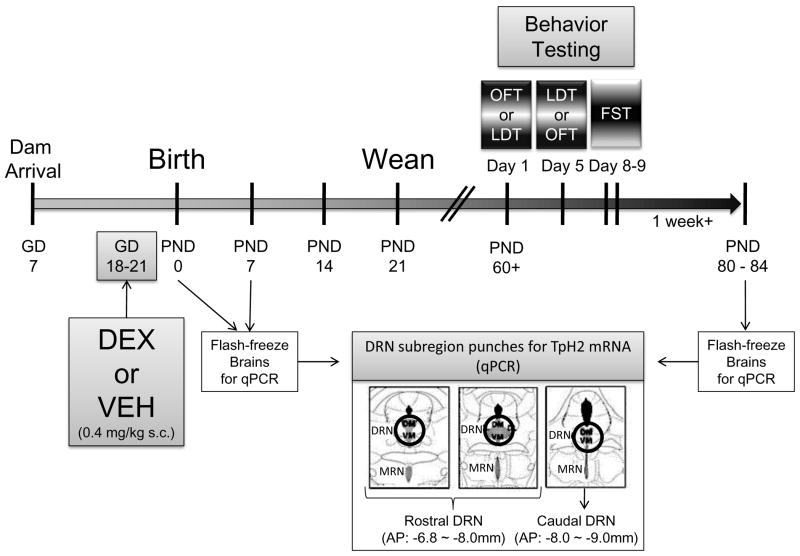
Figure 1.
Timeline of the experimental procedures. Time-pregnant dams arrived at gestational day (GD) 7. Dexamethasone (DEX) or vehicle (VEH) were administered from GD 18–21. Brains from postnatal day (PND) 0 and PND 7 were extracted for analysis of TpH2 mRNA in the subdivisions of the dorsal raphe nucleus (DRN) using quantitative PCR (qPCR). Behavioral testing in open field test (OFT), light dark transition (LDT) and forced swim test (FST; a 2-day testing procedure with a pretest on the first day and a test on second day) began starting PND 60 with a 4-day resting period between each test. Females were tested in the OFT, LDT, and day 2 (test day) of FST on the day of diestrus. Following at least one week of rest, all animals were sacrificed and brains were extracted and flash frozen for TpH2 mRNA analysis using qPCR.
2.1.1 Adult behavioral experiments
A group of pups were weaned at P21 and allowed to grow to 60 days of age, when they began testing in the open field test (OFT) and light-dark transition box (LDT) and forced swim test (FST). A total of 50 animals were assigned to the following groups: Male vehicle, Male DEX, Female vehicle, Female DEX. Vehicle-treated animals came from 6 separate litters, and DEX-treated animals came from another 7 separate litters. Each animal was tested in all three measures of behavior with a 4-day rest period between each test. Animals were housed 2 rats per cage, and cagemates were tested simultaneously. Females were monitored daily for estrous cyclicity for at least two weeks prior to behavioral testing. On the day of testing, vaginal smears were taken at least one hour before being placed into the behavioral apparatus.
Females were tested on the day of diestrus I or II (henceforth referred to collectively as diestrus) and were omitted from further analysis if they were not in diestrus. All behavioral testing was performed before 12:00 pm and video-recorded for later analysis by an observer blind to the experimental condition. Animals had at least one week of rest following behavioral testing to minimize any sub-acute stress effects on gene expression. Animals at ages P80-84 were euthanized with CO2. Care was taken to ensure that females were euthanized on the day of diestrus. Brains were flash frozen in 2-methylbutane at −30°C and stored at −80°C until analysis.
2.1.2 Adult stress response experiments
A separate cohort of male and female adult animals, naïve to behavioral testing, was examined at P81-82 to measure changes in the DRN TpH2 mRNA in response to 20 min of restraint stress. A total of 24 animals were assigned to the following groups: Male vehicle, Male DEX, Female vehicle, Female DEX. Vehicle-treated animals came from 3 separate litters, and DEX-treated animals came from another 3 separate litters. Females were monitored daily for estrous cyclicity for at least two weeks prior to behavioral testing. On the day of testing, vaginal smears were taken at least one hour before being placed into the restraint stress apparatus. Females were tested on the day of diestrus and were omitted from further analysis if they were not in diestrus. Immediately following the restraint stress, animals were euthanized and brains were removed from the skull, flash frozen with 2 methylbutane at −30°C and stored at −80°C until analysis. Again, care was taken to ensure that all females were stressed and sacrificed on the day of diestrus. These stress challenged animals received no behavioral testing and were a separate cohort of animals from the aforementioned group of animals with no stress challenge that were tested in behavioral assays. Given these differences, the current study did not analyze any comparisons between the animals with versus without stress.
For the developmental time course experiments, separate cohorts of P0 and P7 pups were euthanized and brains were removed from the skull, flash frozen with 2-methylbutane at −30°C, and stored at −80°C until further analysis.
2.2 Vaginal smears and analysis
Vaginal smears were obtained and analyzed as described previously (Hiroi and Neumaier, 2006a) for two weeks prior to the behavioral tests and at least one hour before any behavioral measures on test days. Briefly, smears were obtained daily with a cotton swab soaked in sterile water. Shortly after the vaginal cells were collected on glass slides, samples were fixed with 100% methanol (5 min), air dried for 10 min, and stained for 15 min in 5% Giemsa stain (GS500, Sigma, St. Louis, MO). Stained cells were analyzed for estrous cycle stages using light microscopy according to a previously published criteria (Hiroi and Neumaier, 2006a).
2.3 Behavior testing
2.3.1 Anxiety-like behaviors
An open field arena was set up adjacent to the light dark box, and at age of P60-63, cage-mates were run simultaneously in the two tests on day 1 of behavior testing. On day 5, rats that were tested in OFT on day 1 were measured in LDT, while rats that were tested in LDT on day 1 were measured in OFT.
The open field apparatus, 100 × 100 × 40 cm, was made of black plexiglass and divided into 25 squares of equal size by thin white lines. A light bulb positioned directly above the center of the apparatus illuminated the center at 80 lux. The animals were placed in the center of the apparatus and behavior was videotaped for later analysis. Time spent in the center and total line crossings were measured during the 10 min test session.
The light dark box consisted of a rectangular Plexiglass box with a dark region with black walls and lid (21 cm length × 24 cm width × 19 cm height) and a light region with transparent walls (21 cm length × 24 cm width × 19 cm height) illuminated at 80 lux by a light bulb above the apparatus. The dark and light regions were connected by a 10 cm height × 5 cm width opening, which allowed the animals to freely enter either area. Animals were placed in the light side of the chamber facing the opening to the dark chamber and behavior was measured for 10 min. The time spent in each compartment, the number of entries into each of the two compartments, and the latency to re-enter the light compartment were recorded over a 10-min duration.
2.3.2 Despair-like behavior
The FST was performed as previously described (Weiser et al., 2009) on behavioral days 8 and 9. Briefly, on the first day (i.e., the pre-test day), animals were placed into a glass cylinder (40cm × 27cm) which was filled with 30cm of 25°C fresh water. Animals were left in the cylinder for 10 min. and then removed and dried before they were returned to their home cage. Twenty-four hours later, animals were again placed in the cylinder and time spent immobile, swimming, and climbing was measured for 5 min. Female rats were in diestrus on day 9 (i.e., on the test day).
2.4 Restraint Stress
Animals were placed into a Plexiglas restraint tube (Plas-Laboratories, Lansing, MI, USA) and blood was collected from tail nick within 1 min to obtain baseline corticosterone (CORT) levels. Animals were sacrificed by decapitation directly after being removed from the tube 20 min later. Trunk blood was collected and centrifuged and plasma was retained and stored at −20°C for later analysis of hormone levels. Brains were flash frozen in 2-methylbutane at −30°C and stored at −80°C until analysis. Females were monitored daily for estrous cyclicity for at least two weeks prior to behavioral testing. Female rats were in diestrus on the day of restraint stress and were omitted from the analysis if they were not in diestrus. Vaginal smears were obtained at least one hour prior to the restraint stress.
2.5 CORT Radioimmunoassay (RIA)
Plasma samples were diluted in 0.01 M PBS (1:25) and corticosteroid binding globulin was inactivated by incubation at 65 °C for 1 h. Samples (20 μl) and standards (5–700 ng/ml) were incubated overnight at 4 °C with antiserum (rabbit anti-CORT; MP Biomedicals, Solon, OH, USA) and [3H] CORT (PerkinElmer, Boston, MA, USA) in 0.1% gelatin 0.01 M PBS. Free CORT was separated from antibody-bound CORT with 1.0 ml dextran-coated charcoal. After centrifugation, the supernatant containing antibody-bound CORT was mixed with 4 mL of scintillation fluid and counted with a Packard 2900 TR liquid scintillation counter (Meriden, CT, USA).
2.6 RNA isolation and quantitative Reverse-Transcriptase Polymerase Chain Reaction (qRT-PCR)
Frozen brains were cryosectioned at −20°C; 100–200 μm-thick coronal sections containing the DRN were punched using a 1-mm diameter stainless steel cannula from the mid-rostral (AP: −6.8 to −8.0 mm, hereafter referred to as “rostral DRN”) and caudal (AP: −8.1 to −9.3 mm) subregions of the DRN according to Paxinos rat brain atlas (Paxinos and Watson, 1986). Given that caudal DRN is known to have distinct properties compared to mid and rostral DRN, and has been shown to play important roles in rat models of stress disorders, such as anxiety and despair (Maier et al., 1995, Maswood et al., 1998, Lowry, 2002, Greenwood et al., 2003, Day et al., 2004, Hiroi et al., 2006a, Hiroi and Neumaier, 2006b, Hiroi et al., 2011), care was taken to separate caudal DRN from mid and rostral DRN. A more detailed set of anatomical criteria described in Clark et al. (Clark et al., 2006) was used to assist with demarcation of rostral versus caudal regions. Rostral DRN consisted of regions ranging from the nearly linear structure dorsal in the far rostral end (AP −6.8mm) up to the point wherein the dorsolateral wings of the DRN are distinct from the dorsomedial DRN (AP −8.0mm); whereas, the caudal DRN consisted of regions ranging from the point wherein the dorsal lateral wings of the DRN faded completely and the dorsomedial DRN exists as a pair of short horns immediately ventral to the aqueduct (AP −8.1mm) up to the point wherein the DRN becomes a nearly linear structure in the far caudal end (AP −9.3mm). If there were any concerns regarding the identification of these criteria during tissue collection, the sample was removed from further analysis. Tissue punches were kept frozen at all times and stored at −80°C until RNA extraction. Total RNA was isolated by the phenol extraction protocol described previously (Chomczynski and Sacchi, 1987). Total RNA (1 μg per sample) was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), qRT-PCR was performed using the Quant-iT Oligreen ssDNA Reagent (Molecular Probes, Eugene, OR), gene expression was measured with a LightCycler 480 (Roche Diagnostics, Indianapolis, IN), and absolute target mRNA was calculated based on comparison between the crossing point of individual sample and the standard curve constructed from known amounts of purified PCR product. Target genes were amplified using primer pairs that corresponded to the rat Tph2 (NM173839), using forward primer sequence 5′-AAA TAC TGG GCC AGG AGA GG-3′ and reverse primer sequence 5′-GGA GAA CAC AAC CGC AGT CT-3′.
2.7 Data Analysis
Data were analyzed using a two-way ANOVA for Sex and Treatment effects for each behavior or for each subregion of the DRN. If ANOVA was significant, Bonferroni multiple comparisons test was used for post hoc examination. All statistical analyses were performed using GraphPad Prism software version 5.0b (Graphpad Software, Inc., La Jolla, CA).
3. Results
3.1 Prenatal DEX treatment increased anxiety-like behavior in the open field selectively in females during adulthood
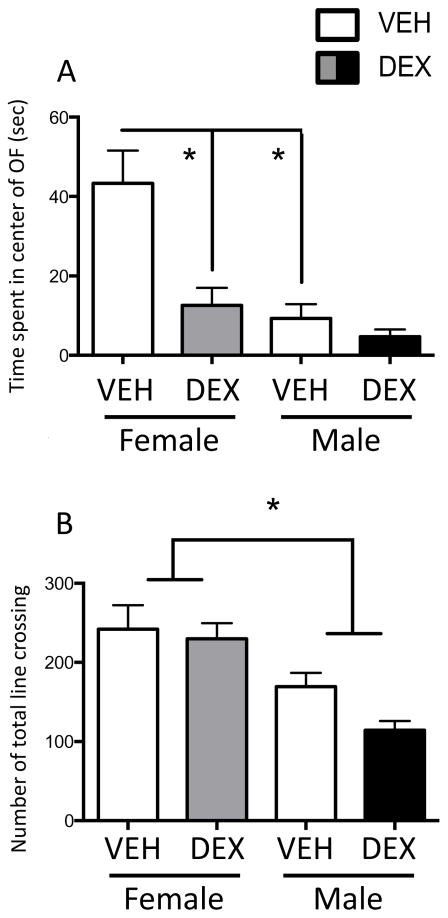
We examined anxiety-like behaviors in the OFT and LDT in adult males and normally cycling females in diestrus. A two-way ANOVA showed main effects of Treatment (F(1,39)=12.01; p=0.0013) and Sex (F(1,39)=17.82; p=0.0001), as well as a Treatment x Sex interaction (F(1,39)=5.887; p=0.02) in the time spent in the center of the open field (Figure 2A). Post hoc tests revealed that DEX treatment in females, but not males, significantly decreased time spent in the center, suggesting that prenatal DEX treatment increased anxiety-like behaviors selectively in females. Post hoc tests also showed that vehicle females spent more time in the center than the vehicle males. Finally, there was a main effect of Sex (F(1,39)=23.49; p<0.0001) in the total line crossings in the open field, suggesting that overall activity in males was lower than in females (Figure 2B).
Figure 2.
Prenatal DEX treatment increased anxiety-like behaviors in the open field test (OFT) selectively in females in adulthood. Duration in the center of the open field (A) and total line crossings (B) in the open field during the 10-min recording period. *Significant difference between groups, p<0.05. All values are expressed as Mean ± SEM. n=6–12 per group.
There were no significant effects of Treatment or Sex on any of the measures analyzed in the LDT (data not shown). Lack of anxiety-like effects in the LDT may be due to lighting conditions, such as different levels of light can have a significant impact on the anxiety-like behaviors (Prut and Belzung, 2003; Bertoglio and Carobrez, 2002; Bouwknecht et al., 2007; Hale et al., 2006). The LDT apparatus was located in a dark room and the light region of the box was illuminated at 80 lux. Although the same illumination of light was used for the LDT as the OFT, due to the inherent differences in the testing condition (ex. the presence of the dark compartment in the LDT, whereas there is no dark compartment in the OFT; the differences in the dimensions and color of the apparatus of the OFT vs LDT), dimmer lighting condition may be needed for the LDT to overcome any light-induced ceiling effect on anxiety-like behaviors that may have masked treatment effects in our study.
3.2 Prenatal DEX treatment increased despair-like behavior in the forced swim test selectively in females during adulthood
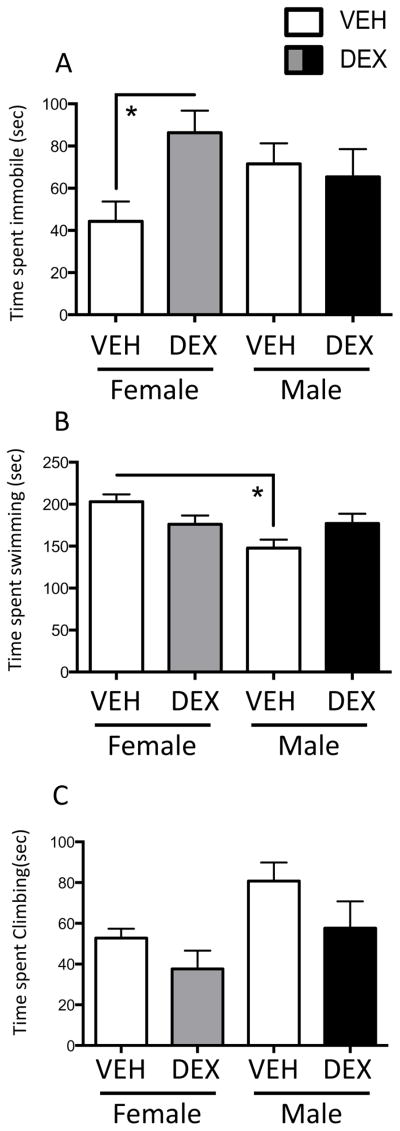
We evaluated depressive-like behaviors in the FST in adult males and normally cycling females in diestrus. A two-way ANOVA showed a Treatment x Sex interaction (F(1,42)=4.285; p=0.0446) in time spent immobile in the FST (Figure 3A). Post hoc tests revealed that prenatal DEX treatment increased time spent immobile in females, but not in males, suggesting that prenatal DEX treatment increased depressive-like behaviors selectively in females.
Figure 3.
Prenatal DEX treatment increased depressive-like behaviors in the forced swim test (FST) selectively in females in adulthood. Time spent immobile (A), swimming (B) and climbing (C) in the FST. *Significant difference between groups, p<0.05. All values are expressed as Mean ± SEM. n=11–13 per group.
There was also a main effect of Sex (F(1,44)=4.285; p=0.0113) and a Treatment x Sex interaction (F(1,44)=7.475; p=0.009) in swimming behavior in the FST (Figure 3B). Post hoc tests showed that males spent less time swimming than females in the vehicle group, but not the DEX group. Finally, there was a main effect of Treatment (F(1,42)=4.215; p=0.046) and Sex (F(1,44)=6.644; p=0.0134) in climbing behavior in the FST (Figure 3C).
3.3 Prenatal DEX treatment increased TpH2 mRNA selectively in the female caudal DRN during development
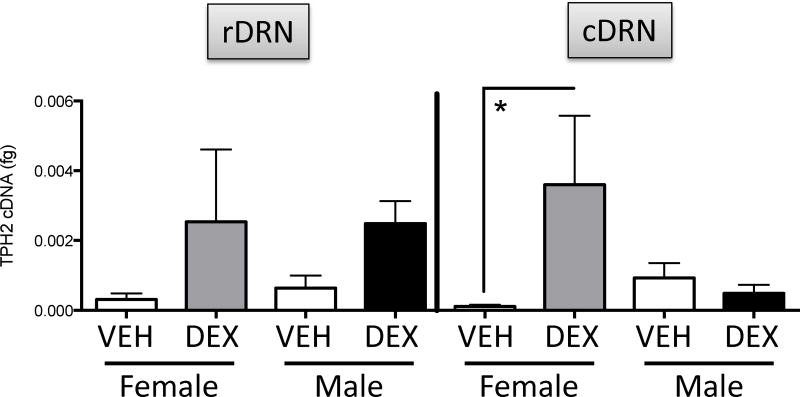
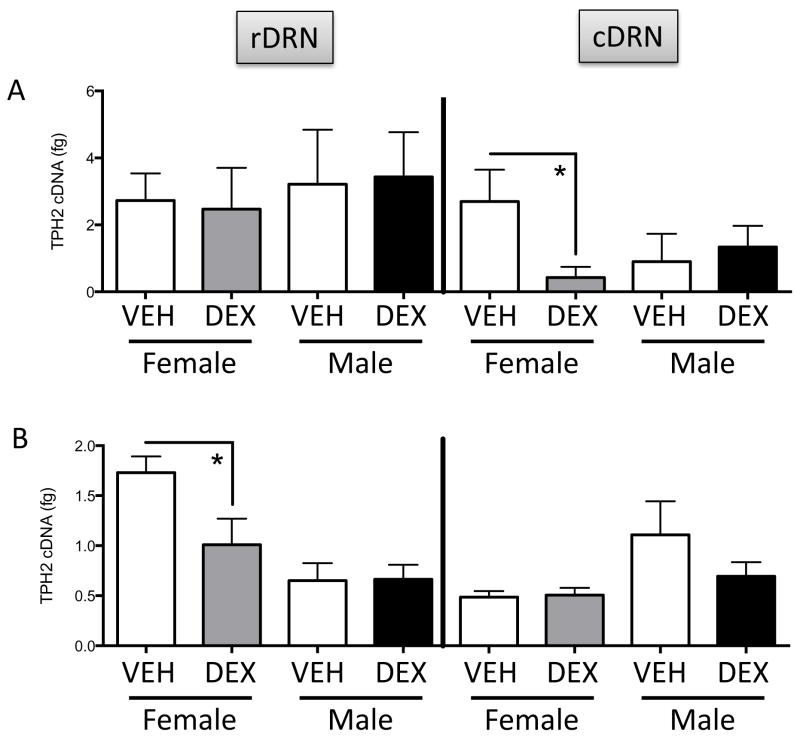
We examined TpH2 mRNA levels in rostral vs caudal subdivisions of the DRN of males and females at P0, P7, and in adulthood. There were no significant differences for Treatment or Sex in TpH2 mRNA in either rostral or caudal DRN at P0 (data not shown). There were no significant differences for Treatment or Sex in TpH2 mRNA in rostral DRN at P7 (Figure 4, left panel). In contrast, a two-way ANOVA showed a Treatment x Sex interaction (F(1,15)=5.067; p=0.0398) in TpH2 mRNA in caudal DRN. Post hoc tests showed that prenatal DEX treatment increased TpH2 mRNA selectively in female, but not male, caudal DRN at P7 (Figure 4, right panel).
Figure 4.
The effects of prenatal DEX treatment on the TPH2 mRNA in the rostral dorsal raphe nucleus (rDRN; left panel) and caudal DRN (cDRN; right panel) in P7 neonates. *Significant difference between groups, p<0.05. All values are expressed as Mean ± SEM. n=4–7 per group.
3.4 Prenatal DEX treatment decreased TpH2 mRNA selectively in the female caudal DRN in adulthood
In adulthood, there were no significant differences between Treatment or Sex groups in TpH2 mRNA in rostral DRN (Figure 5A, left panel) nor in caudal DRN (Figure 5A right panel); however, two-way ANOVA showed a Treatment x Sex interaction (F(1,16)=7.049; p=0.0173) in TpH2 mRNA in caudal DRN. Post hoc tests revealed that prenatal DEX treatment decreased TpH2 mRNA selectively in female, but not male, caudal DRN in adulthood (Figure 5A, right panel).
Figure 5.
The effects of prenatal DEX treatment on the TPH2 mRNA in the rostral dorsal raphe nucleus (rDRN; left panels) and caudal DRN (cDRN; right panels) in adults with no stress challenge (A) or with stress challenge (B). *Significant difference between groups, p<0.05. All values are expressed as Mean ± SEM. n=4–8 per group.
3.5 Prenatal DEX treatment decreased TpH2 mRNA selectively in the female rostral DRN in adult animals challenged with restraint stress
To examine whether the effects of prenatal DEX on the 5-HT system were exacerbated when the animals were challenged with a processive stressor in adulthood, we also examined TpH2 mRNA in the DRN of adult animals following 20 min of restraint stress. A two-way ANOVA showed a main effect of Sex (F(1,15)=13.06; p=0.0026) and a marginal effect of Treatment (F(1,15)=3.226; p=0.0926) and a marginal Treatment x Sex interaction (F(1,15)=3.475; p=0.0820) in TpH2 mRNA in rostral DRN (Figure 5B, left panel). Post hoc tests revealed that prenatal DEX treatment decreased TpH2 mRNA selectively in female, but not male, rostral DRN (Figure 5B, left panel). On the other hand, TpH2 mRNA levels in the caudal DRN were not significantly different between Treatment or Sex groups (Figure 5B, right panel).
Prenatal DEX treatment had no effect on restraint stress-induced plasma CORT levels in adulthood
There was no difference in the plasma CORT levels between vehicle and DEX treatment either before (baseline) or after 20 min restraint stress (data not shown). We did, however, find a significantly higher stress-induced plasma CORT level in females than males. Two-way ANOVA showed significant main effect of sex (F(1,20)=42.54; p<0.0001) and post-hoc test revealed that female plasma CORT (ng/ml) following stress is higher than that of males in both treatment groups (Female Vehicle = 335.539 ± 20.855, Female DEX = 323.790 ± 17.844, Male Vehicle = 180.731 ± 13.801, Male Vehicle = 208.895 ± 27.654), consistent with the literature that stress responsivity of the hypothalamo-pituitary-adrenal axis is higher in females than males (Brett et al., 1983, Weinstock et al., 1992, Handa et al., 1994, McCormick et al., 1995, Richardson et al., 2006).
4. Discussion
In these studies, we examined the effects of late gestation overexposure to the synthetic GC, DEX, on TpH2 gene expression in the DRN during development and in adulthood, and on anxiety- and despair- like behaviors in adulthood. We demonstrated that prenatal DEX treatment increased anxiety- and depressive- like behavior in the open field and the forced swim tests, respectively, and these effects were specific for females. In addition, we report here that the effects of prenatal DEX treatment on TpH2 mRNA were also limited to females and were age and subregion specific. At P7, prenatal DEX increased TpH2 mRNA selectively in the female caudal DRN, while in adulthood, prenatal DEX decreased TpH2 mRNA selectively in the female caudal DRN. When animals were challenged with restraint stress in adulthood, DEX history resulted in a decrease in TpH2 mRNA selectively in the rostral DRN in females.
These results add to the growing evidence that females may be more sensitive to the effects of prenatal overexposure to stress hormones. Consistent with our present findings, exposure to prenatal stress has been shown to increase anxiety- and despair- like behaviors selectively in females (Richardson et al., 2006, Zagron and Weinstock, 2006, Weinstock, 2007, Baker et al., 2008, Van den Hove et al., 2014). In contrast to these female-specific findings, others have also reported increases (Welberg and Seckl, 2001, Oliveira et al., 2006, Darnaudery and Maccari, 2008, Hossain et al., 2008, Mueller and Bale, 2008, Nagano et al., 2008, Brunton and Russell, 2010) or decreases (Welberg et al., 2001, Cannizzaro et al., 2006) in anxiety- and despair- like behaviors in adult males following prenatal GC overexposure.
These differences may be due to the nature of maternal stress, and consequently, the intensity, duration, and timing of the fetal GC exposure. For instance, while prolonged maternal restraint stress during the last week of gestation caused increased anxiety-like behaviors in adults of both sexes (Fride and Weinstock, 1988, Vallee et al., 1997), a similar stress applied only once a day increased anxiety-like behaviors selectively in females (Zagron and Weinstock, 2006). These findings implicate a lower threshold at this particular stage of development in females compared to males for the anxiogenic effects of prenatal stress and suggest that females may be more sensitive to the effects of prenatal stress or GC exposure (Weinstock, 2007, 2008) during late gestation of the rodent. It is noteworthy, however, that very low levels of stress may be beneficial, as a shorter regimen of maternal stress has anxiolytic and pro-cognitive effects on behavior (Fujioka et al., 2001, Cannizzaro et al., 2006).
In addition, there may also be an important interaction between the GC and sex hormones that lead to sex-dependent effects. In the present study, we controlled for the variations in hormone levels across the estrous cycle by evaluating females in the diestrus phase only. There may be, however, distinct effects of prenatal GC at other stages of the cycle when estradiol (E2) or progesterone is elevated. For instance, there are reported differences in anxiety-like behaviors between distinct stages of the estrous cycle (Blizard et al., 1975, Mora et al., 1996, Frye et al., 2000, Marcondes et al., 2001), which is likely due to the fluctuating levels of ovarian hormones. Estradiol has been shown to decrease anxiety-like and depressive- like behaviors (Frye and Walf, 2004, Hiroi and Neumaier, 2005, Lund et al., 2005). On the other hand, progesterone has been shown to have antiestrogenic effects on anxiety-like behaviors (Diaz-Veliz et al., 1994, Arpels, 1996, Hiroi and Neumaier, 2005, Hiroi et al., 2006b). Future studies exploring the interaction between the effects of prenatal DEX treatment and sex hormones are needed to address this important issue.
Ovarian hormones may also affect overall locomotion, which in turn can have a significant impact on the evaluated behaviors in the present study. Estradiol has been shown to increase locomotion (Diaz-Veliz et al., 1991, Morgan and Pfaff, 2002, Morgan et al., 2004). It is also well established that there is a sex difference in overall locomotor activity, in which males show lower overall activity in the open field than females (Blizard et al., 1975). This sex difference emerges during puberty and may be a result of neonatal exposure to sex hormones (Blizard et al., 1975). Given that males have lower overall locomotion than females, this lower activity level may impact the ability of the current behavioral assays to detect the effects of prenatal DEX on male center time in the open field and immobility in the forced swim test, due to a possible floor/ceiling effect, respectively. Therefore, in addition to evaluating the interaction between sex hormones and prenatal DEX, further testing with lower lighting conditions may increase the sensitivity of these assays for possible anxiogenic effects of prenatal DEX in males.
In addition, there is some evidence suggesting that the timing of the maternal GC overexposure may differentially impact the programming of the fetal HPA axis and adult behavior. Specifically, maternal stress during late, rather than early, human gestation was more likely to be associated with emotional problems in the offspring (O’Connor et al., 2002, O’Connor et al., 2003). On the other hand, early maternal anxiety at 12–22 weeks of pregnancy was associated with deficits in cognitive functioning in children (Laplante et al., 2004, Van den Bergh and Marcoen, 2004). These differential effects of GC overexposure may depend on the timing of the exposure, as the neural circuitry responsible for regulating emotion, compared to other functions, such as cognition, may undergo developmental plasticity at a distinct gestational period, thereby making that time period more susceptible to the programming effects of GC (Weinstock, 2008). Levels of GC exposure may also be an important modulating factor for the effects on the brain 5-HT system. Slotkin and colleagues have shown both increase and no change in 5-HT turnovers in the forebrain following prenatal DEX, depending on the dose of DEX treatment. For example, prenatal DEX treatment (0.05 – 0.8mg/kg at GD 17–19) had no effect on 5-HT turnover in the cortex and hippocampus, while it increased 5-HT1A, 5-HT2 and SERT binding in the cortex (Slotkin et al., 2006) and increased SERT binding in the brainstem (McGrath et al., 1997) in adulthood. In another study, a higher dose of prenatal DEX (0.2mg/kg at GD 17–19) increased 5-HT turnover in the adult midbrain, and also in striatum and cortex (Slotkin and Seidler, 2010). Taken together, these studies suggest that the level and/or timing of changes seen on the brain 5-HT activity may reflect the behavioral outcome.
The DRN 5-HT system may serve as a critical brain region responsible for the observed sex-specific behavioral findings reported in the present study. Studies have shown a decreased rate of 5-HT synthesis in women compared to men (Okazawa et al., 2000) and in depressed compared to non-depressed patients (Rosa-Neto et al., 2004). Animal studies also show that baseline firing rate of 5-HT neurons in the DRN is higher in male rats compared to ovariectomized (OVX) female rats (Klink et al., 2002, Robichaud and Debonnel, 2005), while E2 stimulates 5-HT activity, as shown by increased serotonergic neurotransmission during pregnancy and following exogenous E2 treatment (Klink et al., 2002, Robichaud and Debonnel, 2005). Together, these studies suggest that decreased serotonergic function in women, compared to men, may contribute to their vulnerability to depression and anxiety and hormone status plays an important role. Of relevance to this idea, in our current study, we found that males spent less time swimming in the forced swim test, a measure associated with enhancement of 5-HT neurotransmission (Detke et al., 1995). This is consistent with the idea that the circulating E2 levels in the cycling females may contribute to higher levels of swimming in the females compared to males in the present study. Interestingly, we also found that males, compared to females, spent more time climbing, which is a measure associated with enhancement of the noradrenergic system (Detke et al., 1995). Prenatal DEX decreased climbing in both sexes. Together, these findings suggest an intricate interaction between the serotonergic and noradrenergic systems in regulating behavior and that prenatal DEX may have distinct effects on these systems. Further studies delineating the differential functions of these systems may reveal important orchestrating effects of prenatal DEX on the brain neurotransmitter systems.
Supporting this notion, in the present study, concomitant to the female-specific behavioral effects, we also found that there were female-specific changes in the DRN TpH2 mRNA. Prenatal DEX exposure increased TpH2 mRNA expression selectively in the female caudal DRN during early development at P7, but not P0. Lack of effect of prenatal DEX at P0 compared to the increased TpH2 mRNA at P7 may imply that there is an important developmental switch or an altered developmental trajectory that occurs during the first week of life. There is a rapid brain growth that occurs in the first week of postnatal life in rodents that corresponds with the growth spurt seen in human brain development during the last trimester of pregnancy through the first few years of life (Ikonomidou et al., 1999, Bandeira et al., 2009). The first week of life in rodent development also signifies a critical period marked by a variety of changes in the expression of number of substances, including neurotransmitters, receptors, synaptic proteins, enzymes, and genes in the central, peripheral, and sensory systems (Burgin et al., 1990, Nakazawa et al., 1992, Geula et al., 1993, Hunter et al., 1995, Villacres et al., 1995, Yamagata et al., 1995, Bennett-Clarke et al., 1996, Nadarajah et al., 1997, Venero et al., 1997, Lim et al., 1999, Olavarria and Hiroi, 2003). Prenatal DEX exposure may have sex- and region-specific effects on the developmental trajectory, given that the stimulatory effects of prenatal DEX treatment on TpH2 expression are only apparent in females at P7, but not P0. Collectively, these findings suggest that the time course of the stimulatory effects of prenatal DEX treatment on TpH2 expression, at least in females, corresponds to a period of rapid brain growth in rodents. Further evaluation of different developmental time periods may reveal a unique pattern of prenatal DEX effects on TpH2 expression in males.
Previous literature, however, show that the overexposure to GC in utero decreases 5-HT activity in the brain regions that receive DRN inputs. Maternal DEX exposure decreased 5-HT turnover in the midbrain and pons-medulla in the offspring at 3 and 14 weeks of life (Muneoka et al., 1997), supporting the idea that prenatal DEX may decrease 5-HT neurotransmission. Furthermore, these authors also showed reductions of 5-HT turnover in hippocampus, neocortex and hypothalamus, all of which are regions that receive serotonergic efferents from the caudal DRN. It is possible that the DEX-induced increase in the TpH2 mRNA at P7 in our current study reflects a compensatory action resulting from decreased 5-HT tone in the forebrain during development. Alternatively, it is also possible that there are further changes in the TpH2 levels at 3 to 14 weeks of life, as the current study did not analyze these developmental time periods. Finally, the differences in dose and timing of the prenatal DEX exposure between the previous (0.05mg/kg daily at GD17–19) and current (0.4mg/kg daily at GD18–22) studies may also contribute to the discrepant findings.
As the serotonergic system plays a critical role in the regulation of stress response and the etiology of affective disorders, these changes in the 5-HT function throughout development may have significant impact on behavior in adulthood. Mueller and Bale (Mueller and Bale, 2008) demonstrated that prenatally stressed adult mice have a heightened behavioral response to a class of antidepressants targeting the 5-HT system, along with a decreased SERT binding in the hippocampus. Other studies also illustrate that the exposure to prenatal DEX or behavioral stress alters 5-HT neurotransmission and 5-HT receptor binding in various forebrain regions that receive major input from the raphe nucleus (Slotkin et al., 1996, McGrath et al., 1997, Muneoka et al., 1997, Slotkin et al., 2006, Slotkin and Seidler, 2010, Van den Hove et al., 2014). Evidence that pre-pubertal treatment with an antidepressant is able to block despair-like behavior in adulthood (Poltyrev and Weinstock, 2004, Weinstock, 2008) also is consistent with the idea that changes during development can impact behavior later in life.
Particularly, subregion-specific changes in the DRN 5-HT system have been shown to impact the regulation of anxiety-like behavior. Previous studies showed that higher levels of TpH2 mRNA in the caudal DRN were associated with decreased anxiety-like behaviors in the open field (Hiroi et al., 2006a). Overexpression of TpH2 mRNA in the caudal DRN decreased anxiety-like behaviors in the open field, while knockdown of TpH2 mRNA in the caudal DRN increased anxiety-like behaviors (Hiroi et al., 2011). Collectively, these studies suggest that TpH2 mRNA in the caudal DRN is important for the generation of anxiolytic behaviors. In the present study, the prenatal DEX resulted in decreased TpH2 mRNA selectively in adult female caudal DRN and correspondingly increased anxiety- and depressive- like behaviors, consistent with the idea that prenatal DEX may increase anxiety-like behaviors in females by decreasing the capacity of 5-HT synthesis in, and thereby output from, the caudal DRN.
The DRN subregions have distinct sensitivity to stress stimuli. Subdivisions of the DRN have differential responses to stress exposure (Molliver, 1987, Price et al., 1998, Kirby et al., 2000, Hammack et al., 2002, Lowry, 2002, Roche et al., 2003, Abrams et al., 2004) and exposure to diverse stressors differentially affects 5-HT efflux in projection regions of the DRN (Kirby et al., 1995, Adell et al., 1997, Kirby et al., 1997, Kirby and Lucki, 1998). One type of stressor can elicit a complex pattern of responses. For instance, forced swim stress increased extracellular 5-HT in the striatum, decreased it in amygdala and lateral septum, but had no effect in the hippocampus or frontal cortex (Kirby et al., 1995). Moreover, different stressors can have distinct effects on a particular forebrain region, as illustrated by increased 5-HT in the striatum and hippocampus following tail pinch but not immobilization stress (Kirby et al., 1997). Furthermore, the serotonergic neurons in the subregions of DRN have distinct projections to diverse forebrain regions involved in stress responses (van der Kooy and Hattori, 1980, Imai et al., 1986, Molliver, 1987, Vertes, 1991, Jacobs and Azmitia, 1992, Kazakov et al., 1993, Lowry, 2002). Midrostral DRN preferentially project to forebrain regions, such as caudate putamen, substantia nigra, ventral tegmental area and diverse cortical targets, and is thought to be important for motor function and behavioral arousal (Lowry, 2002). In contrast, caudal DRN efferents preferentially project to limbic regions, such as hippocampus, locus coeruleous and lateral septum, suggesting that activation of caudal DRN may be important for regulating emotional coping responses (Lowry, 2002). Therefore, depending on the nature of the stimulus, the DRN is equipped to regulate distinct aspects of stress-related behaviors.
Another factor that may modulate the effects of prenatal DEX is whether the animals have a history of stress exposure as an adult. We found that in females, prenatal DEX treatment decreased TpH2 expression in rostral DRN following restraint stress. This is in contrast to the findings in animals with no restraint stress, in which we found no effects in rostral DRN. A recent study reported that chronic variable stress reversed the prenatal stress-induced increases in the TpH2 immunoreactivity in the dorsal hippocampal regions of male and female rat adult offspring (Van den Hove et al., 2014). This study also reported that prenatal stress decreased 5-HT immunoreactivity in the DRN, specifically in males, and this decrease was reversed by chronic mild stress challenge in adulthood. At first glance, these results may appear contradictory to our findings that TpH2 mRNA decreased selectively in females exposed to prenatal DEX treatment following restraint stress challenge during adulthood. However, a closer analysis reveals at least three important differences between the previous and the current study that may explain the apparent contradictory findings. First, the previous study examined the entire DRN, whereas the current study focused on a subregion specific analysis of the DRN. As the distinct DRN subregions have differential responses to stress, subregion-specific analysis in our study may have revealed differences undetectable in females in the previous study. Second, changes found in TpH2 mRNA may not necessarily correspond in the same direction of changes seen in TpH2 and 5-HT immunoreactivity, due to compensatory mechanisms. Third, the levels of GC exposure may differ between the two studies, as the previous study examined the effects of prenatal exposure to behavioral stress applied from gestational day 14–21, while our current study examined the effects of a shorter course of prenatal DEX treatment from gestational day 18–22. As discussed earlier, dose- and time-dependent effects of prenatal GC are demonstrated. Further, prenatal stress can lead to rises in corticosterone levels and thereby affect other neural systems, such as the mineralocorticoid receptor system or plasma epinephrine from the adrenal medulla, and these may have distinct effects from that of the GC receptor system alone. This may partly explain why DEX treatment may not always recapitulate the effects of maternal stress. Therefore, it is important to evaluate and understand the impact of critical factors, such as region-specific analysis and the regimen of GC exposure.
The prenatal GC-induced effects on the 5-HT system may have adaptive values when animals encounter a stressful situation during adulthood. Van den Hove and colleagues (Van den Hove et al., 2014) found that although prenatal stress increased anxiety-like behavior selectively in the females, chronic mild stress challenge in adulthood reversed these anxiogenic behaviors. These authors also reported that the prenatal stress-induced increases of TpH2 immunoreactivity in the hippocampal regions were reversed by chronic variable stress. In the context of our current findings showing DRN subregion specific effects, it is important to consider the DRN-forebrain neurocircuitry involved in regulation of stress-related behaviors. The midrostral DRN sends its afferents to hippocampus and is thought to be involved in behavioral arousal and anxiety (Lowry, 2002). Moreover, a previous report demonstrated a positive correlation between TpH2 mRNA levels in the female rostral DRN and anxiogenic behavior in the open field (Hiroi et al., 2006a), suggesting that increased capacity for 5-HT activity from the rostral DRN is associated with anxiogenic behavior. Collectively, these studies suggest that prenatal exposure to GC results in adaptive responses to stress challenge in adulthood that result in decreased 5-HT neurotransmission from the rostral DRN to the hippocampal regions and a subsequent resilience to stress. Further studies are required to confirm this idea, as anxiety-like behavior in response to stress challenge in the context of prenatal DEX exposure was not examined in the current study.
The mechanisms underlying the effects of prenatal DEX treatment on the DRN 5-HT system is unknown. The DRN expresses GC receptors in serotonergic neurons (Harfstrand et al., 1986), which may serve as a direct mechanism underlying the DEX-induced changes of TpH2 expression. However, the effects of DEX are also likely mediated through indirect and/or epigenetic mechanisms since DEX was only delivered during prenatal life and changes occurred at later ages. Namely, early developmental programming and epigenetic mechanisms have been reported to have important biological consequences in adulthood. Through a series of studies, Seckl and Meaney (For review of these and other studies, see (Seckl and Meaney, 2004, 2006)) have elegantly shown that the epigenetic effects of early life experiences may underlie the behavioral changes seen in adulthood. Further studies evaluating these potential programming and epigenetic mechanisms are warranted.
The sex-specific nature of our findings implicates differential underlying mechanisms in which prenatal DEX impacts the brain and behavior in males vs females. A potential mechanism underlying these sex differences may be related to the differences in perinatal sex steroid hormone levels. Sex steroid hormones, including E2 and androgens, have been shown to regulate GC receptors in the brain (Ahima and Harlan, 1992, Burgess and Handa, 1993, Kerr et al., 1996, Blanco et al., 2002, Sheng et al., 2003). Sex differences in the GC receptors and GC receptor function in the brain have also been reported (McCormick et al., 1995, Goel and Bale, 2010, Zavala et al., 2011, Bourke et al., 2012, Kikuchi et al., 2015). Thus, the differential levels of sex hormones in males vs females during development may organize the brain GC system in a sex-specific manner. Sex differences in maternal care may be another potential underlying mechanism contributing to our sex-specific findings. Sex-specific maternal care is linked to sex-dependent effects of early stress on DNA methylation levels of hippocampal GC receptor genes and anxiety-like and fear behaviors (Weaver et al., 2004, Kosten et al., 2014). Further studies exploring these avenues of endocrine and epigenetic mechanisms may be key to revealing the underlying mechanisms for the sex-specific effects of prenatal GC on brain and behavior.
Although there are significant clinical implications that can be drawn from the present study, there are also important caveats that warrant discussion. As depression and anxiety are heterogeneous syndromes, it should be noted that a single animal model does not adequately recapitulate all levels of these disorders. In addition, the behavioral models utilized in the present study were originally developed to test the efficacy of anxiolytic and antidepressant drugs. Thus, it is crucial to review multiple tests to reveal the regulation of distinct aspects of the syndrome. It is also noteworthy that the present findings on the effects on 5-HT system are only a part of the much broader impact that prenatal GC exposure has on a variety of systems, including on the dopamine system (Slotkin et al., 2006), on the norepinephrine system (Muneoka et al., 1997), and on the peripheral organs such as skeletal muscles, liver, and adipose tissues (Cleasby et al., 2003, Drake et al., 2010, Carbone et al., 2012). Elucidating the impact of GC on the intricate relationship between these systems will likely be key to understanding the effects on behavior in a holistic manner.
5. Conclusions
In conclusion, the present study revealed that the effects of prenatal DEX overexposure on anxiety- and depressive- like behaviors in adulthood were selective to females. These behavioral effects were accompanied by a selective long-term decrease in TpH2 mRNA in female caudal DRN. Although there is no doubt that prenatal GC can impact multiple brain regions and various genes, this study highlights the subregion-specific changes in the DRN TpH2 mRNA as potential factors in understanding the mechanisms underlying the sex differences in the effects of prenatal stress hormone overexposure on the anxiety- and depressive-behaviors. Understanding the sex-specific behavioral effects and the underlying mechanisms of prenatal exposure to GC may lead to a potentially novel preventative treatment for anxiety and affective disorders.
Highlights.
Prenatal dexamethasone increased anxiety- and despair- like behaviors in females
Prenatal dexamethasone increased TpH2 mRNA in female caudal DRN at P7
Prenatal dexamethasone decreased TpH2 mRNA in female caudal DRN in adulthood
Stress challenged females with prenatal dexamethasone had lower TpH2 in rostral DRN
All prenatal dexamethasone effects were found selectively in females but not males
Acknowledgments
We thank Alicia Quihuis and Anthony Lacagnina for assisting in brain extraction and behavioral procedures and Laura Hinds for providing technical consultation for primer designing and qPCR. This study was supported by NIH MH082679 (RJH) and NIH MH093145 (RH).
Comprehensive list of the abbreviations used (in order of appearance)
- GC
glucocorticoids
- DEX
dexamethasone
- TpH2
tryptophan hydroxylase-2
- DRN
dorsal raphe nucleus
- 5-HT
serotonin
- P or PND
postnatal day
- GD
gestational day
- OFT
open field test
- LDT
light-dark transition box
- FST
forced swim test
- qRT-PCR
quantitative reverse-transcriptase polymerase chain reaction
- rDRN
rostral dorsal raphe nucleus
- cDRN
caudal dorsal raphe nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Annals of the New York Academy of Sciences. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology. 1997;36:735–741. doi: 10.1016/s0028-3908(97)00048-8. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE. Regulation of glucocorticoid receptor immunoreactivity in the rat hippocampus by androgenic-anabolic steroids. Brain research. 1992;585:311–314. doi: 10.1016/0006-8993(92)91226-5. [DOI] [PubMed] [Google Scholar]
- Alonso SJ, Arevalo R, Afonso D, Rodriguez M. Effects of maternal stress during pregnancy on forced swimming test behavior of the offspring. Physiology & behavior. 1991;50:511–517. doi: 10.1016/0031-9384(91)90538-y. [DOI] [PubMed] [Google Scholar]
- Arpels JC. The female brain hypoestrogenic continuum from the premenstrual syndrome to menopause. A hypothesis and review of supporting data. J Reprod Med. 1996;41:633–639. [PubMed] [Google Scholar]
- Baker S, Chebli M, Rees S, Lemarec N, Godbout R, Bielajew C. Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain research. 2008;1213:98–110. doi: 10.1016/j.brainres.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Bandeira F, Lent R, Herculano-Houzel S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14108–14113. doi: 10.1073/pnas.0804650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Chiaia NL, Rhoades RW. Thalamocortical afferents in rat transiently express high-affinity serotonin uptake sites. Brain research. 1996;733:301–306. doi: 10.1016/0006-8993(96)00791-3. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Carobrez AP. Behavioral profile of rats submitted to session 1-session 2 in the elevated plus-maze during diurnal/nocturnal phases and under different illumination conditions. Behavioural brain research. 2002;132:135–143. doi: 10.1016/s0166-4328(01)00396-5. [DOI] [PubMed] [Google Scholar]
- Blanco CE, Peltz A, Staley R, Kim F. Effects of pharmacologic androgen treatment duration on glucocorticoid receptor alpha immunoreactivity of lumbosacral motor neurons in the male rat. Neuroscience. 2002;115:941–949. doi: 10.1016/s0306-4522(02)00338-x. [DOI] [PubMed] [Google Scholar]
- Blizard DA, Lippman HR, Chen JJ. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiology & behavior. 1975;14:601–608. doi: 10.1016/0031-9384(75)90188-2. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Horm Behav. 2012;62:210–218. doi: 10.1016/j.yhbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA. Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain research bulletin. 2007;72:32–43. doi: 10.1016/j.brainresbull.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett LP, Chong GS, Coyle S, Levine S. The pituitary-adrenal response to novel stimulation and ether stress in young adult and aged rats. Neurobiology of aging. 1983;4:133–138. doi: 10.1016/0197-4580(83)90037-4. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Aetiology of anxiety and depressive disorders in an inner-city population. 1. Early adversity. Psychological medicine. 1993;23:143–154. doi: 10.1017/s0033291700038939. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO, Eales MJ. Social factors and comorbidity of depressive and anxiety disorders. Br J Psychiatry Suppl. 1996:50–57. [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. Journal of neuroendocrinology. 2010;22:258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Estrogen-induced alterations in the regulation of mineralocorticoid and glucocorticoid receptor messenger RNA expression in the female rat anterior pituitary gland and brain. Mol Cell Neurosci. 1993;4:191–198. doi: 10.1006/mcne.1993.1023. [DOI] [PubMed] [Google Scholar]
- Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC, Kelly PT. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10:1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannizzaro C, Plescia F, Martire M, Gagliano M, Cannizzaro G, Mantia G, Cannizzaro E. Single, intense prenatal stress decreases emotionality and enhances learning performance in the adolescent rat offspring: interaction with a brief, daily maternal separation. Behavioural brain research. 2006;169:128–136. doi: 10.1016/j.bbr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Zuloaga DG, Hiroi R, Foradori CD, Legare ME, Handa RJ. Prenatal dexamethasone exposure potentiates diet-induced hepatosteatosis and decreases plasma IGF-I in a sex-specific fashion. Endocrinology. 2012;153:295–306. doi: 10.1210/en.2011-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark MS, McDevitt RA, Neumaier JF. Quantitative mapping of tryptophan hydroxylase-2, 5-HT1A, 5-HT1B, and serotonin transporter expression across the anteroposterior axis of the rat dorsal and median raphe nuclei. The Journal of comparative neurology. 2006;498:611–623. doi: 10.1002/cne.21073. [DOI] [PubMed] [Google Scholar]
- Cleasby ME, Kelly PA, Walker BR, Seckl JR. Programming of rat muscle and fat metabolism by in utero overexposure to glucocorticoids. Endocrinology. 2003;144:999–1007. doi: 10.1210/en.2002-220559. [DOI] [PubMed] [Google Scholar]
- Darnaudery M, Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev. 2008;57:571–585. doi: 10.1016/j.brainresrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. The Journal of comparative neurology. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Diaz-Veliz G, Urresta F, Dussaubat N, Mora S. Effects of estradiol replacement in ovariectomized rats on conditioned avoidance responses and other behaviors. Physiology & behavior. 1991;50:61–65. doi: 10.1016/0031-9384(91)90498-d. [DOI] [PubMed] [Google Scholar]
- Diaz-Veliz G, Urresta F, Dussaubat N, Mora S. Progesterone effects on the acquisition of conditioned avoidance responses an other motoric behaviors in intact and ovariectomized rats. Psychoneuroendocrinology. 1994;19:387–394. doi: 10.1016/0306-4530(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Raubenheimer PJ, Kerrigan D, McInnes KJ, Seckl JR, Walker BR. Prenatal dexamethasone programs expression of genes in liver and adipose tissue and increased hepatic lipid accumulation but not obesity on a high-fat diet. Endocrinology. 2010;151:1581–1587. doi: 10.1210/en.2009-1088. [DOI] [PubMed] [Google Scholar]
- Fride E, Weinstock M. Prenatal stress increases anxiety related behavior and alters cerebral lateralization of dopamine activity. Life Sci. 1988;42:1059–1065. doi: 10.1016/0024-3205(88)90561-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacology, biochemistry, and behavior. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behavioral neuroscience. 2004;118:306–313. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- Frye CA, Wawrzycki J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav. 2003;44:319–326. doi: 10.1016/s0018-506x(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Fujioka A, Tan N, Chowdhury GM, Mouri H, Sakata Y, Nakamura S. Mild prenatal stress enhances learning performance in the non-adopted rat offspring. Neuroscience. 2001;103:301–307. doi: 10.1016/s0306-4522(00)00582-0. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam MM, Tokuno H, Kuo CC. Developmentally transient expression of acetylcholinesterase within cortical pyramidal neurons of the rat brain. Brain research Developmental brain research. 1993;76:23–31. doi: 10.1016/0165-3806(93)90119-u. [DOI] [PubMed] [Google Scholar]
- Goel N, Bale TL. Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress axis. Endocrinology. 2010;151:1784–1794. doi: 10.1210/en.2009-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiology & behavior. 2003;79:471–478. doi: 10.1016/s0031-9384(03)00166-5. [DOI] [PubMed] [Google Scholar]
- Hale MW, Bouwknecht JA, Spiga F, Shekhar A, Lowry CA. Exposure to high- and low-light conditions in an open-field test of anxiety increases c-Fos expression in specific subdivisions of the rat basolateral amygdaloid complex. Brain research bulletin. 2006;71:174–182. doi: 10.1016/j.brainresbull.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, et al. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Morcos PA, Clark MS, Neumaier JF. Overexpression or knockdown of rat tryptophan hyroxylase-2 has opposing effects on anxiety behavior in an estrogen-dependent manner. Neuroscience. 2011;176:120–131. doi: 10.1016/j.neuroscience.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat dorsal raphe nucleus: association between gene expression and anxiety behavior in the open field. Biological psychiatry. 2006a;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biological psychiatry. 2006b;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF. Differential effects of ovarian steroids on anxiety versus fear as measured by open field test and fear-potentiated startle. Behavioural brain research Electronic publication. 2005 doi: 10.1016/j.bbr.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF. Differential effects of ovarian steroids on anxiety versus fear as measured by open field test and fear-potentiated startle. Behavioural brain research. 2006a;166:93–100. doi: 10.1016/j.bbr.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF. Estrogen selectively decreases 5-HT1B mRNA in distinct subregions of rat dorsal raphe nucleus: Inverse association between gene expression and anxiety behavior in the open field. Annual Northwest Chapter Meeting of the Society for Neuroscience. 2006b doi: 10.1016/j.neuroscience.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain A, Hajman K, Charitidi K, Erhardt S, Zimmermann U, Knipper M, Canlon B. Prenatal dexamethasone impairs behavior and the activation of the BDNF exon IV promoter in the paraventricular nucleus in adult offspring. Endocrinology. 2008;149:6356–6365. doi: 10.1210/en.2008-0388. [DOI] [PubMed] [Google Scholar]
- Hunter SE, Seibenhener ML, Wooten MW. Atypical zeta-protein kinase c displays a unique developmental expression pattern in rat brain. Brain research Developmental brain research. 1995;85:239–248. doi: 10.1016/0165-3806(94)00219-p. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. The Journal of comparative neurology. 1986;243:363–380. doi: 10.1002/cne.902430307. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Kazakov VN, Kravtsov P, Krakhotkina ED, Maisky VA. Sources of cortical, hypothalamic and spinal serotonergic projections: topical organization within the nucleus raphe dorsalis. Neuroscience. 1993;56:157–164. doi: 10.1016/0306-4522(93)90570-6. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Beck SG, Handa RJ. Androgens selectively modulate C-fos messenger RNA induction in the rat hippocampus following novelty. Neuroscience. 1996;74:757–766. doi: 10.1016/0306-4522(96)00219-9. [DOI] [PubMed] [Google Scholar]
- Keshet GI, Weinstock M. Maternal naltrexone prevents morphological and behavioral alterations induced in rats by prenatal stress. Pharmacology, biochemistry, and behavior. 1995;50:413–419. doi: 10.1016/0091-3057(94)00289-u. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Hosono K, Yamashita J, Kawabata Y, Okubo K. Glucocorticoid receptor exhibits sexually dimorphic expression in the medaka brain. Gen Comp Endocrinol. 2015;223:47–53. doi: 10.1016/j.ygcen.2015.09.031. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Allen AR, Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain research. 1995;682:189–196. doi: 10.1016/0006-8993(95)00349-u. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain research. 1997;760:218–230. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Lucki I. The effect of repeated exposure to forced swimming on extracellular levels of 5-hydroxytryptamine in the rat. Stress. 1998;2:251–263. doi: 10.3109/10253899809167289. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Klink R, Robichaud M, Debonnel G. Gender and gonadal status modulation of dorsal raphe nucleus serotonergic neurons. Part I: effects of gender and pregnancy. Neuropharmacology. 2002;43:1119–1128. doi: 10.1016/s0028-3908(02)00219-8. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Huang W, Nielsen DA. Sex and litter effects on anxiety and DNA methylation levels of stress and neurotrophin genes in adolescent rats. Dev Psychobiol. 2014;56:392–406. doi: 10.1002/dev.21106. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier JF, Zelazo PR, King S. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatric research. 2004;56:400–410. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- Lim S, Naisbitt S, Yoon J, Hwang JI, Suh PG, Sheng M, Kim E. Characterization of the Shank family of synaptic proteins. Multiple genes, alternative splicing, and differential expression in brain and development. J Biol Chem. 1999;274:29510–29518. doi: 10.1074/jbc.274.41.29510. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. Journal of neuroendocrinology. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biological psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behavioral neuroscience. 1995;109:404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiology & behavior. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain research. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain research Developmental brain research. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- McGrath KE, Seidler FJ, Slotkin TA. Convergent control of serotonin transporter expression by glucocorticoids and cocaine in fetal and neonatal rat brain. Brain research Developmental brain research. 1997;104:209–213. doi: 10.1016/s0165-3806(97)00144-2. [DOI] [PubMed] [Google Scholar]
- Molliver ME. Serotonergic neuronal systems: what their anatomic organization tells us about function. J Clin Psychopharmacol. 1987;7:3S–23S. [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Estrogen’s effects on activity, anxiety, and fear in two mouse strains. Behavioural brain research. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Schulkin J, Pfaff DW. Estrogens and non-reproductive behaviors related to activity and fear. Neuroscience and biobehavioral reviews. 2004;28:55–63. doi: 10.1016/j.neubiorev.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneoka K, Mikuni M, Ogawa T, Kitera K, Kamei K, Takigawa M, Takahashi K. Prenatal dexamethasone exposure alters brain monoamine metabolism and adrenocortical response in rat offspring. Am J Physiol. 1997;273:R1669–1675. doi: 10.1152/ajpregu.1997.273.5.R1669. [DOI] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Jones AM, Evans WH, Parnavelas JG. Differential expression of connexins during neocortical development and neuronal circuit formation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:3096–3111. doi: 10.1523/JNEUROSCI.17-09-03096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Ozawa H, Suzuki H. Prenatal dexamethasone exposure affects anxiety-like behaviour and neuroendocrine systems in an age-dependent manner. Neurosci Res. 2008;60:364–371. doi: 10.1016/j.neures.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Nakazawa M, Koh T, Kani K, Maeda T. Transient patterns of serotonergic innervation in the rat visual cortex: normal development and effects of neonatal enucleation. Brain research Developmental brain research. 1992;66:77–90. doi: 10.1016/0165-3806(92)90143-k. [DOI] [PubMed] [Google Scholar]
- Nash MW, Sugden K, Huezo-Diaz P, Williamson R, Sterne A, Purcell S, Sham PC, Craig IW. Association analysis of monoamine genes with measures of depression and anxiety in a selected community sample of siblings. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2005;135:33–37. doi: 10.1002/ajmg.b.30063. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Glover V, Team AS. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. Journal of child psychology and psychiatry, and allied disciplines. 2003;44:1025–1036. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- Okazawa H, Leyton M, Benkelfat C, Mzengeza S, Diksic M. Statistical mapping analysis of serotonin synthesis images generated in healthy volunteers using positron-emission tomography and alpha-[11C]methyl-L-tryptophan. J Psychiatry Neurosci. 2000;25:359–370. [PMC free article] [PubMed] [Google Scholar]
- Olavarria JF, Hiroi R. Retinal influences specify cortico-cortical maps by postnatal day six in rats and mice. The Journal of comparative neurology. 2003;459:156–172. doi: 10.1002/cne.10615. [DOI] [PubMed] [Google Scholar]
- Oliveira M, Bessa JM, Mesquita A, Tavares H, Carvalho A, Silva R, Pego JM, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. Induction of a hyperanxious state by antenatal dexamethasone: a case for less detrimental natural corticosteroids. Biological psychiatry. 2006;59:844–852. doi: 10.1016/j.biopsych.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neuroscience and biobehavioral reviews. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press, INC; 1986. [Google Scholar]
- Pigott TA. Anxiety disorders in women. Psychiatr Clin North Am. 2003;26:621–672. vi–vii. doi: 10.1016/s0193-953x(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Poltyrev T, Weinstock M. Gender difference in the prevention of hyperanxiety in adult prenatally stressed rats by chronic treatment with amitriptyline. Psychopharmacology. 2004;171:270–276. doi: 10.1007/s00213-003-1577-9. [DOI] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropinreleasing factor on brain serotonergic activity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European journal of pharmacology. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depression and anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- Robichaud M, Debonnel G. Oestrogen and testosterone modulate the firing activity of dorsal raphe nucleus serotonergic neurones in both male and female rats. Journal of neuroendocrinology. 2005;17:179–185. doi: 10.1111/j.1365-2826.2005.01292.x. [DOI] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-Neto P, Diksic M, Okazawa H, Leyton M, Ghadirian N, Mzengeza S, Nakai A, Debonnel G, Blier P, Benkelfat C. Measurement of brain regional alpha-[11C]methyl-L-tryptophan trapping as a measure of serotonin synthesis in medication-free patients with major depression. Archives of general psychiatry. 2004;61:556–563. doi: 10.1001/archpsyc.61.6.556. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. Glucocorticoid programming. Annals of the New York Academy of Sciences. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. Glucocorticoid “programming” and PTSD risk. Annals of the New York Academy of Sciences. 2006;1071:351–378. doi: 10.1196/annals.1364.027. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Yanai A, Fujinaga R, Kawano J, Tanaka M, Watanabe Y, Shinoda K. Gonadal and adrenal effects on the glucocorticoid receptor in the rat hippocampus, with special reference to regulation by estrogen from an immunohistochemical view-point. Neurosci Res. 2003;46:205–218. doi: 10.1016/s0168-0102(03)00056-7. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Barnes GA, McCook EC, Seidler FJ. Programming of brainstem serotonin transporter development by prenatal glucocorticoids. Brain research Developmental brain research. 1996;93:155–161. doi: 10.1016/0165-3806(96)00027-2. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Kreider ML, Tate CA, Seidler FJ. Critical prenatal and postnatal periods for persistent effects of dexamethasone on serotonergic and dopaminergic systems. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:904–911. doi: 10.1038/sj.npp.1300892. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Mimicking maternal smoking and pharmacotherapy of preterm labor: interactions of fetal nicotine and dexamethasone on serotonin and dopamine synaptic function in adolescence and adulthood. Brain research bulletin. 2010;82:124–134. doi: 10.1016/j.brainresbull.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. Journal of affective disorders. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- Sun HS, Tsai HW, Ko HC, Chang FM, Yeh TL. Association of tryptophan hydroxylase gene polymorphism with depression, anxiety and comorbid depression and anxiety in a population-based sample of postpartum Taiwanese women. Genes Brain Behav. 2004;3:328–336. doi: 10.1111/j.1601-183X.2004.00085.x. [DOI] [PubMed] [Google Scholar]
- Tobe I, Ishida Y, Tanaka M, Endoh H, Fujioka T, Nakamura S. Effects of repeated maternal stress on FOS expression in the hypothalamic paraventricular nucleus of fetal rats. Neuroscience. 2005;134:387–395. doi: 10.1016/j.neuroscience.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:2626–2636. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 2004;75:1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Van den Hove DL, Leibold NK, Strackx E, Martinez-Claros M, Lesch KP, Steinbusch HW, Schruers KR, Prickaerts J. Prenatal stress and subsequent exposure to chronic mild stress in rats; interdependent effects on emotional behavior and the serotonergic system. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2014;24:595–607. doi: 10.1016/j.euroneuro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Hattori T. Dorsal raphe cells with collateral projections to the caudate-putamen and substantia nigra: a fluorescent retrograde double labeling study in the rat. Brain research. 1980;186:1–7. doi: 10.1016/0006-8993(80)90250-4. [DOI] [PubMed] [Google Scholar]
- Venero JL, Vizuete ML, Machado A, Cano J. Developmental expression of 5-HT7 receptor mRNA in rat brain visual structures after neonatal enucleation. Neuroreport. 1997;8:1531–1535. doi: 10.1097/00001756-199704140-00042. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. The Journal of comparative neurology. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Villacres EC, Wu Z, Hua W, Nielsen MD, Watters JJ, Yan C, Beavo J, Storm DR. Developmentally expressed Ca(2+)-sensitive adenylyl cyclase activity is 33 disrupted in the brains of type I adenylyl cyclase mutant mice. J Biol Chem. 1995;270:14352–14357. doi: 10.1074/jbc.270.24.14352. [DOI] [PubMed] [Google Scholar]
- Watson JB, Mednick SA, Huttunen M, Wang X. Prenatal teratogens and the development of adult mental illness. Dev Psychopathol. 1999;11:457–466. doi: 10.1017/s0954579499002151. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res. 2007;32:1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neuroscience and biobehavioral reviews. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain research. 1992;595:195–200. doi: 10.1016/0006-8993(92)91049-k. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150:1817–1825. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. Journal of neuroendocrinology. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Herman JP, Sanes JR. Lamina-specific expression of adhesion molecules in developing chick optic tectum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:4556–4571. doi: 10.1523/JNEUROSCI.15-06-04556.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You JS, Hu SY, Chen B, Zhang HG. Serotonin transporter and tryptophan hydroxylase gene polymorphisms in Chinese patients with generalized anxiety disorder. Psychiatric genetics. 2005;15:7–11. doi: 10.1097/00041444-200503000-00002. [DOI] [PubMed] [Google Scholar]
- Zagron G, Weinstock M. Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behavioural brain research. 2006;175:323–328. doi: 10.1016/j.bbr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Zavala JK, Fernandez AA, Gosselink KL. Female responses to acute and repeated restraint stress differ from those in males. Physiology & behavior. 2011;104:215–221. doi: 10.1016/j.physbeh.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, Schwartz DA, Krishnan KR, Caron MG. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Carbone DL, Hiroi R, Chong DL, Handa RJ. Dexamethasone induces apoptosis in the developing rat amygdala in an age-, region-, and sex-specific manner. Neuroscience. 2011;199:535–547. doi: 10.1016/j.neuroscience.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Carbone DL, Quihuis A, Hiroi R, Chong DL, Handa RJ. Perinatal dexamethasone-induced alterations in apoptosis within the hippocampus and paraventricular nucleus of the hypothalamus are influenced by age and sex. Journal of neuroscience research. 2012;90:1403–1412. doi: 10.1002/jnr.23026. [DOI] [PubMed] [Google Scholar]