Summary
Ethinylestradiol (EE) is an endocrine disruptor (ED) which acts as an oestrogen agonist; this compound is known as an oral contraceptive. Male and female rodents exposed to EE during critical time points of development, such as in the prenatal period, show alterations in their reproductive tract during adulthood. Few studies have placed an emphasis on the effects of EE during ageing. Thus, this study had as it's objective the analysis of the morphological and immunohistochemical effects of exposure to EE in the prenatal period on ventral male prostate and female prostate of gerbils (Meriones unguiculatus) during ageing. The animals were exposed to EE (15 μg/kg/day) during the 18–22th days of prenatal life (EE/PRE group), and the analyses were performed when the male and female reached 12 months of age. Our results showed an increase in the development of prostatic intraepithelial neoplasia (PIN), which was observed in the male and female prostate of EE/PRE groups. Immunohistochemistry showed a rise in prostatic epithelial and basal cells immunoreactivity, respectively, and to AR and p63 in the male EE/PRE. There were alterations in the morphological pattern of the prostatic glands and increase in predisposition to emergence of prostatic lesions of both sexes during ageing. Despite male and female having been exposed to the same doses of EE, the “exposure to EE promoted modifications” more accentuated in the male prostate. Thus the male gland is more sensitive to the action of this synthetic oestrogen than the female prostate.
Keywords: ageing, ethinylestradiol, female prostate, male prostate, prenatal period
Endocrine disruptors (EDs) are exogenous chemical compounds cable of disrupting the function of the endocrine system, thus affecting the reproductive systems of animals and humans (Diamanti‐Kandarakis et al. 2009; Casals‐Casas & Desvergne 2011). Many EDs have the potential to mimic, antagonize or alter the levels of endogenous steroid hormones (androgens and oestrogens) (Frye et al. 2012). Worldwide, people are exposed to ED, which are found in products such as bisphenol A (BPA) which is present in polycarbonate plastic, glycidyl methacrylate for dental use and ethinylestradiol (EE) used in medicines (Soto & Sonnenschein 2010; Frye et al. 2012).
Exposure to ED during development alters the male and female reproductive systems, resulting in changes in the neuroendocrine system, reproductive behaviour (Frye et al. 2012) and susceptibility to prostate cancer (Prins 2008). The prenatal, neonatal and pubertal period are vulnerable to the action of ED, because during these phases, the levels of steroid hormones are essential for the development of male and female reproductive organs (Maffini et al. 2006; Ryan et al. 2010). The hormonal dysregulation caused by exposure to ED during critical stages of development increases the risks of the prostate diseases in adulthood, such as cancer (Prins et al. 2007a,b; Prins 2008; Hu et al. 2012).
The prostate is a gland that depends on the action of steroid hormones for its development, and these effects begin in the prenatal period (Cunha et al. 2004). Exposure to ethinylestradiol, a synthetic oestrogen in contraceptive pills, during the prenatal period, increases susceptibility to the emergence of preneoplastic lesions in the prostate in male and female gerbils in adulthood (Perez et al. 2011, 2012).
The female prostate of adult gerbil (Meriones unguiculatus), an experimental model utilized in our research group, presents morphofunctional similarity to the human gland. It is located para‐urethrally, (Santos et al., 2003; Santos & Taboga 2006). It consists of ducts and alveoli surrounded by a development stroma (Santos et al., 2003). The alveolar region is coated by secretory cells, which produce a secretion in which acid phosphatase is abundant (Custódio et al. 2004). Studies show that female gerbil prostate is sensitive to actions of steroid hormones and undergoes morphological changes, similar to the male gerbil prostate (Perez et al. 2011, 2012; Biancardi et al. 2012, 2014).
Millions of women consume contraceptive pills daily. Some women may not know they are pregnant and may continue to take birth control during the first months of pregnancy (Li et al. 1995). According to Thayer et al. (2001), exposure to low doses of ethinylestradiol (0.002 mg/kg/day; substantially less than the 0.5 mg/kg found in contraceptive pills consumed by women) during pregnancy caused rodents to have decreased daily sperm production during adolescence and increased prostate size in males in the neonatal period. ED exposure during pregnancy, even at low doses, promotes changes in the prostate of male and female rodents in the neonatal phase, and such modifications remain into adulthood (Perez et al. 2011, 2012).
Most studies involving ED emphasize the consequences of intrauterine exposure on the male and female prostate of rodents during the neonatal and adult phases (Prins et al. 2001; Biancardi et al. 2012; Perez et al. 2012). However, few studies have evaluated the effects of this exposure in senile individuals (Prins et al. 2007a,b). Senescence has been characterized as reproductive ageing, and this, in turn, is associated with the loss of reproductive capacity and deregulation of the hypothalamic–pituitary–gonadal axis. This process may be regulated by genetic and environmental factors (Basaria 2013). In rodents there is a decrease in the feedback of gonadal steroid hormones on the hypothalamus and pituitary gland during the senile period (Kermath & Gore 2012; Walker et al. 2013). This hormonal deregulation is a major cause for the emergence of prostatic diseases, especially during ageing (Wigle et al. 2008). Therefore, it is of fundamental importance to study the effects of exposure to ED during critical phases of development on the prostate gland of rodents during ageing. Thus, this study aimed to evaluate the morphological effects of prenatal exposure to ethinylestradiol on the ventral male prostate and female prostate of gerbils during ageing.
Material and method
Animals and experimental design
The male and females gerbils (Meriones unguiculatus) used in this experiment were maintained in a biotherium in the IBILCE/UNESP (São José do Rio Preto‐SP) in polyethylene boxes with wood shavings substrate, under controlled light conditions, and at an average temperature 23°C. They were supplied filtered water in glass bottles and food ad libitum (composition: 23% protein, 12% minerals, 5% fibre and 4% total lipids). Animal handling and experiments were in accordance with ethical principles of animal research and were approved by Committee for Animal Research (CEEA Protocol n° 020/09) of UNESP.
In this experiment, we used eight adult virgin female gerbils (90–120 days), and each was maintained with a male of the same age for the formation of different families and separated into two different groups. As shown in Figure 1, in the EE/PRE group, four pregnant females received 15 μg/kg/day ethinylestradiol (EE, 17α‐ethinylestradiol; Sigma, St. Louis, MO, USA) diluted in 100 μl of Nujol® mineral oil (CAS 8020‐83‐5; Sigma‐Aldrich) by gavage. The dose of EE used in the experiment is similar to the dose found in the oral contraceptive (Thayer et al. 2001). Pregnant females in the EE/PRE groups received ethinylestradiol from the 18th to the 22nd day, taking into account that the gestational time of the gerbil is 26 days. The treatment was performed during the prostatic morphogenesis of the gerbils, which starts between the 20th and 21st days of the prenatal period (Sanches et al. 2014). In the control group, four pregnant females received no treatment. This study did not use vehicle controls because previous studies by our research group demonstrated the absence of significance between the vehicle group and intact animals (Scarano et al. 2006, 2008; Santos et al. 2007).
Figure 1.
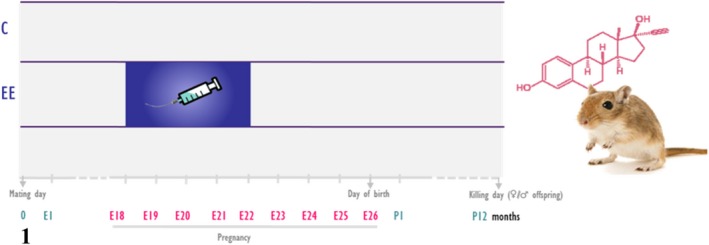
Schematic of the experimental design. Males and females in EE/PRE group received 15 μg/kg/day of ethinylestradiol (EE) during the embryonic period (E). The animals were killed at 12 months of postnatal life (P). In the control group (C), the pregnant females were not treated.
This experiment utilized one male and one female offspring of each pregnant female (n = 8) in the experimental groups; the rest of the offspring we utilized in the experimental design of another study. A total of eight males from the control group and eight males from the EE/PRE group, .i.e. the same number of animals were utilized for the experimental groups of female. Thus, five males and five females of each experimental group were utilized for optical microscopy and serological analyses and three males and three females for ultrastructural analysis.
Eight males and eight females from each experimental group were euthanized at 12 months for blood collection, by anaesthesia in CO2 followed by decapitation. After death, the male and female prostate complexes were removed and weighed, along with the testis, the ovary and the adrenal gland. The female offspring of the experimental group were cycled and killed during the proestrus phase when they were 12 months in age (Nishino & Totsukawa 1996).
Ethical approval statement
UNESP/IBILCE Ethical Committee for Animal Research (CEEA) hereby certify that the scientific investigation entitled ‘Evaluation of estrogenic exposure in utero and pubertal development as a predisposing factor of prostatic lesions in male and female gerbils senile’ (protocol n°. 020/09 CEEA), under Sebastiao Roberto Taboga's responsibility, is performed in accordance with the Ethical Principles in Animal Research adopted by Brazilian College of Animal Experimentation (COBEA) and was approved by the Committee of this Institute on December 10th, 2009.
Biometric analysis
Before collection and weighing of the biological materials, males and females of the experimental groups (12 months of age) were submitted to measurement of the anogenital distance (DAG) using a calliper, King Tools Digital Caliper (0–300 mm).
Morphological, morphometric and histophathological analyses
Male (n = 5) and female (n = 5) prostates from the experimental groups were fixed in 4% buffered paraformol and methacarn (at 1:3:6 of acetic acid, chloroform and methanol, respectively), dehydrated in ethanol, clarified with xylene and then embedded in Paraplast (Histosec, Dermstadt, Germany). The organs were sectioned at 5 μm and stained with haematoxylin and eosin (HE) for morphological, morphometric and histopathological analysis of the prostate (Behmer et al. 1976). In addition, we used the Gömöri's reticulin reaction for the analysis of stromal reticular and collagen fibres (Gӧmӧri 1937). For morphological analysis, 25 sections of each experimental group were utilized.
For morphometric analysis, we collected 200 data points of five sections of each prostate of the experimental groups (n = 5) and these were used to measure the thickness (μm) of the epithelial height of the muscular layer of ventral male prostate and female prostate. The analysis was performed using a photomicroscope Olympus BX60, and the images were captured for photographic documentation and morphometric measurement using the image‐pro plus© average cybernetics software (Silver Spring, MA, USA).
Histopathological analysis of the ventral male prostate and female prostate from the control and EE/PRE groups (n = 5) was carried out through the multiplicity of prostatic intraepithelial neoplasia (PIN). The identification of these lesions was performed according to the classification of Shappell et al. (2004). The multiplicity was evaluated by determining the number of lesions throughout the section using five animals from each group, analysing 25 sections of the each group in Olympus optical microscope (Olympus, Hamburg, Germany).
The histological images for morphological, morphometric and histopathological analyses were captured using a microscope Zeiss Jenaval (Jena, Germany), and scanned using the image‐pro plus software version 4.5 for Windows (Media Cybernetics, Inc., Silver Spring, MA, USA).
Immunohistochemistry
The ventral male prostates and female prostates from the experimental groups were fixed in 4% buffered paraformol or methacarn and submitted to histological processing by immersion in Paraplast (Histosec, Merck), followed by sectioning (5 μm). The sections were subjected to immunohistochemical detection of several antigens: proliferating cell nuclear antigen (PCNA; mouse monoclonal IgG2a, P‐10: SC 56; Santa Cruz Biotechnology, Paso Robles, CA, USA) (Custódio et al. 2010; p63 protein (basal cell marker; mouse monoclonal IgG2a, 4A4: sc‐8431; Santa Cruz Biotechnology) (Biancardi et al. 2012), androgen receptor (AR; rabbit polyclonal IgG, N‐20: sc‐816; Santa Cruz Biotechnology) (Santos et al. 2006), oestrogen receptor‐alpha (ERα; rabbit polyclonal IgG, H‐184: sc‐7207; Santa Cruz Biotechnology) and oestrogen receptor‐beta (ERβ; rabbit polyclonal IgG, H‐150: sc‐8974; Santa Cruz Biotechnology) (Rochel‐Maia et al. 2013). Antigenic detection was performed in citrate buffer (pH 6.0) at a high temperature (98°C) for 45 min, followed by three 5‐min washes in phosphate‐buffered saline (PBS). Peroxidase was blocked against using 12% H2O2 in methanol for 20 min (high peroxidase blocking because the antibodies are not specific for gerbils). Then the sections were blocked with non‐specific protein (milk powder diluted in PBS) for 30 min and then incubated with antibodies diluted in 1% bovine serum albumin (BSA) in PBS overnight (4°C; 1:100 PCNA and p63; 1:75 AR; 1:50 ERα and ERβ) and incubated with polymer (Novolink, Novocastra) at 37°C for 45 min, then visualized with diaminobenzidine (DAB; Sigma) and counterstained with Harris haematoxylin.
For the reactions for PCNA, p63 and AR, we determined the number of positive nuclei in prostatic epithelial cells in the experimental groups using fields of microscopic images (400×; Olympus BX60) of the prostate from each group (n = 3; 2000 cells total). The values of positive nuclei were divided by the total number of cells counted in each field and expressed as the percentage (%).
Immunofluorescence
Prostates from the control and EE/PRE groups were fixed in methacarn, subjected to histological processing, embedded in Paraplast (Histosec, Merk) and sectioned into 5‐μm slices. The sections were subjected to immunofluorescence imaging in order to detect smooth muscle α‐actin. Antigenic detection was performed in citrate buffer (pH 6.0) at a high temperature (98°C) for 1 h, followed by three 5‐min washes with PBS. Non‐specific binding was blocked by incubation in 5% BSA in PBS for 1 h. Sections were incubated with primary α‐actin antibody (1:100; mouse monoclonal IgG2a 1C4: sc‐32251; Santa Cruz Biotechnology) diluted in 1% BSA in PBS overnight at 4°C (Biancardi et al. 2014). After this procedure, the sections were incubated with secondary antibody (1:200 goat anti‐mouse IgG‐FITC: sc‐2010; Santa Cruz Biotechnology) for 2 h in the dark. After that, we used a mounting means with DAPI (Ultra CruzTM Mounting Medium: sc‐24941, with DAPI fluorescence; Santa Cruz Biotechnology). The images were analysed using a fluorescence microscope Zeiss Axio Imager M2.
Serum hormonal analysis
The blood of male and female senile gerbils from the experimental groups was collected at the time of decapitation. The serum was centrifuged (3000 rpm for 20 min) and stored at −80°C until analysis. Hormonal dosages were performed in duplicate by ELISA and using kits with high sensitivity (testosterone and 17β‐estradiol, Cayman Chemical Company, MI, USA) following the manufacturer's instructions. The detection limits for testosterone and 17β‐estradiol were 6 and 19 pg/ml respectively. The readings were performed using the lector SpectraMax Plus 384, at 405 ηm (Molecular Devices, Sunnyvale, CA, USA).
Transmission Electronic Microscopy
After removal, the prostate glands (n = 3) were cut into small fragments and fixed for 24 h in 3% glutaraldehyde solution, 0.25% tannic acid and 0.54% glucose in Milloning's buffer pH 7.3 (Cotta‐Pereira et al. 1976). After being washed, the fragments were postfixed with 1% osmium tetroxide for 2 h, washed again, dehydrated in increasing concentrations of acetone and then embedded in Araldite resin. Subsequently, the fragments were cut into ultrathin sections (50 nm) using diamond knives and then contrasted with 2% uranyl acetate for 20 min and lead citrate for 6 min. Three sections of male and female prostates of each experimental group were observed and evaluated using the LEO‐Zeiss 906 transmission electronic microscope (Zeiss, Cambridge, UK).
Statistical analysis
For statistical analysis of the biometric, morphometric, histopathological frequency of immunoreactivity, serological data utilized the spreadsheets and graphics of graphpad instat (GraphPad Software, Inc., La Jolla, CA, USA). To prove the significance of the parametric results, we used the Tukey test and nonparametric data, and the Mann–Whitney U test. The level of significance was 5% (P ≥ 0.05) and was expressed as the mean ± SD.
Results
Table 1 shows the biometric data from the experimental groups of the male and female senile gerbils. These data showed no significant differences in the experimental groups of male and female senile gerbils. The serological data showed no significant differences in the levels of testosterone and estradiol between the males in the control and EE/PRE groups (Figure 2a and b). However, the EE/PRE female group showed a significant increase in testosterone and estradiol levels when compared to the control group (Figure 2a and b).
Table 1.
Biometric data of the experimental male and female groups of senile gerbils
| Parameters | Experimental groups | |
|---|---|---|
| Control | EE/PRE | |
| Male | ||
| Body weight (g) | 90.44 ± 14.38 | 87.11 ± 10.30 |
| Prostatic complex (g) | 1.06 ± 0.23 | 1.06 ± 0.11 |
| Ventral prostate (g) | 0.024 ± 0.006 | 0.023 ± 0.006 |
| Relative prostate of ventral prostate (×10−3) | 0.26 ± 0.07 | 0.26 ± 0.04 |
| Right testis (g) | 0.65 ± 0.06 | 0.62 ± 0.06 |
| Left testis (g) | 0.64 ± 0.05 | 0.61 ± 0.05 |
| Adrenal (g) | 0.05 ± 0.01 | 0.05 ± 0.01 |
| Anogenital distance (AGD) (mm) | 12.50 ± 1.50 | 12.31 ± 1.40 |
| Female | ||
| Body weight (g) | 66.20 ± 6.54 | 72.20 ± 7.00 |
| Urethra and prostate (g) | 0.03 ± 0.01 | 0.02 ± 0.01 |
| Relative prostate (×10−3) | 0.44 ± 0.22 | 0.34 ± 0.08 |
| Ovary (g) | 0.04 ± 0.01 | 0.07 ± 0.02 |
| Adrenal (g) | 0.04 ± 0.01 | 0.04 ± 0.01 |
| Anogenital distance (AGD) (mm) | 3.92 ± 0.55 | 4.21 ± 0.40 |
Values are expressed as the mean ± SD. EE/PRE: Senile male and female exposed to ethinylestradiol during the prenatal period (n = 5). The significant difference between the groups was considered with P ≤ 0.05.
Figure 2.
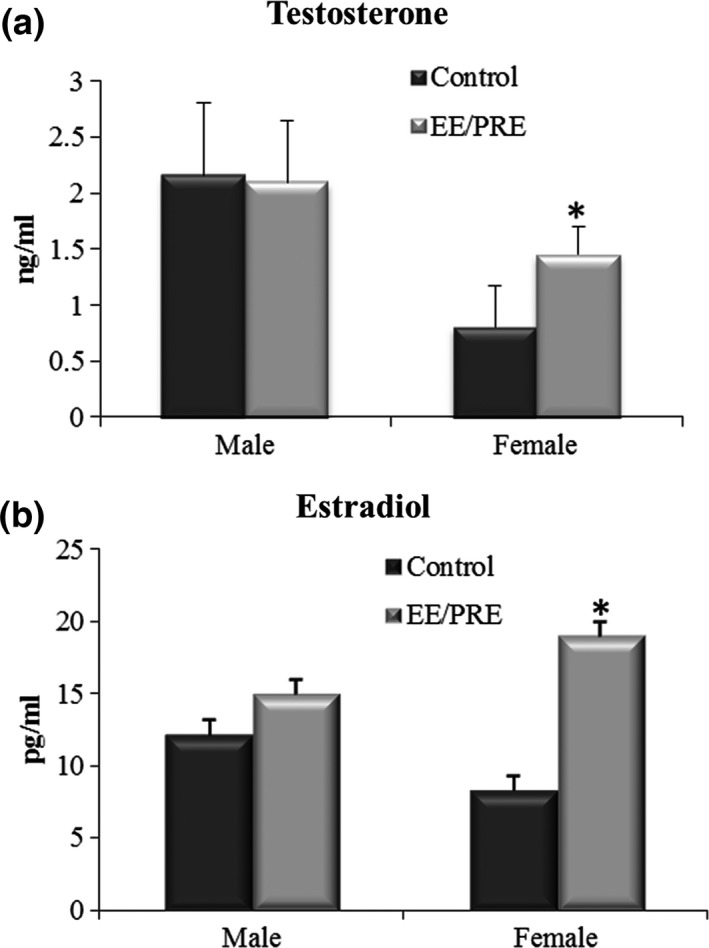
Serological data for the male and female of senile gerbils from the experimental groups. (a) Testosterone levels (ng/ml). (b) Estradiol levels (pg/ml). Values expressed as mean ± SD (n = 5). *Significant difference between the groups with P ≤ 0.05.
During analysis, we noted no anatomical differences between the testes, adrenal and ventral prostate of the EE/PRE male group when compared to the control group. However, histologically, (Figure 3) the ventral prostate of EE/PRE male group showed the presence of acinar atrophy within the smallest lumen area (Figure 3e). Furthermore, we found the presence of stratified epithelium and an increase in the stromal density, which are characteristics of benign prostatic hyperplasia (BPH; Figure 3e). The epithelial height and the thickness of the muscle layer that surrounds the prostatic acini decreased significantly in EE/PRE group males (10.52 ± 3.10; 7.93 ± 4.00 μm) when compared to control group males (16.30 ± 3.80; 8.80 ± 3.53 μm; Figure 3b, G and 3l).
Figure 3.
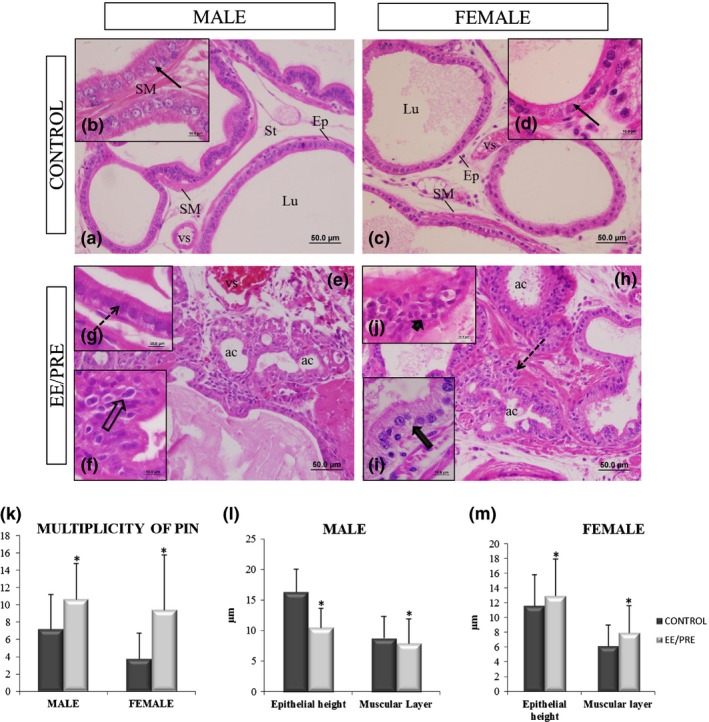
Histological sections of the ventral male prostate and female prostate from senile gerbils stained by haematoxylin and eosin (HE). Control group: (a, c) Prostatic acini surrounded by a simple prismatic epithelium (Ep) with the presence of luminal region (Lu), smooth muscle layer (SM) and stroma (St) with vessels (vs). (b, d) Detail of the epithelial height (arrows). EE/PRE group: (e, h) Acinar atrophy (ac). (f, j) Presence of prostatic intraepithelial neoplasia and atypical nuclei (broad arrow and arrowhead). (g) Decrease in epithelial height in the ventral male prostate (dashed arrow). (i) An increase in epithelial height (broad arrow) has been observed in the female prostate. (k) Multiplicity (specific number) of prostatic neoplasia intraepithelial (PIN) for each experimental senile male and female. (l, m) Morphometric of epithelial heights and thicknesses of the muscle layers of the ventral male prostate and female prostate from senile gerbils of the experimental groups. Values of multiplicity of PIN and morphometry are expressed as the mean ± SD. EE/PRE: exposure to ethinylestradiol during the prenatal period. *Significant difference between the groups with P ≤ 0.05.
The morphological analysis of the female prostates (Figure 3) revealed stratified epithelium and an increase in stromal densities, which are characteristics of BPH (Figure 3h). During the morphometric analysis, we found that the epithelial height and the thickness of muscle layer prostatic increased in the EE/PRE group females (13.00 ± 5.00; 8.00 ± 3.62 μm) when compared to the control group (11.66 ± 4.15; 6.20 ± 2.80 μm; Figure 3d, I and 3m).
EE exposure during the prenatal period also promoted the increase in (PIN; 10.64 ± 4.16; Figure 3l) in the glands of senile males (Figure 3f) and females (9.44 ± 6.33; Figure 3j, k) when compared to their respective controls groups (7.2 ± 4.00 and 3.76 ± 2.95).
The Gömöri's reticulin technique allowed us to observe the extracellular matrix component of male and female prostate from the experimental groups. We noted irregular aspects in the arrangement of reticular fibres and collagen of male ventral prostate of EE/PRE group when compared to controls group (Figure 4a, b, d and e). For the female prostate stromal compartments of the EE/PRE group, we observed plenty of collagen and reticular fibres and disturbance in the arrangement of reticular fibres, principally in inflammation foci (Figure 4f) compared to control group (Figure 4c).
Figure 4.
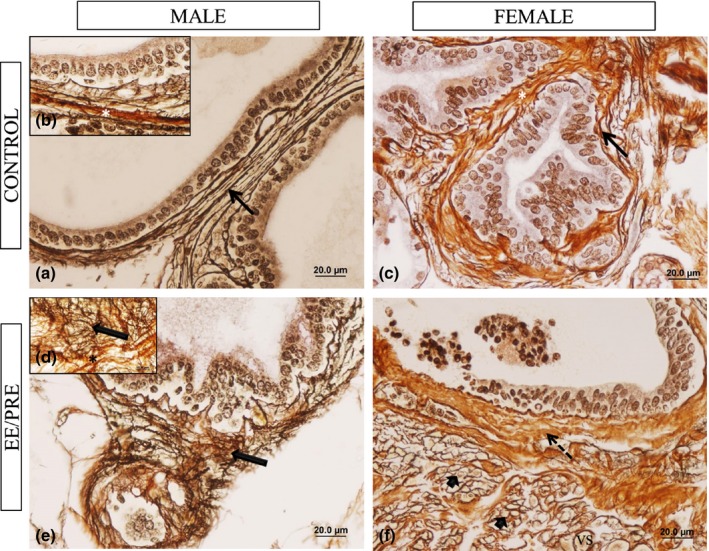
Histological sections of the ventral male prostates and female prostates from senile gerbils stained by Gömori reticulin. Control group: (a–c) Elements of prostatic stromal: collagen (white asterisks) and reticular fibres (arrows). EE/PRE group: (d and e). Disorder of arrangement of reticular fibres (large arrow) and collagen fibres (*). (f) Apparent increase in collagen fibres (dashed arrow) and disturbance in the arrangement of reticular fibres (arrowhead) in the inflammatory foci and abundance of blood vessels (vs).
Figure 5 shows immunofluorescence results for α‐actin reaction surrounding the alveoli of the prostates from the males and females in the senile groups. In areas that had prostatic buds, the muscle layer exhibited ruptures, showing the absence immunoreactivity in the EE/PRE male group (Figure 5c). In the ventral male prostates from the EE/PRE group, we observed increased immunoreactivity in regions with stromal lesions (Figure 5d), while in the prostate of the EE/PRE female group, we noted an increase in this immunoreactivity in regions with PIN (Figure 5e).
Figure 5.
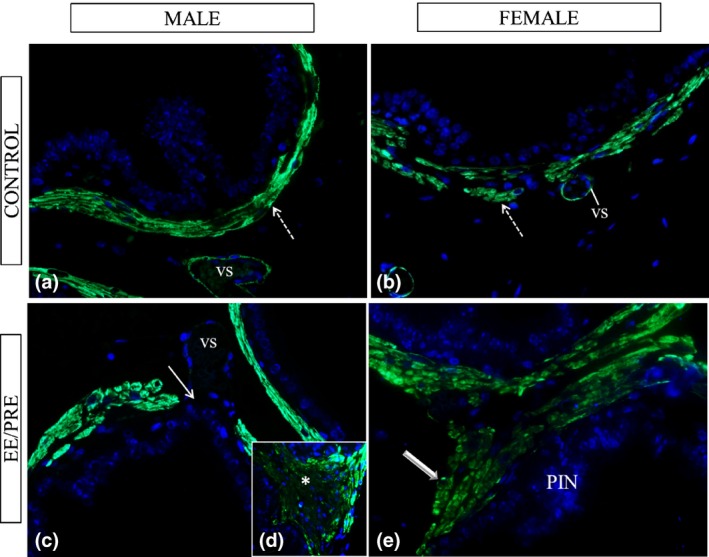
Histological sections of the ventral male prostates and female prostates from senile gerbils subjected to immunofluorescence for α‐actin of smooth muscle. Control group: (a and b) Immunoreactivity in the prostatic muscle layer (dashed arrows) and in the vessels (vs). EE/PRE group: (c–e) Region with prostatic buds noted absence of this immunoreactivity (arrow). Observed an increase in the α‐actin immunolocalization in lesions in the stroma (*) and in the regions with PIN (large arrow).
Table 2 shows the quantification (%) of prostatic epithelial cells in male and female that showed positive immunoreactivity for androgen receptors (AR), p63 (basal cells) and PCNA (cell proliferation). These results revealed increased immunoreactivity for AR (72.53 ± 10.14%) in the prostates from the EE/PRE male group when compared to the control group (51.17 ± 14.26%; Figure 6a, b, g and h). However, we saw decreased immunoreactivity for p63 in the prostates from the EE/PRE male group (8.61 ± 3.60%) when compared to the control group (11.23 ± 3.25%; Figure 6e, f, g, and l), in which the basal cells of prostatic epithelium were counted. Immunohistochemistry for PCNA revealed an increase in immunoreactivity in the ventral prostates, especially in areas with PIN, from the EE/PRE male group (62.82 ± 13.54%) when compared to the control group (34.03 ± 18.52%) (Figure 6c, d, i and k).
Table 2.
Frequency of cells labelled by AR, PCNA and p63 immunohistochemistry in the male and female prostates from senile gerbils in experimental group
| % Epithelial cells | Experimental group | |
|---|---|---|
| Control | EE/PRE | |
| AR | ♂ 51.17 ± 14.26 | 72.53 ± 10.14* |
| ♀ 80.00 ± 15.44 | 83.22 ± 12.66 | |
| p63 (basal cell) | ♂ 11.23 ± 3.25 | 8.61 ± 3.60* |
| ♀ 14.00 ± 5.24 | 18.03 ± 7.60 | |
| PCNA | ♂ 34.03 ± 18.52 | 62.82 ± 13.54* |
| ♀ 32.11 ± 15. 33 | 59. 435 ± 14.23* | |
Values expressed as the mean ± SD (n = 5). *Significant difference between the groups with P ≤ 0.05.
Figure 6.
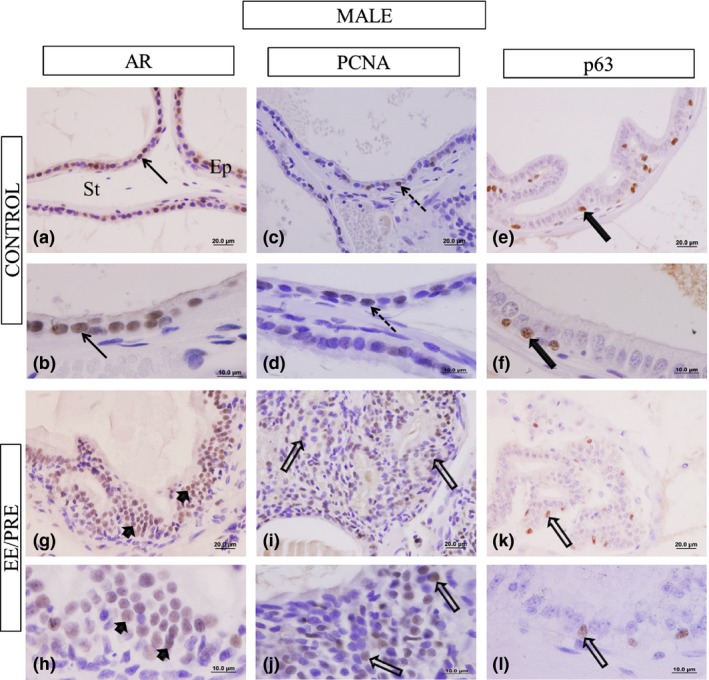
Histological sections of the ventral male prostate subjected to immunohistochemistry of the AR, PCNA and p63. Control group: (a and b). We observed immunoreactivity in the prostatic epithelial cells (Ep) (arrows). Presence of the prostatic stroma (St). EE/PRE group: (g and h) Increase in AR immunoreactivity in the epithelial cells (arrowhead). (i) PCNA immunohistochemistry. Control group: (c and d) Marcation in the prostatic epithelial cell (dashed arrows). EE/PRE group: (i and j) Increase in the PCNA immunolocalization in regions with PIN (large arrow). Immunohistochemistry for p63. Control group: (e and f) Labelling of basal cells (large filled arrows). EE/PRE group: (k and l) Decrease in the p63 immunoreactivity (large arrow).
In the prostates from the EE/PRE female group, we did not note any significant differences in the frequency of cells positive for p63 and AR when compared with the control group, as shown in Table 2 and Figure 7a, b, g and h. However, the immunoreactivity of PCNA was higher in the female prostates from the EE/PRE group (59.43 ± 14.23%) when compared to the control group (32.11 ± 15.33%) (Figure 7c, d, i and k).
Figure 7.
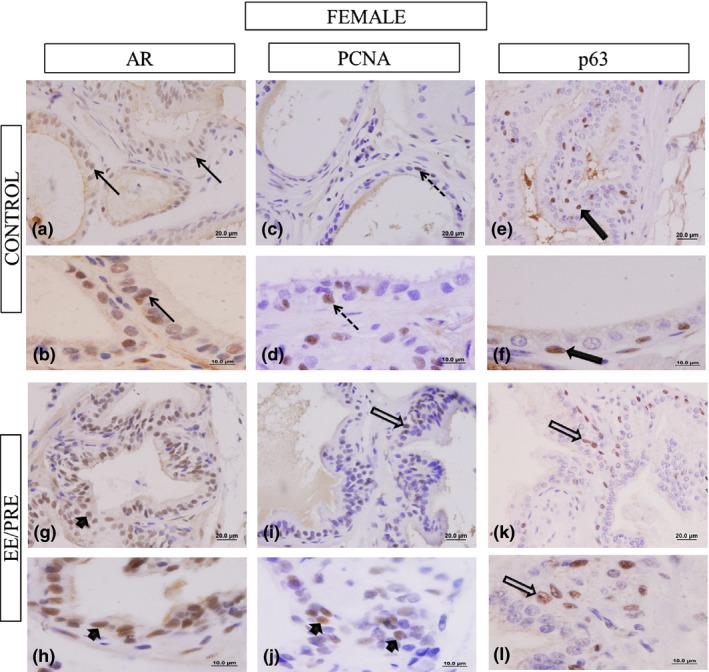
Histological sections of the female prostate subjected to immunohistochemistry for the AR, PCNA and p63. Control group: (a and b) Marcation in the epithelial cells (arrows). EE/PRE group: (g and h) Evidence for AR immunoreactivity in the prostatic epithelial cell (arrowhead). Immunohistochemistry for PCNA. Control group: (c and d) EE/PRE group (i and j) Immunoreactivity of cellular proliferation markers (dashed arrows). p63 immunohistochemistry. Control group: (e and f) Details of the immunoreactivity in the prostatic basal cells (large filled arrow). EE/PRE group: (k and l) Increase in p63 immunoreactivity (large arrows).
The immunoreactivity for oestrogen receptors type alpha (ERα) has been observed in the stromal cells of the ventral male prostate of control and EE/PRE group (Figure 8). However, in the group treated with EE during pregnancy, this immunoreactivity was intense in the cytoplasmic region of prostatic epithelial cells of senile males (Figure 8c). Regarding ERα, immunoreactivity was noted to be increased in the stromal cells of the prostate females in the EE/PRE group compared to control (Figure 8b, d).
Figure 8.
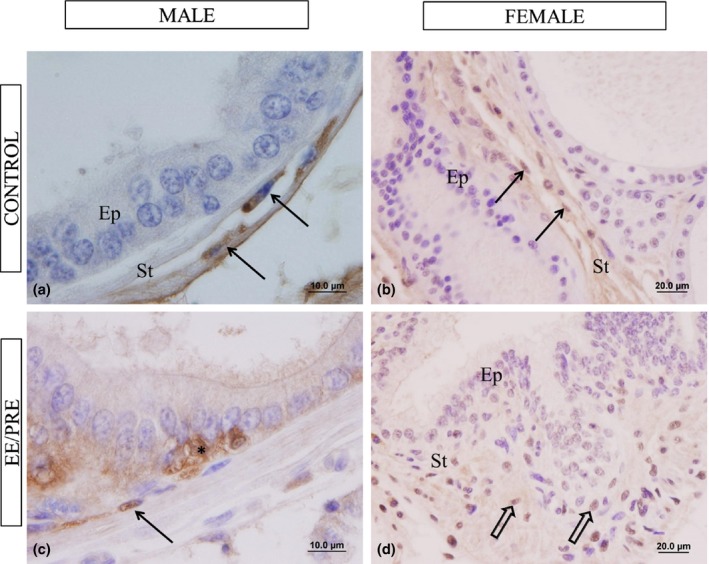
Histological sections of the ventral male prostate and female prostate from senile gerbils subjected to immunohistochemistry for ERα. Control group: (a and b). Presence of immunoreactivity in the prostatic stroma (St) cell (arrows). Epithelium (Ep). EE/PRE group: (c) Cytoplasm labelling (*) and epithelial cell (arrow). (d) We noted an increase in ERα immunoreactivity in the stromal cell (large arrows).
The oestrogen receptors of type beta (ERβ) were observed primarily as nuclear immunoreactivity in the male prostate epithelial cells in the experimental groups (Figure 9). In the prostate of the EE/PRE male group this immunoreactivity was higher compared to control group (Figure 9a and d). There was no observed difference in the immunoreactivity for ERβ in the prostate of the experimental female groups (Figure 9b, c and e).
Figure 9.
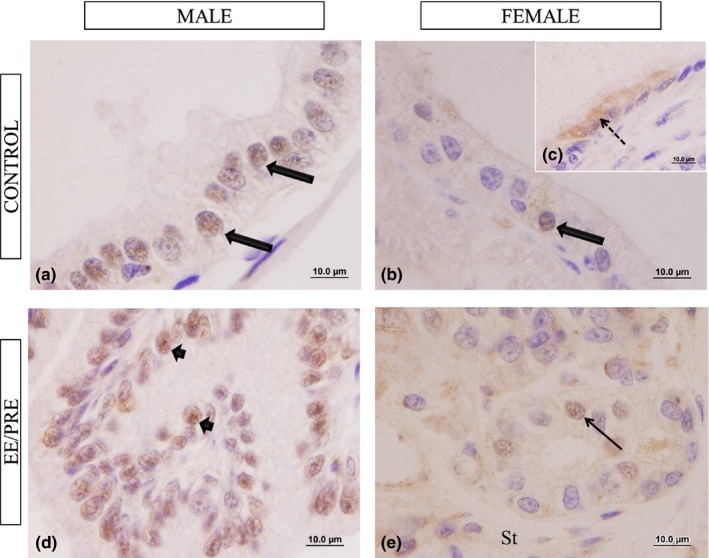
Histological sections of the ventral male prostate and female prostate of senile gerbils subjected to immunohistochemistry for ERβ. Control group: (a) (male) and (b–c) (female). Details of the nuclear immunoreactivity in the epithelial cells (large arrows). Cytoplasmic marcation (dashed arrow). EE/PRE male group: (d) Increase in nuclear immunoreactivity in the epithelial cell (arrowhead). EE/PRE female group (e). Labelled epithelial cell (arrow).
In the ultrastructural analysis of the ventral male prostate of the senile gerbil columnar epithelial cells were observed with the presence of dense nuclear chromatin condensation, and with nuclei mainly distributed in the periphery (Figure 10a). Under the basal membrane collagen fibres and smooth muscle cells were observed in the prostatic stroma, (Figure 10a).
Figure 10.
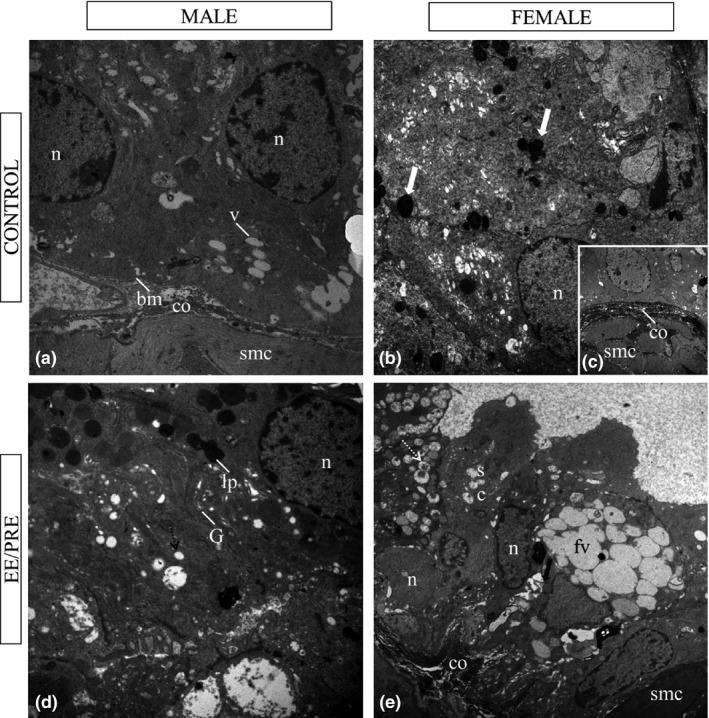
Ultrastructural aspects of ventral male prostate and female prostate of senile gerbil. Control group: (a) (male), (b) and (c) (female). We observed large nuclei (n) and deposit of lipofuscin in the cytoplasm (large white arrow) of the epithelium. Basal membrane (bm). Dense stroma with abundant collagen fibres (co), and smooth muscle cells (smc). EE/PRE group: (d) (male). Increase in lipofuscin deposit (lp), amount of vesicle (v), Golgi complex (g) in the cytoplasm. (e) (female). Observed the presence of vesicle (dashed white arrow) in the secretory cells (sc). Note the presence of fusion of the vesicles (fv) and alterations in the cellular adhesion (large arrow). a: 7750X; b: 3597X; c, d: 6000X; e: 4646X.
With regard to the ultrastructure of the male prostate of the EE/PRE group, there was an apparent increase in lipofuscin and organelles as the Golgi complex when compared to the control group, and we observed the fusion of secretory vesicles (Figure 10d).
During the ultrastructural analysis of the prostate from female senile gerbils, highly secretory cells with large nuclei and lipofuscin‐like deposits in the prostatic cytoplasm were observed (Figure 10b and c). In the EE/PRE group, the increase in lipid droplets in the prostatic cytoplasm, as well as vesicle fusion with the accumulation of secretion compared to control group, was evident. Further, a decrease in adhesion between epithelial cells in the EE/PRE group was noticed (Figure 10e).
Discussion
The present study shows that prenatal exposure to ethinylestradiol (15 μg/kg/day) causes changes in the epithelium and stroma of the prostate of the gerbil ‐ both males and females ‐ during ageing. Senile females exposed to EE showed a significant increase in testosterone and estradiol levels. The female prostate develops in an environment with high levels of endogenous oestrogen (Santos & Taboga 2006), but the increase in testosterone stimulates growth and secretory activity of this gland (Santos et al. 2006). In this study, it was observed that exposure to EE interfered in the process of synthesis of steroid hormones (androgen), increasing hormone levels in the circulation of rodent female; this fact was noted in the exposure to other synthetic oestrogens such as biphenyl A (BPA) (Xi et al. 2011). Early exposure to EE, a synthetic oestrogen affects testosterone and estradiol levels in senile females, resulted in increased epithelial height and in changes in the muscle layer of the female prostate, thereby altering the morphology of this gland and increasing the development of PIN during ageing.
Oestrogen plays an important role in the development and growth of the prostate (McPherson et al. 2008); however, the exposure to synthetic oestrogens during critical periods of development causes changes in prostate health (Ho et al. 2006; Perez et al. 2012). The EE exposure during the prenatal period resulted in increased (PIN) in the ventral male prostate and female prostate of senile gerbil. Low doses of endocrine disruptors that act as oestrogen agonists exposed during development alter the prostate epigenome; this process is called oestrogenic imprint, predisposing the animal to precursor lesions of prostate (Prins et al. 2007a,b). Ethinylestradiol, as an oestrogen agonist which induces oestrogenic activity (Ryan et al. 2010), promotes imprint prenatal permanent when there is exposure during development, and the consequences are accentuated during ageing.
The stromal–epithelial interaction is essential for normal prostate development (Hayward et al. 1997; Cunha et al. 2004). This interaction is mediate by ERα, which is a receptor that acts in the muscle cells and fibroblasts, and influences the proliferation of epithelial cells (Vitkus et al. 2013). Our data showed an increase in ERα immunoreactivity in stromal cells and the cytoplasm of epithelial cells in the prostates from male and female senile gerbils that were exposed to EE. One of the mechanisms of action of EE, or oestrogen agonists, is the interference in oestrogen signalling and the interaction with the oestrogen receptors (De Coster & van Larebeke 2012). Interference in the action of ERα during development due to EE exposure promoted morphological changes in the structures of the prostatic stroma of senile males and females. We observed areas where the collagen and reticular fibres were disorganized and do not establish a boundary between the epithelial structures and the stroma, primarily in regions with the presence of PIN in the female prostate and male ventral prostate of EE/PRE group. These modifications in the arrangements of fibres are observed in prostatic stroma of adenocarcinoma (Taboga & Vidal 2003). Alterations in the prostatic stroma contribute to the development of an invasive phenotype in the ventral male prostate and female prostate during ageing promoted by EE exposure during the development of the prostate gland.
Similar to oestrogen, androgen plays an important role in prostate development, in stromal–epithelial interactions, in epithelial cell proliferation and in the development of prostatic buds (Thomas & Keenan 1994; Cunha et al. 2004). During immunohistochemical analysis, we observed an increase in the frequency of AR in the senile ventral male prostate in the EE/PRE group. Studies with male mice exposed to EE (Thayer et al. 2001) and diethylstilbestrol (DES – medicament utilized in the 70s for women with high‐risk pregnancies) (Herbst & Bern 1981; Gupta 2000) during the embryonic stage showed an increased in the frequency of AR in the prostate of the neonatal rodent. During ageing, the increase in AR in the male and female prostates of gerbils exposed to EE contributed to the development of alterations in stromal–epithelial interactions, including rupture of the muscular layer in invasive lesions and the increase in this layer in regions with PIN. Both ERα and the AR seem to contribute to the adverse effects on prostatic morphology in senile animals exposed to ethinylestradiol, data observed too in male and female prostate of adult gerbil exposed to EE during the prenatal period (Perez et al. 2011).
Ethinylestradiol exposure during the prenatal period increased the proliferation of epithelial cells, resulting in the formation of neoplastic lesions in the male and female prostates in gerbils during ageing. This change was shown by the increase in the PCNA immunoreactivity, mainly in regions with PIN. The expression levels of PCNA are not only associated with the pathways involved in cell cycle control and replication, but also with DNA repair processes (Strzalka & Ziemienowicz 2011). Chemicals can alter the levels of PCNA, interfering with cell cycle control pathways, thus promoting the increase in cell proliferation and the formation of neoplasms (Strzalka & Ziemienowicz 2011; Zhang et al. 2011).
The pattern of immunolocalization for p63 showed decreased immunolocalization in basal cells in the ventral male prostates from the EE/PRE group. The p63 has been shown to play an important role in prostate development, and this gene is essential for normal stem cell function in the prostate as well as other epithelial organs, so when there is a progressive loss of the basal cell layer in high‐grade PIN, p63 expression is altered (Grisanzio & Signoretti 2008). Exposure to high doses of testosterone during the prenatal period decreases the immunoreactivity of p63 in regions with PIN in the ventral male prostate of the adult gerbil (Biancardi et al. 2012). Even when using low doses, we noted that exposure to DE during development is enough to cause neoplastic lesions and the loss of the basal cell layer in the ventral male prostate in the senile stage.
While the functions of AR and ERα receptors are crucial during the early phases of prostate development (Cunha et al. 2004; McPherson et al. 2008), the action of ERβ on the prostate is more significant in adulthood, during which it has antiproliferative functions (Weihua et al. 2002), while ERα has proliferative functions (McPherson et al. 2008). According to Rochel‐Maia et al. (2013), ERβ decreases in the prostates of male and female gerbils during ageing, predisposing these rodents to prostate proliferative diseases. Meanwhile, in the male ventral prostates from the EE/PRE senile group, we observed an increase in the immunoreactivity of ERβ in epithelial cells. This increase in the male prostate may be an attempt of the gland to counteract the proliferative action exerted by ERα effects.
The structural analyses of the ventral male prostate and female prostate of senile gerbils emphasized the morphological changes caused by exposure to EE during development. However, we also observed changes in the ultrastructure of the glands of the EE/PRE group for both sexes. The analyses revealed modifications to the secretory pattern of the prostatic epithelial cells, including the increase in vesicles containing secretions and organelles directly related to this function, such as the Golgi apparatus. The presence of lipofuscin deposits and changes in the arrangement of collagen fibres are characteristics observed in the prostates of both male (Campos et al. 2008) and female (Custódio et al. 2010) senile gerbils. The exposure to EE during the prenatal period accentuated the alterations in the prostatic gland that are typical of senile gerbil.
Senescence is a period marked by the deregulation of steroid hormones (Chahal & Drake 2007), and this feature is one of the main factors for predisposition to prostate disease in senile males and females (Campos et al. 2008; Custódio et al. 2010). Our study showed that exposure to EE during critical periods of development, such as prenatally, interferes with the normal morphology of male and female prostates in senile gerbils. This exposure altered the stromal–epithelial interaction and contributed to the emergence of PIN, widely modifying the prostate morphology.
The exposure to synthetic oestrogen during development alters the levels of sex hormones, causing reproductive disorders through interference in the feedback that regulates the hypothalamic–pituitary–gonadal axis (Xi et al. 2011). Changes, such as the increase in the proliferative index of epithelial cells, alterations in the expression patterns of steroid receptors and potentiation of ultrastructural characteristics typical of the ageing in gerbils caused by early exposure to EE. Furthermore, the exposure to EE increases the predisposition to emergence of premalignant lesions in male and female gerbils during ageing.
The male and female prostates of adult gerbils respond differently to exposure to oestrogen disruptors during development. Additionally, the ventral male prostate is more sensitive to the action of ethinylestradiol than the female prostate, as well as showing a predisposition to the emergence of neoplastic lesions (Perez et al. 2011, 2012). The mammalian brain is extremely sensitive to the action of hormones during developmental phases (prenatal, puberty), and differences in brain development between males and females may result in different effects due to EE exposure (Gore 2008; De Coster & van Larebeke 2012). The action of oestrogen disruptors during the critical phases of development exacerbates the development of precancerous lesions in the male prostate, damaging the health of this gland during ageing.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Funding source
This work was financed by the State of Sao Paulo Research Foundation – FAPESP (Process Nr. 09/16790‐5 and 11/06335‐9) and CNPq – National Research Council (fellowship to SRT – Grant Nr. 301596/2011‐5).
References
- Basaria S. (2013) Reproductive aging in men. Endocrinol. Metab. Clin. North Am. 42 , 255–270. [DOI] [PubMed] [Google Scholar]
- Behmer A.O., Tolosa E.M.C., Neto A.G.F. (1976). Manual de práticas para histologia normal e patológica. p. 329 São Paulo, SP: Edart‐Edusp. [Google Scholar]
- Biancardi M.F., Perez A.P., Góes R.M., Santos F.C., Vilamaior P.S. & Taboga S.R. (2012) Prenatal testosterone exposure as a model for the study of endocrine‐disrupting chemicals on the gerbil prostate. Exp. Biol. Med. 11, 1298–1309. [DOI] [PubMed] [Google Scholar]
- Biancardi M.F., Perez A.P., Caires C.R. et al (2014) Prenatal exposure to testosterone masculinises the female gerbil and promotes the development of lesions in the prostate (Skene's gland). Reprod. Fertil. Dev. in press. Apr 4. doi: 10.1071/RD13387. [DOI] [PubMed] [Google Scholar]
- Campos S.G.P., Zanetoni C., Scarano W.R., Vilamaior P.S.L. & Taboga S.R. (2008) Age‐related histopathological lesions in the Mongolian gerbil ventral prostate as a good model for studies of spontaneous hormone‐related disorders. Int. J. Exp. Pathol. 89, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals‐Casas C. & Desvergne B. (2011) Endocrine disruptors: from endocrine to metabolic disruption. Annu. Rev. Physiol. 73, 135–162. [DOI] [PubMed] [Google Scholar]
- Chahal H.S. & Drake W.M. (2007) The endocrine system and aging. J. Pathol. 211, 173–180. [DOI] [PubMed] [Google Scholar]
- Cotta‐Pereira G., Rodrigo F.G. & David‐Fereira J.F. (1976) The use of tannic acid glutaraldehyde in the study of elastic related fibers. Stain Technol. 51, 7–11. [DOI] [PubMed] [Google Scholar]
- Cunha G.R., Ricke W., Thomson A. et al (2004) Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J. Steroid Biochem. Mol. Biol. 92, 221–236. [DOI] [PubMed] [Google Scholar]
- Custódio A.M.G., Góes R.M. & Taboga S.R. (2004) Acid phosphatase activity in gerbil prostate:comparative study in male and female during postnatal development. Cell Biol. Int. 28, 335–344. [DOI] [PubMed] [Google Scholar]
- Custódio A.M.G., Santos F.C.A., Campos S.G.P. et al (2010) Disorders related with ageing in the gerbil female prostate (Skene′s paraurethral glands). Int. J. Exp. Pathol. 91, 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coster S. & van Larebeke N. (2012) Endocrine‐disrupting chemicals: associated disorders and mechanism of action. J. Environ. Public Health 2012, 713696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti‐Kandarakis E., Bourguignon J.P., Giudice L.C. et al (2009) Endocrine‐disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 30, 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye C.A., Bo E., Calamandrei G. et al (2012) Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J. Neuroendocrinol. 24, 144–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A.C. (2008) Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front. Neuroendocrinol. 29, 358–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanzio C. & Signoretti S. (2008) p63 in prostate biology and pathology. J. Cell. Biochem. 103, 1354–1368. [DOI] [PubMed] [Google Scholar]
- Gupta C. (2000) Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc. Soc. Exp. Biol. Med. 224, 61–68. [DOI] [PubMed] [Google Scholar]
- Gӧmӧri G. (1937) Silver impregnation for reticulin in paraffin section. Am. J. Pathol. 13, 993–1002. [PMC free article] [PubMed] [Google Scholar]
- Hayward S.W., Rosen M.A. & Cunha G.R. (1997) Stromal‐epithelial interactions in the normal and neoplastic prostate. Br. J. Urol. 79, 18–26. [DOI] [PubMed] [Google Scholar]
- Herbst A.L. & Bern H.A. (1981) Development effects of diethylstilbestrol (DES) in pregnancy. New York: Thieme‐Stratton. [Google Scholar]
- Ho S.M., Tang W.Y., Belmonte de Frausto J. & Prins G.S. (2006) Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 66, 5624–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W.Y., Shi G.B., Hu D.P., Nelles J.L. & Prins G.S. (2012) Actions of estrogens and endocrine disrupting chemicals on human prostate stem/progenitor cells and prostate cancer risk. Mol. Cell. Endocrinol. 354, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermath B.A. & Gore A.C. (2012) Neuroendocrine control of the transition to reproductive senescence: lessons learned from the female rodent model. Neuroendocrinology 96, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.K., Daling J.R., Mueller B.A., Hickok D.E., Fantel A.G. & Weiss N.S. (1995) Oral contraceptive use after conception in relation to the risk of congenital urinary tract anomalies. Teratology 51, 30–36. [DOI] [PubMed] [Google Scholar]
- Maffini M.V., Rubin B.S., Sonnenschein C. & Soto A.M. (2006) Endocrine disruptors and reproductive health: the case of bisphenol‐A. Mol. Cell. Endocrinol. 25, 179–186. [DOI] [PubMed] [Google Scholar]
- McPherson S.J., Ellem S.J. & Risbridger G.P. (2008) Estrogen‐regulated development and differentiation of the prostate. Differentiation 76, 660–670. [DOI] [PubMed] [Google Scholar]
- Nishino N. & Totsukawa K. (1996) Study on the estrous cycle in the Mongolian gerbil (Meriones unguiculatus). Exp. Anim. 45, 283–288. [DOI] [PubMed] [Google Scholar]
- Perez A.P., Biancardi M.F., Dos Santos F.C.A., Góes R.M. & Taboga S.R. (2011) Exposure to ethinylestradiol during prenatal development and postnatal supplementation with testosterone causes morphophysiological alterations in the prostate of male and female adult gerbils. Int. J. Exp. Pathol. 92, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez A.P., Biancardi M.F., Vilamaior P.S., Góes R.M., Santos F.C. & Taboga S.R. (2012) Microscopic comparative study of the exposure effects of testosterone cypionate and ethinylestradiol during prenatal life on the prostatic tissue of adult gerbils. Microsc. Res. Tech. 75, 1084–1092. [DOI] [PubMed] [Google Scholar]
- Prins G.S. (2008) Endocrine disruptors and prostate cancer risk. Endocr. Relat. Cancer 15, 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins G.S., Birch L., Habermann H. et al (2001) Influence of neonatal estrogens on rat prostate development. Reprod. Fertil. Dev. 13, 241–252. [DOI] [PubMed] [Google Scholar]
- Prins G.S., Tang W.Y., Belmonte J. & Ho S.M. (2007a) Perinatal exposure to oestradiol and Bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin. Pharmacol. Toxicol. 102, 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins G.S., Birch L., Tang W.Y. & Ho S.M. (2007b) Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod. Toxicol. 23, 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochel‐Maia S.S., Santos F.C., Alonso‐Magdalena P. et al (2013) Estrogen receptors alpha and beta in male and female gerbil prostates. Biol. Reprod. 88, 7. [DOI] [PubMed] [Google Scholar]
- Ryan B.C., Hotchkiss A.K., Crofton K.M. & Gray L.E. Jr (2010) In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol. Sci. 114, 133–148. [DOI] [PubMed] [Google Scholar]
- Sanches B.D., Biancardi M.F., Santos F.C., Góes R.M., Vilamaior P.S. & Taboga S.R. (2014) Budding process during the organogenesis of the ventral prostatic lobe in Mongolian gerbil. Microsc. Res. Tech. 77, 458–466. [DOI] [PubMed] [Google Scholar]
- Santos F.C.A., Hernandes H.F., Góes R.M. & Taboga S.R. (2003) Struture, histochemistry, and ultrastructure of the epithelium and stroma in the gerbil (Meriones unguiculatus) female prostate. Tissue & Cell. 35, 447‐457. [DOI] [PubMed] [Google Scholar]
- Santos F.C.A. & Taboga S.R. (2006) Female prostate: a review about the biological repercussions of this gland in humans and rodents. Anim. Reprod. 3, 3–18. [Google Scholar]
- Santos F.C.A., Leite R.P., Custódio A.M.G. et al (2006) Testosterone stimulates growth and secretory activity of the adult female prostate of the gerbil (Meriones unguiculatus). Biol. Reprod. 75, 370–379. [DOI] [PubMed] [Google Scholar]
- Santos F.C.A., Falleiros‐Júnior L.R., Corradi L.S., Vilamaior P.S. & Taboga S.R. (2007) Experimental endocrine therapies promote epithelial cytodifferentiation and ciliogenesis in the gerbil prostate. Cell Tissue Res. 328, 617–624. [DOI] [PubMed] [Google Scholar]
- Scarano W.R., Vilamaior P.S. & Taboga S.R. (2006) Tissue evidence of the testosterone role on the abnormal growth and aging effects reversion in the gerbil (Meriones unguiculatus) prostate. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 288, 1190–1200. [DOI] [PubMed] [Google Scholar]
- Scarano W.R., de Souza D.E., Campos S.G., Corradi L.S., Vilamaior O.S. & Taboga S.R. (2008) Oestrogen supplementation following castration promotes stromal remodelling and histopathological alterations in the Mongolian gerbil ventral prostate. Int. J. Exp. Pathol. 89, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shappell S.B., Thomas G.V., Roberts R.L. et al (2004) Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the mouse models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 64, 2270–2305. [DOI] [PubMed] [Google Scholar]
- Soto A.M. & Sonnenschein C. (2010) Environmental causes of cancer: endocrine disruptors as carcinogens. Nat. Rev. Endocrinol. 6, 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzalka W. & Ziemienowicz A. (2011) Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Ann. Bot. 107, 1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboga S.R. & Vidal B.C. (2003) Collagen fibers in human prostatic lesions: histochemistry and anisotropies. J. Submicrosc. Cytol. Pathol. 35, 11–16. [PubMed] [Google Scholar]
- Thayer K.A., Ruhlen R.L., Howdeshell K.L. et al (2001) Altered prostate growth and daily sperm production in male mice exposed prenatally to subclinical doses of 17α‐ethinyl oestradiol. Hum. Reprod., 16, 988–996. [DOI] [PubMed] [Google Scholar]
- Thomas J.A. & Keenan E.J. (1994) Effects of estrogens on the prostate. J. Androl. 15, 97–99. [PubMed] [Google Scholar]
- Vitkus S., Yeh C.R., Lin H.H. et al (2013) Distinct function of estrogen receptor α in smooth muscle and fibroblast cells in prostate development. Mol. Endocrinol. 27, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.M., Kermath B.A., Wolker M.J. & Gore A.C.D. (2013) Disruption of reproductive aging in female and male rats by gestational exposure to estrogenic endocrine disruptors. Endocrinology 154, 2129–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihua Z., Warner M. & Gustafsson J.A. (2002) Estrogen receptor beta in the prostate. Mol. Cell. Endocrinol. 193, 1–5. [DOI] [PubMed] [Google Scholar]
- Wigle D.T., Turner M.C., Gomes J. & Parent M.E. (2008) Role of hormonal and other factors in human prostate cancer. J. Toxicol. Environ. Health B Crit. Rev. 11, 242–259. [DOI] [PubMed] [Google Scholar]
- Xi W., Lee C.K.F., Yeung W.S.B. et al (2011) Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus–pituitary–gonadal axis of CD‐1 mice. Reprod. Toxicol. 31, 409–417. [DOI] [PubMed] [Google Scholar]
- Zhang W., Qin Z., Zhang X. & Xiao W. (2011) Roles of sequential ubiquitination of PCNA in DNA‐damage tolerance. FEBS Lett. 585, 2786–2794. [DOI] [PubMed] [Google Scholar]


