Summary
Permethrin (PM), a synthetic pyrethroid insecticide, has broad toxicity spectra. We aimed to investigate the effects of PM on the testes of adult albino rats, examine the recovery response and evaluate the efficacy of naringenin (NG) supplementation. Adult male albino rats were randomly assigned to five groups of six each: control, NG (50 mg/kg), PM (70 mg/kg), recovery (after subsequent withdrawal of PM) and NG‐PM group. All treatments were given by oral gavage for 6 weeks and another 3 weeks for the recovery group. At the time of sacrifice, each testis was weighed. Biochemical analysis of epididymal sperm count and serum testosterone level was performed. Testes were processed for histological, ultrastructural and c‐Kit immunohistochemical study. PM toxicity was evidenced by a highly significant decrease in testicular weight, epididymal sperm count and serum testosterone level compared to control. Furthermore, testicular structure abnormalities and reduced c‐Kit immunoreactions were observed. Stoppage of PM in the recovery group partially reversed PM‐induced changes. There was a mild decrease in testicular weight and biochemical parameters compared to control. The structure of seminiferous tubules was partially retained. The NG‐PM group showed an overall improvement in testicular weight and biochemical alterations which were confirmed by light and electron microscopic examination. In conclusion, PM induced testicular toxicity, which was ameliorated by NG co‐administration. However, stoppage of PM exposure was associated with partial recovery.
Keywords: c‐Kit, naringenin, permethrin, rats, testicular toxicity
Pesticides represent an important group of environmental pollutants (Pogribny & Rusyn 2013). Environmental contamination of pesticide is a threat to life and the hazard goes beyond the farming community because pesticide residues are found in the food chain, soil and water supplies (Roma et al. 2012).
Permethrin (PM) is a synthetic pyrethroid with a broad‐spectrum insecticidal activity, which is used for indoor/outdoor pest control (Bradberry et al. 2005). In medicine it is a component of commonly used skin applications for scabies and head lice (Kitchen et al. 2009).
The general population could be exposed to PM via the inhalation of ambient air or the ingestion of contaminated food (HSDB 2003). Occupational exposure may occur by both inhalation and dermal contact at workplaces where this compound is produced or used (ATSDR 2005).
Although safer than organophosphorus pesticides (Shafer & Meyer 2004), previous studies of PM effects confirmed both some male reproductive organ damage (Jin et al. 2012) and genotoxic effects (Falcioni et al. 2010). Other adverse effects include carcinogenicity (Rusiecki et al. 2009), immunosuppressive potential (Fedeli et al. 2012), liver damage (Roma et al. 2012), heart damage (Vadhana et al. 2011), neurological disorders and behavioural changes (Wolansky & Harrill 2008; Carloni et al. 2012).
c‐Kit is a type III receptor of the tyrosine kinase family (Figueira et al. 2014). Together with its ligand stem cell factor (SCF), c‐Kit is a key controlling receptor for a number of cell types, including haematopoietic stem cells, melanocytes, mast cells and germ cells (Ronnstrand 2004). Complete loss of c‐Kit expression leads to death in utero or perinatally, most likely due to severe anaemia. Heterozygous animals display defects in pigmentation, reduced fertility and anaemia (Lennartsson et al. 2005). In certain cases, infertility has been attributed to functional defects in c‐Kit (Sikarwar & Reddy 2008).
Naringenin (NG; 4, 5, 7‐trihydroxyflavone) is a glycone form of naringin, one of the naturally occurring flavanones (Podder et al. 2014). NG is widely distributed in citrus fruits, tomatoes, cherries, grapefruit, and cocoa (Jain et al. 2011). It is known as a safe natural product with various bioactive effects, including anticancer (Chen et al. 2007; Du et al. 2009), anti‐oxidative (Kannappan et al. 2010), anti‐inflammatory (Fang et al. 2010), nephron‐protective (Badary et al. 2005; Renugadevi & Prabu 2009) and hepato‐protective activities (Renugadevi & Prabu 2010).
As the complete avoidance of exposure to PM is very difficult, chemoprevention is an attractive strategy for protecting the human and animals from the risks caused by the exposure to this insecticide (Türkez & Aydin 2013). Many approaches were employed to counteract the PM problem (Etang et al. 2007; Koutros et al. 2010; Rossbach et al. 2010).
As far we know, there is no study concerning the effect NG on PM‐induced toxicity. Additionally, the testicular toxicity of PM exposure, particularly in relation to the ultrastructural injuries, remains poorly understood. Thus, this study aimed to investigate the effect of PM on the testes of adult albino rats, examine the recovery response and evaluate the efficacy of NG supplementation. This was conducted using biochemical and histological methods.
Materials and methods
Chemicals
Permethrin (PM)
(Crystalline colourless odourless powder; CAS No. 52645‐53‐1; purity of 93%) was purchased from Sigma‐Aldrich (St. Louis, MO, USA). It is an isomer blend of cis and trans isomers, in a nominal isomeric ratio of 25:75.
Naringenin (NG; 5, 7, 4′‐trihydroxyflavanone)
(CAS No. 67604‐48‐2; purity of 95%) was purchased from Sigma‐Aldrich.
Experimental animals
Thirty adult male albino rats, weighing 180–200 g, were maintained on PM‐free rat chow and water ad libitum at the Breading Animal House of the Faculty of Medicine, Zagazig University. Acclimatization period lasted for 2 weeks. The animals were housed in filter‐top plastic cages in a controlled temperature (23 ± 1°C), humidity (55 ± 5%) and artificially illuminated (12:12‐h light:dark cycle) room, free from any source of chemical contamination. All rats received humane care in compliance with the guidelines of the medical research ethics committee of Zagazig University (Egypt).
Experimental protocol
The rats were randomly assigned to five groups of six animals each. They were caged separately. All treatments were given by oral gavage.
Group I (control group) received corn oil (0.5 ml/day) for six weeks. Corn oil was used as a vehicle to dissolve PM and NG.
Group II (NG group) was treated with NG at a dose of 50 mg/kg b.w./day in 0.5 ml corn oil for 6 weeks. The dose level for NG was based on previously published reports (Prabu et al. 2011; Wang et al. 2012; Hermenean et al. 2013).
Group III (PM group) received PM at a dose of 70 mg/kg b.w./day, in 0.5 ml corn oil for 6 weeks. The dose corresponded to 1/20 of the LD50 value of PM. The dosage was chosen based on the ‘no observed adverse effect level’ (NOAEL) for PM of 25 mg/kg (Vadhana et al. 2013).
Group IV (recovery group) was given the same dose of PM for the same period as group III and then left without treatment for another 3 weeks for follow‐up.
Group V (NG‐PM group) was treated with PM along with NG at the same doses for 6 weeks. NG was administered during PM exposure following a previously published method (Gnanasoundari & Pari 2006; Prabu et al. 2011; Wang et al. 2012).
Measurement of body and testis weights
At the time of sacrifice, the rats were weighed using a digital balance and then anesthetized with ether inhalation. Intracardiac perfusion was carried out with 2.5% glutaraldehyde for partial fixation of the testes. Each testis was dissected by abdominal incision and cleared from adhering connective tissue, and the epididymides were carefully trimmed free from the testes which were weighed.
Biochemical analysis
Epididymal sperm count: The sperm samples, obtained from the distal region of the right vas deferens by excision of a small piece (1.0 cm) of the vas just distal to the cauda epididymis, were placed in a Petri dish containing an aliquot of buffer (Hank's balanced salt solution) and agitated gently at 37°C for 15 min. Specimens were spread on a hemocytometer, and the heads were counted manually under an optical microscope. Total sperm count was determined after calculating the per cent of dilution, and the data were expressed as total number of sperm/ml (Karbalay‐Doust et al. 2007).
Serum testosterone level: Blood samples were collected from the retro‐orbital venous plexus of all animals into heparinized tubes. The serum was separated by centrifugation at 1600 × g for 10 min and stored at −70°C until use. Total testosterone was analysed using solid‐phase radioimmunoassay method by Coat‐A‐Count Total Testosterone Kit (Diagnostic Products Corporation, Los Angeles, CA, USA) (Kim et al. 2012).
Histopathological study
Haematoxylin and eosin (H&E) stain: Specimens for light microscope examination were fixed in Bouin's fixative. They were processed to prepare 5‐μm‐thick paraffin sections for H&E staining (Kiernan 2000).
Ultrastructural study: Specimens for electron microscopy were immediately fixed in 2.5% phosphate‐buffered glutaraldehyde (pH 7.4), post‐fixed in 1% osmium tetroxide in the same buffer at 4°C, dehydrated and embedded in epoxy resin. Ultrathin sections were obtained using Leica Ultracut UCT, stained with uranyl acetate and lead citrate (Ayache et al. 2010), examined and photographed using a JEOL JEM 1010 electron microscope (Jeol Ltd, Tokyo, Japan) in the Histology and Cell Biology Department, Faculty of Medicine, Zagazig University (Egypt).
Immunohistochemical study: The immunohistochemical staining for c‐Kit protein in testicular tissue was carried out by means of streptavidin–biotin–peroxidase method (DAKO LSAB® 2 System, HRP, Code No. K0673; DAKO Corporation, Carpinteria, CA, USA) following the manufacturer's instructions. Paraffin sections (4 μm) were deparaffinized in xylene, rehydrated in a descending series of ethanol and immersed in 0.3% H2O2 for 10 min to block endogenous peroxidases. Antigens were retrieved by microwaving for 15 min in citrate buffer (pH 6.0). Non‐specific binding was blocked with 10% goat serum (Dako Ltd, Cambridge shire, UK) for 30 min. The sections were rinsed in phosphate‐buffered saline (PBS) and then incubated with an anti‐c‐Kit antibody (anti‐CD117 – rabbit polyclonal antibody – Catalog ID: LS‐C78828; Lifespan Biosciences, Inc., Seattle, WA, USA; at a dilution of 1:100) overnight at 4°C. After washing in PBS, a biotinylated anti‐rabbit antibody was applied for 10 min followed by a peroxidase‐marked streptavidin for an additional 10 min. The reaction was visualized using 3, 3′‐diaminobenzidine (DAB, chromogen). Negative control sections were incubated with PBS instead of the primary antibody. Sections were counterstained with haematoxylin, dehydrated, mounted and visualized by light microscope (Ramos‐Vara et al. 2008).
Morphometrical study
The data were obtained using a Leica QWin500 image analyzer (Leica Ltd, Cambridge, UK) in the image‐analysing unit of the Pathology department, Faculty of Dentistry, Cairo University (Egypt). The mean epithelial height of the seminiferous tubules was measured in H&E‐stained sections at a total magnification ×100. The area percentage of positive c‐Kit immune reactivity in anti‐c‐Kit immune‐stained sections was also assessed at a total magnification ×400. All measurements were made in 10 non‐overlapping fields from five sections for each rat in all groups using the interactive measure menu.
Statistical analysis
Data for all groups were expressed as mean ± standard deviation (X ± SD). The data obtained were subjected to spss program, version 19 (Chicago, IL, USA). Statistically significant difference was determined by one‐way analysis of variance, followed by the post hoc test for multiple comparisons between different groups. The probability values (P) were considered significant when <0.05 and highly significant when <0.001 (Petrie & Sabin 2005).
Ethical approval
Our investigation conformed to the guidelines of the medical research ethics committee of Zagazig University (Egypt).
Results
Body and testis weights
The initial body weight, final body weight, absolute testis weight and relative testis weight are given in Table 1. Body weight did not significantly differ between groups. There was a highly significant decrease in both absolute and relative testis weights in groups III (PM group) and IV (recovery) compared with group I (control). Group V (NG‐PM group) showed a highly significant increase compared with PM group but a non‐significant decrease compared to the control. Group II (NG group) revealed a non‐significant difference compared to control.
Table 1.
Initial body weight, final body weight, absolute testis weight and relative testis weight in the study groups
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| Control | NG | PM | Recovery | NG‐PM | |
| Initial body weight (g) | 191.17 ± 5.04 | 190 ± 3.85 | 191.83 ± 4.88 | 192.67 ± 5.75 | 189.67 ± 4.8 |
| Final body weight (g) | 217.83 ± 4.17 | 220.67 ± 4.32 | 212.17 ± 4.83 | 213.33 ± 4.84 | 215.83 ± 4.58 |
| Absolute testis weight (g/100 g final body weight) | 1.77 ± 0.06 | 1.75 ± 0.076 | 1.30 ± 0.052**,a | 1.51 ± 0.042**,a,**,b | 1.66 ± 0.06**,b |
| Relative testis weight (g) | 0.813 ± 0.038 | 0.806 ± 0.05 | 0.614 ± 0.033**,a | 0.706 ± 0.018**,a,*,b | 0.771 ± 0.032**,b |
Biochemical results
The biochemical assay of epididymal sperm counts and serum testosterone levels is given in Table 2. The NG group showed a non‐significant difference compared to the control group. There was a highly significant decrease in both parameters in the PM and recovery groups compared with the control. The NG‐PM group showed a highly significant increase compared with the PM group but a non‐significant decrease compared with the control.
Table 2.
Epididymal sperm count and serum testosterone level in the study groups
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| Control | NG | PM | Recovery | NG‐PM | |
| Epididymal sperm count (×106/ml) | 92.55 ± 3.55 | 89.73 ± 4.57 | 40.66 ± 11.83**,a | 61.29 ± 3.74**,a,**,b | 81.77 ± 5.63**,b |
| Serum testosterone level (ng/ml) | 4.03 ± 0.11 | 4.13 ± 0.18 | 1.78 ± 0.27**,a | 3.11 ± 0.19**,a,**,b | 3.65 ± 0.14**,b |
PM, permethrin; NG, naringenin. Values are expressed as mean ± standard deviation (SD) of n = 6 animals; a P value compared with control group; b P value compared with PM group; *Significant difference (P < 0.05); ∗∗Highly significant difference (P < 0.001).
Histopathological results
The light and electron microscopic examinations of the control group and NG group revealed similar results. Thus only the morphological results of the control group are presented for comparison with the other three groups.
H&E stain results
Light microscope examination of the control group showed that the testicular parenchyma consisted of densely packed seminiferous tubules lined by stratified germinal epithelium and separated by narrow interstitium. The stratified germinal epithelium was formed of spermatogonia, primary spermatocytes and spermatids. Sertoli cells were observed between these cells. The seminiferous tubules were separated by narrow interstitium containing interstitial cells of Leydig with acidophilic cytoplasm, and their lumina were filled with sperms (Figure 1a and b).
Figure 1.

H&E‐stained sections of testis of albino rats of the study groups. (a and b) Control. (a) Densely packed seminiferous tubules (T) lined by stratified germinal epithelium (G) and separated by a narrow interstitium (I) [×100]. (b) Parts of seminiferous tubules (T) with regular basement membrane (arrow) separated by narrow interstitium (I) containing interstitial cells of Leydig with acidophilic cytoplasm (arrowhead). The stratified germinal epithelium is formed of spermatogonia (g), primary spermatocytes (p) and spermatids (Sd). The lumen is filled with sperms (Sp). Sertoli cells (S) are observed between the spermatogenic cells [×400]. (c and d): Permethrin (PM) group. (c) Markedly distorted shrunken tubules (T) with narrow lumina. Homogenous material appeared in the lumen (*), while spermatozoa are almost absent [×100]. (d) Widely separated seminiferous tubules (T) by the interstitium which is filled with acidophilic hyaline material (*). The germinal epithelium is disorganized with decreased number of layers and irregular basement membrane (arrow). The germinal epithelial cells appear apoptotic (thick arrow), sloughed (arrowhead), or vacuolated (v) [×400]. (e and f) Recovery group. (e) Some distorted seminiferous tubules (T) with separated germinal epithelium (*) and regular basement membrane (arrow) [×100]. (f) Some germinal epithelial cells are apoptotic with darkly stained nuclei (arrow) and others are vacuolated (V). The interstitium is still filled with acidophilic hyaline material (*) [×400]. (g and h) Naringenin (NG)‐PM group. (g) Closely packed germinal epithelial cells (G), regular basement membrane (arrow) and some acidophilic hyaline material in the interstitium (*) [×100]. (h) Mostly normal cells with vesicular nuclei (curved arrow). Only few apoptotic (arrow) and vacuolated cells (v) can be seen. Notice Sertoli cells (S) [×400].
Examination of group III (PM group) revealed markedly distorted shrunken tubules with irregular basement membrane. Homogenous material appeared in the lumen, while spermatozoa were almost absent. The germinal epithelium was disorganized with decreased number of layers. The germinal epithelial spermatogenic cells appeared apoptotic, sloughed or vacuolated. The interstitium was increased and filled with acidophilic hyaline material (Figure 1c and d).
Group IV (recovery group) showed that some of seminiferous tubules were still distorted with separated germinal epithelial cells while the basement membrane retained its regularity. Some cells were apoptotic with darkly stained nuclei. Others revealed vacuolations. The interstitium was still filled with acidophilic hyaline material (Figure 1e and f).
Examination of group V (NG‐PM group) illustrated that most of the tubules regained its normal appearance with closely packed cells and regular basement membrane. Most of the spermatogenic cells were normal with vesicular nuclei except for few apoptotic and vacuolated cells. Some acidophilic hyaline material was still noticed in the interstitium (Figure 1g and h).
Ultrastuctural results
Electron microscopic examination of the control group showed seminiferous tubules with closely packed cells resting on a thin regular basement membrane. Sertoli cells with pale notched euchromatic nuclei, many mitochondria and electron‐dense granules were seen. Spermatids appeared with euchromatic rounded nuclei, numerous peripherally arranged mitochondria and well‐formed acrosomal cap. Leydig cells were observed in the interstitium with euchromatic nuclei and peripheral heterochromatin. The cytoplasm contained mitochondria with tubular cristae, lipid droplets and Golgi apparatus (Figure 2a–c).
Figure 2.

Electron micrograph of the testis of albino rat of control and permethrin (PM) groups. (a–c) Control. (a) A part of a seminiferous tubule with closely packed cells resting on a thin regular basement membrane (bm). A Sertoli cell (S) with a pale notched euchromatic nucleus (N), many mitochondria (m) and electron‐dense granules (arrow) can be seen [×3000]. (b) Spermatids (sd) with euchromatic rounded nuclei (N), numerous peripherally arranged mitochondria (m) and a well‐formed acrosomal cap (arrow) [×3000]. (c) Leydig cell (Ld) with euchromatic nucleus (N) and peripheral heterochromatin. The cytoplasm contains mitochondria (m) with tubular cristae, lipid droplets (L) and Golgi apparatus (G) [×6000]. (d–g) PM group. (d) Many intercellular spaces (*) between the germinal epithelial cells (g). Sertoli cells (S) with irregular nucleus (N) and prominent nucleolus (n) [×3000]. (e) Aspermatogenic cell (sp) separated from the surrounding cells by large space (*) with distorted nuclear envelope (short arrow) and cytoplasmic vacuoles (v). The basement membrane (bm) is thickened and irregular with enclosing flat myoid cell (my) [×4000]. (f) Spermatids (sd) with abnormally distributed mitochondria (arrow) and pyknotic nuclei (N) [×2000]. (g) Leydig cells (Ld) with irregular euchromatic nuclei and peripheral heterochromatin (N). The cytoplasm contains few lipid droplets (L), mitochondria with ruptured cristae (m), electron‐dense granules (arrowhead) and multiple cytoplasmic processes (arrow) [×4000].
Examination of the ultrathin sections of the PM group showed many intercellular spaces between the germinal epithelial cells. Sertoli cells appeared with irregular nuclei and prominent nucleoli. Most spermatogenic cells were separated by large spaces and appeared with distorted nuclear envelope and cytoplasmic vacuoles. The basement membrane appeared thickened and irregular with enclosing flat myoid cells. Some spermatids showed abnormally distributed mitochondria and pyknotic nuclei. Leydig cells revealed irregular euchromatic nucleis and peripheral heterochromatin. The cytoplasm contained few lipid droplets, mitochondria with ruptured cristae, electron‐dense granules and multiple cytoplasmic processes (Figure 2d–g).
The recovery group showed Sertoli cells with regular euchromatic nuclei resting on a regular thin basement membrane with enclosing flat myoid cells. Spermatids appeared with euchromatic rounded nuclei and numerous peripherally arranged mitochondria. Intercellular spaces were seen. Leydig cells revealed irregular euchromatic nuclei and peripheral heterochromatin. The cytoplasm contained some lipid droplets, mitochondria with intact cristae and electron‐dense granules of different sizes and shapes. Multiple cytoplasmic processes were still seen (Figure 3a and b).
Figure 3.
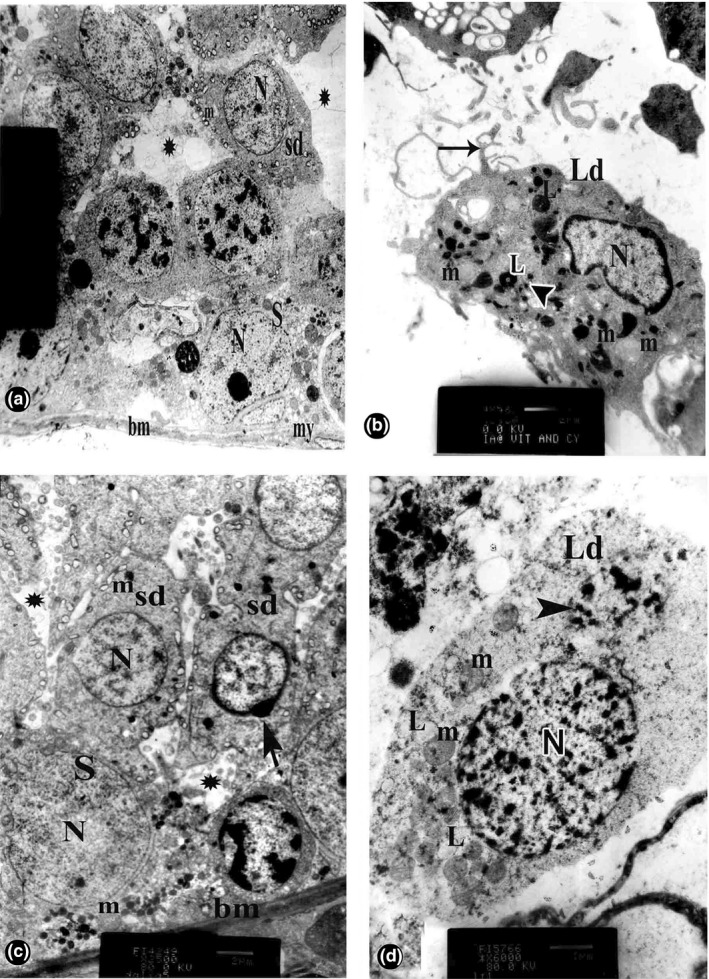
Electron micrograph of the testis of albino rat of recovery and naringenin (NG)–permethrin (PM) groups. (a and b) Recovery group. (a) Sertoli cells (S) with regular euchromatic nuclei (N) resting on a thin regular basement membrane (bm) with flat myoid cell (my). Spermatids (sd) appear with euchromatic rounded nuclei (N) and numerous peripherally arranged mitochondria (m). Intercellular spaces (*) are seen [×2000]. (b): Leydig cells (Ld) with irregular euchromatic nucleus (N) and peripheral heterochromatin. The cytoplasm contains mitochondria (m) with intact cristae, some lipid droplets (L) and variable‐sized electron‐dense bodies (arrowhead). Multiple cytoplasmic processes (arrow) are still noticed [×4500]. (c and d) NG‐PM group. (c) Sertoli cells (S) with pale euchromatic nucleus (N) and normal mitochondria (m) resting on regular thin basement membrane (bm). Spermatids (sd) with euchromatic rounded nuclei (N), numerous peripherally arranged mitochondria (m) and well‐formed acrosomal cap (arrow), but minimal intercellular spaces (*) are still present [×2500]. (d) Leydig cell (Ld) with euchromatic nucleus (N) and peripheral heterochromatin. The cytoplasm contains mitochondria (m) with tubular cristae, lipid droplets (L) and electron‐dense bodies (arrowhead). No cytoplasmic processes are observed [×6000].
Ultrathin sections of NG‐PM group revealed Sertoli cells with pale euchromatic nuclei and normal mitochondria resting on regular basement membrane. Spermatids were seen with euchromatic rounded nuclei, numerous peripherally arranged mitochondria and well‐formed acrosomal cap, but minimal intercellular spaces were still present. Leydig cells appeared with euchromatic nuclei and peripheral heterochromatin. The cytoplasm contained mitochondria with tubular cristae, lipid droplets and electron‐dense bodies. No cytoplasmic processes were observed (Figure 3c and d).
Immunohistochemical results
Immunohistochemically stained sections for c‐Kit of the control group showed brown positive immunoreactions in the cytoplasm of most germinal epithelial cells (Figure 4a).
Figure 4.

c‐Kit immunohistochemically stained sections of testis of albino rats of all studied groups. (a) Control group with brown positive immunoreactions in the cytoplasm of most germinal epithelial cells (arrow) [×400]. (b) Permethrin (PM) group with few immunoreactive cells (arrow) [×400]. (c) Recovery group with mild decrease in the immunoreactive cells (arrow) compared with the control group [×400]. (d) Naringenin (NG)‐PM group with nearly normal immunoreactions (arrow) [×400].
The PM group showed a marked decrease in the brown positively stained cells, compared with the control group (Figure 4b).
Sections of the recovery group showed a mild decrease in the immune reactive cells, compared with the control group (Figure 4c).
Group IV sections showed nearly normal positive immune reactions (Figure 4d).
Morphometrical results
Epithelial height
The epithelial height measurements showed a non‐significant difference between the NG group and the control. There were a highly significant decrease in the PM group and a significant decrease in the recovery group compared with the control. NG‐PM group showed a highly significant increase compared with PM group but a non‐significant decrease compared with the control (Table 3).
Table 3.
Epithelial height of the seminiferous tubules and area percentage of positive c‐Kit immune reactivity of the study groups
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| Control | NG | PM | Recovery | NG‐PM | |
| Epithelial height (µm) | 85.97 ± 8.21 | 87.08 ± 9.77 | 52.72 ± 5.48**,a | 71.58 ± 3.41**,a,*,b | 80.47 ± 6.99**,b |
| c‐Kit immunoreactivity (area %) | 75.74 ± 4.30 | 76.66 ± 7.38 | 23.69 ± 9.74**,a | 52.86 ± 6.65**,a,**,b | 71.768 ± 8.05**,b |
Values are expressed as mean ± standard deviation (SD) of n = 6 animals; a P value compared with control group; b P value compared with PM group; ∗Significant difference (P < 0.05); ∗∗Highly significant difference (P < 0.001). PM, permethrin; NG, naringenin
c‐Kit immune reactivity
The area percentage of positive c‐Kit immune reactivity showed a non‐significant difference between NG group and the control. There was a highly significant decrease in the PM group and the recovery group compared with the control, but the NG‐PM group showed a highly significant increase compared with the PM group and a non‐significant decrease compared with the control (Table 3).
Discussion
Exposure to environmental toxicants including pesticides is a proven factor in impairment of male reproductive system and infertility (Sharma et al. 2014). Permethrin (PM), the most popular insecticide among the synthetic pyrethroids, has been used worldwide (Zhang et al. 2008).
Animal studies showed quick absorption of 60% of orally administered PM with an absorption half‐life of <1 h. PM was rapidly distributed throughout the body with highest amounts accumulating in fat and the brain (Anadon et al. 1991). This can be explained by the lipophilicity of PM (Tornero‐Velez et al. 2012). The major route of PM metabolism included ester hydrolysis followed by conjugation mainly in the liver. Hydroxylation and oxidation pathways were also involved (Crow et al. 2007; Scollon et al. 2009). 3‐Phenoxybenzoic acid (3‐PBA) is the major metabolite of PM. It was proposed that 3‐PBA had anti‐androgenic activity in monkey kidney cells (Sun et al. 2006). However, Yuan et al. (2010) proved that 3‐PBA did not reduce sperm motility directly at all concentrations and treatment periods. The final metabolites were primarily excreted in the urine. Measurements of metabolites in urine have been used to assess exposure to pyrethroids (Morgan et al. 2007). The excretion half‐life was measured at 12.3 h (Anadon et al. 1991).
PM group
Testicular weight is a valuable index of reproductive toxicity in male animals (Jana et al. 2006). In the current study, PM toxicity was evidenced by a highly significant decrease in testicular weight and epididymal sperm count. This could be implicated to the decrease in serum testosterone level as observed in this study, which is consistent with the findings of other investigators (Zhang et al. 2008; Jin et al. 2012). PM exposure mainly induced Leydig cell mitochondrial membrane damage and disrupted the limiting steps of the steroidogenic process, suppressing mRNA expression levels of peripheral benzodiazepine receptor (PBR), cytochrome P450 and steroidogenic acute regulatory protein (StAR), so as to reduce testicular testosterone biosynthesis (Zhang et al. 2007).
In the present work, PM exposure induced testicular structural abnormalities including distortion and shrinkage of the seminiferous tubules due to a decrease in the number of germ cells. These results were confirmed by morphometrical analysis of the height of the epithelial lining which was significantly decreased in comparison with the control. Similar effects were observed by Issam et al. (2011) after subcutaneous PM treatment. It was speculated that PM has led to DNA damage and mutagenic alterations (Rosita et al. 2004). PM has evoked a genotoxic response upon generation of oxidative stress (Gabbianelli et al. 2009; Vadhana et al. 2010). Moreover, reduction of testosterone level may lead to separation of germ cells from the epithelium and impair the structure of the testis (Elbetieha et al. 2001).
Regarding examination of the germinal epithelium, we found disorganization, vacuolization and apoptosis. These findings were in agreement with those detected by Li et al. (2013) using another pyrethroid: cypermethrin. Ultrastructurally, the vacuolations appeared as intercellular spaces. They were probably a consequence of the death of germinal lineage cells and lack of elements of the seminiferous epithelium resulting in large spaces between contiguous Sertoli cells (Freitas et al. 2002). Leon et al. (2005) hypothesized that mitochondria play an important role in the apoptotic process. They added that mitochondrial dysfunction induced by oxidative stress can lead to the release of cytochrome c and then caspase activation, which results in apoptotic cell death.
Spermatozoa were absent in most of the seminiferous tubules, which was consistent with the reduction in epididymal sperm count found in this work. Ultrastructurally, some spermatids showed abnormally distributed mitochondria. Others had pyknotic nuclei. The disturbance detected in all germinal cells could be explained by lipophilicity of PM, whereby it could penetrate in the cells more easily than other pyrethroids (Issam et al. 2011). The specificity in the structure of PM is attributed to lacking an α‐cyanogroup (Cinzia et al. 2003).
With reference to the basement membrane in the current study, electron microscope examination revealed thickening and irregularity. Some reports have shown that certain stimulants may induce myoid cells to produce more collagen and extracellular matrix (Santoro et al. 1999). Increased production of glycosaminoglycans and proteoglycans has been considered as a defence reaction against the damaging effect of free radicals (Albertini et al. 2000).
In the present work, the interstitial spaces were widened. Similar findings were reported by Jin et al. (2012). The appearance of acidophilic hyaline material could be attributed to excess lymphatic exudates oozing from degenerative lymphatic vessels (Paniagua et al. 1991) or to an increase in vascular permeability (Salama et al. 2003). Electron microscope examination of Leydig cells showed irregular nuclei, few lipid droplets, electron‐dense granules, abnormal mitochondria with ruptured cristae and multiple cytoplasmic processes. Similarly in a previous study, high‐magnification profiles of Leydig cells showed that the mitochondrial inner membrane was disrupted and the cristae were replaced by a denser matrix. The outer membrane was very thin and sometimes fused with the inner membrane (Zhang et al. 2007).
The c‐Kit receptor is expressed by developing germ cells (Unni et al. 2009). It has been considered as a strong marker of the germ cell population (Sa et al. 2013). The c‐Kit receptor is also detected in Leydig cells (Muciaccia et al. 2010). c‐Kit displays distinct isoforms, which differ by their location at the cell membrane or in the cytoplasm (Zhang et al. 2013). Sertoli cells secrete stem cell factor (SCF) which influences the germ cells expressing c‐Kit (Correia et al. 2014). Interestingly, our study revealed a significant decrease in the area percentage of positive immunoreactions for c‐Kit in PM‐treated rats compared with the control. It is speculated that abnormal levels of androgens deregulate the expression of c‐Kit and stem cell factor (SCF), which may be linked with the aetiology of infertility (Figueira et al. 2014). The SCF/c‐Kit system determines the survival of germ cells (Yan et al. 2000a) and protects them from apoptosis (Yan et al. 2000b). It is also needed for the onset and progression of spermatogenesis (Sato et al. 2012).
Recovery group
In the present work, stoppage of PM in the recovery group partially reversed PM‐induced changes. There was a mild decrease in testicular weight, sperm count and serum testosterone level compared to the control group. Examination of the seminiferous tubules showed mild distortion. Some apoptotic and vacuolated cells were observed. Ultrastructurally, large intercellular spaces were still seen. Leydig cells revealed multiple cytoplasmic processes. Moreover, there was a mild decrease in the area percentage of c‐Kit immunoreactions compared with the control. Similar findings were detected in cypermethrin‐exposed fish, in which recovery response was detected in muscle and kidneys. However, enzyme activities did not show much improvement (Begum 2007). In this respect, other investigators observed persistent effects of cypermethrin during brain development in rats even after withdrawal of the treatment (Gupta et al. 1999). According to Nasuti et al. (2014), early‐life PM exposure had long‐lasting consequences on the hippocampus such as impairment of long‐term memory storage and synaptic morphology. Additionally, early‐life exposure to low doses of PM imparted cardiac hypotrophy and increased calcium and some gene expression levels in old age (Vadhana et al. 2013).
NG‐PM group
The involvement of oxidative stress in PM toxicity has been reported (Gabbianelli et al. 2004; Nasuti et al. 2008; Otitoju et al. 2008; Vadhana et al. 2011). The reactive oxygen species (ROS) generation and malondialdehyde (MDA) production were increased by PM treatment, while the activity of antioxidant enzymes such as catalase and superoxide dismutase and glutathione content had declined in the rats (Hu et al. 2010). Increased levels of ROS are in direct connection with oxidative metabolism of PM. During oxidation by microsomal fraction of the liver, a leakage of ROS can occur (Rose et al. 2005).
NG is a plant bioflavonoid richly found in citrus fruits and grapefruits (Prabu et al. 2011). NG can protect against ROS‐mediated cell toxicity through inhibition of intracellular ROS generation and ROS scavenging (Podder et al. 2014). Results of the present study indicated an overall improvement of PM‐induced biochemical alterations and testicular weight upon co‐administration of NG in the NG‐PM group. We recorded enhanced sperm count and testosterone level compared to PM‐treated rats. The increase in testosterone level might be attributed to the increased cell viability and testicular mass (Sharma et al. 2014). In the same group, light and electron microscopic examinations confirmed the biochemical results. The seminiferous tubules retained almost normal structure except for few apoptotic and vacuolated cells. Ultrastructurally small intercellular spaces were still present. Immune expression of c‐Kit was upregulated to a level almost identical with the control. These results were confirmed statistically. In the same context, previous studies have reported NG‐related protection against ROS‐mediated cell toxicity due to arsenic (Mershiba et al. 2013), carbon tetrachloride (Hermenean et al. 2013), lead (Wang et al. 2012), cadmium (Prabu et al. 2011) or cisplatin (Badary et al. 2005) administration in rats.
NG plays a role in cellular protection under stress conditions partly by its radical scavenging and iron‐chelating properties. It is speculated that ROS scavenging by NG may be through its 5‐hydroxy and 4‐carbonyl groups in the C ring, which may interact with Cu and Fe ions (Podder et al. 2014). Interestingly, NG can also effectively induce the expression of many antioxidant‐related genes (Renugadevi & Prabu 2009) and inhibit the activity of ROS‐forming enzymes (e.g. NADPH oxidase) (Ciz et al. 2012). Hermenean et al. (2013) proved the great ability of NG to attenuate lipid peroxidation by decreasing the high MDA level after carbon tetrachloride exposure.
On the other hand, it was suggested that the impact of PM could be related to the direct effect of mitochondrial membrane damage (Betancourt & Reséndiz 2006; Zhang et al. 2007). NG was found to modulate mitochondrial dysfunction by restoring mitochondrial membrane potential. Consequently, the apoptotic signalling cascade would stop by preventing apoptotic proteins release and translocation to the nucleus which precluded DNA damage. Thereby, NG prevented tissue damage by the inhibition of oxidative stress‐induced apoptosis (Kapoor & Kakkar 2014).
A dose of 50 mg/kg NG in rats corresponds to 8 mg/kg in humans. For an average person weighing 60 kg, 480 mg NG will be needed (CDER 2005). The high bioavailability of citrus flavanones in animal models (Felgines et al. 2000) and in man (Erlund et al. 2001, 2002) suggests that this group of flavonoids may reach target cells at high concentrations. Orange juice and other citrus juices provide sufficiently high tissue levels of NG in man to exert a biological effect (Breinholt et al. 2004).
Conclusion
PM induced testicular toxicity, which was evidenced by the reduced testicular weight, biochemical and histopathological changes. Stoppage of PM exposure was associated with partial recovery. However, NG co‐administration was protective. Accordingly, consumption of fruit and vegetables enriched in NG efficiently counteracts toxins and maintains reproductive health. Nonetheless, despite this potential method of counteracting the toxicity, there is a continuing need for countries to balance pesticide use with environmental degradation as well as health safety.
Conflict of interest
There is no conflict of interest.
Funding source
This study was not funded by any source.
References
- Albertini R., Passi A., Abuja P.M. et al (2000) The effect of glycosaminoglycans and proteoglycans on lipid peroxidation. Int. J. Mol. Med. 6, 129–136. [DOI] [PubMed] [Google Scholar]
- Anadon A., Martinez‐Larranaga M.R., Diaz M.J. et al (1991) Toxicokinetics of permethrin in the rat. Toxicol. Appl. Pharmacol. 110, 1–8. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease) (2005) Registry. Toxicologic information about insecticides used for eradicating mosquitoes (West Nile virus control‐permethrin). Available: http://www.atsdr.cdc.gov/consultations/west_nile_virus.
- Ayache J., Beaunier L., Boumendil J. et al (2010) Sample Preparation Handbook for Transmission Electron Microscopy Techniques. New York, USA: Springer Science and Business Media. [Google Scholar]
- Badary O.A., Abdel‐Maksoud S., Ahmed W.A. et al (2005) Naringenin attenuates cisplatin nephrotoxicity in rats. Life Sci. 76, 2125–2135. [DOI] [PubMed] [Google Scholar]
- Begum G. (2007) Cypermethrin‐induced biochemical perturbations in freshwater fish Clarias batrachus at sub‐lethal exposure and after released into freshwater. Drug Chem. Toxicol. 30, 55–65. [DOI] [PubMed] [Google Scholar]
- Betancourt M. & Reséndiz A. (2006) Effect of two insecticides and two herbicides on the porcine sperm motility patterns using computer‐assisted semen analysis (CASA) in vitro. Reprod. Toxicol. 22, 508–512. [DOI] [PubMed] [Google Scholar]
- Bradberry S.M., Cage S.A., Proudfoot A.T. et al (2005) Poisoning due to pyrethroids. Toxicol. Rev. 24, 93–106. [DOI] [PubMed] [Google Scholar]
- Breinholt V.M., Svendsen G.W., Dragsted L.O. et al (2004) The citrus‐derived flavonoid naringenin exerts uterotrophic effects in female mice at human relevant doses. Basic Clin. Pharmacol. Toxicol. 94, 30–36. [PubMed] [Google Scholar]
- Carloni M., Nasuti C., Fedeli D. et al (2012) The impact of early life permethrin exposure on development of neurodegeneration in adulthood. Exp. Gerontol. 47, 60–66. [DOI] [PubMed] [Google Scholar]
- CDER, USFDA (2005) Guidance for Industry: Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078932.pdf
- Chen D., Chen M.S., Cui Q.C. et al (2007) Structure proteasome‐ inhibitory activity relationships of dietary flavonoids in human cancer cells. Front Biosci. 12, 1935–1945. [DOI] [PubMed] [Google Scholar]
- Cinzia N., Franco C., Giancarlo F. et al (2003) Different effects of type I and type II pyrethroids on erythrocyte plasma membrane properties and enzymatic activity in rats. Toxicology 191, 233–244. [DOI] [PubMed] [Google Scholar]
- Ciz M., Denev P., Kratchanova M. et al (2012) Flavonoids inhibit the respiratory burst of neutrophils in mammals. Oxid. Med. Cell Longev., 2012, Article ID 181295, 6 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia S., Alves M.R., Cavaco J.E. et al (2014) Estrogenic regulation of testicular expression of stem cell factor and c‐kit: implications in germ cell survival and male fertility. Fertil. Steril. 102, 299–306. [DOI] [PubMed] [Google Scholar]
- Crow J.A., Borazjani A., Potter P.M. et al (2007) Hydrolysis of pyrethroids by human and rat tissues: examination of intestinal, liver and serum carboxylesterases. Toxicol. Appl. Pharmacol. 221, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Jin L., Han X. et al (2009) Naringenin: a potential immunomodulator for inhibiting lung fibrosis and metastasis. Cancer Res. 69, 3205–3212. [DOI] [PubMed] [Google Scholar]
- Elbetieha A., Da'as S.I., Khamas W. et al (2001) Evaluation of the toxic potentials of cypermethrin pesticide on some reproductive and fertility parameters in the male rats. Arch. Environ. Contam. Toxicol. 41, 522–528. [DOI] [PubMed] [Google Scholar]
- Erlund I., Meririnne E., Alfthan G. et al (2001) Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. 131, 235–241. [DOI] [PubMed] [Google Scholar]
- Erlund I., Silaste M.L., Alfthan G. et al (2002) Plasma concentrations of the flavonoids hesperetin, naringenin and quercetin in human subjects following their habitual diets, and diets high or low in fruit and vegetables. Eur. J. Clin. Nutr. 56, 891–898. [DOI] [PubMed] [Google Scholar]
- Etang J., Chouaibou M., Toto J.C., et al (2007) A preliminary test of the protective efficacy of permethrin‐treated bed nets in an area of Anopheles gambiae metabolic resistance to pyrethroids in north Cameroon. Trans. R. Soc. Trop. Med. Hyg. 101, 881–884. [DOI] [PubMed] [Google Scholar]
- Falcioni M.L., Nasuti C., Bergamini C. et al (2010) The primary role of glutathione against nuclear DNA damage of striatum induced by permethrin in rats. Neuroscience 168, 2–10. [DOI] [PubMed] [Google Scholar]
- Fang F., Tang Y., Gao Z. et al (2010) A novel regulatory mechanism of naringenin through inhibition of T lymphocyte function in contact hypersensitivity suppression. Biochem. Biophys. Res. Commun. 397, 163–169. [DOI] [PubMed] [Google Scholar]
- Fedeli D., Montani M., Carloni M. et al (2012) Leukocyte Nurr1 as peripheral biomarker of early‐life environmental exposure to permethrin insecticide. Biomarkers 17, 604–609. [DOI] [PubMed] [Google Scholar]
- Felgines C., Texier O., Morand C. et al (2000) Bioavailability of the flavanone naringenin and its glycosides in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G1148–G1154. [DOI] [PubMed] [Google Scholar]
- Figueira M.I., Cardoso H.J., Correia S. et al (2014) Hormonal regulation of c‐KIT receptor and its ligand: implications for human infertility? Prog. Histochem. Cytochem. 49, 1–19. [DOI] [PubMed] [Google Scholar]
- Freitas F.E.L., Cordeiro‐Mori F., Sasso‐Cerri E. et al (2002) Alterations of spermatogenesis in etoposide‐treated rats: a stereological study. Interciencia 27, 227–235. [Google Scholar]
- Gabbianelli R., Nasuti C., Falcioni G. et al (2004) Lymphocyte DNA damage in rats exposed to pyrethroids: effect of supplementation with Vitamins E and C. Toxicology 203, 17–26. [DOI] [PubMed] [Google Scholar]
- Gabbianelli R., Falcioni M.L., Cantalamessa F. et al (2009) Permethrin induces lymphocyte DNA lesions at both Endo III and Fpg sites and changes in monocyte respiratory burst in rats. J. Appl. Toxicol. 29, 317–322. [DOI] [PubMed] [Google Scholar]
- Gnanasoundari M. & Pari L. (2006) Impact of naringenin on oxytetracycline‐mediated oxidative damage in kidney of rats. Ren. Fail. 28, 599–605. [DOI] [PubMed] [Google Scholar]
- Gupta A., Agarwal R. & Shukla G.S. (1999) Functional impairment of blood‐brain barrier following pesticide exposure during early development in rats. Hum. Exp. Toxicol. 18, 174–179. [DOI] [PubMed] [Google Scholar]
- Hermenean A., Ardelean A., Stan M. et al (2013) Protective effects of naringenin on carbon tetrachloride‐induced acute nephrotoxicity in mouse kidney. Chem. Biol. Interact. 205, 138–147. [DOI] [PubMed] [Google Scholar]
- HSDB (Hazardous Substances Data Bank. National Library of Medicine) (2003) National Toxicology Program, Bethesda, MD: HSDB; Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB. [Google Scholar]
- Hu F., Li L., Wang C. et al (2010) Enantioselective induction of oxidative stress by permethrin in rat adrenal pheochromocytoma (PC12) cells. Environ. Toxicol. Chem. 29, 683–690. [DOI] [PubMed] [Google Scholar]
- Issam C., Zohra H., Monia Z. et al (2011) Effects of dermal sub‐chronic exposure of pubescent male rats to permethrin (PRMT) on the histological structures of genital tract, testosterone and lipoperoxidation. Exp. Toxicol. Pathol. 63, 393–400. [DOI] [PubMed] [Google Scholar]
- Jain A., Yadav A., Bozhkov A.I. et al (2011) Therapeutic efficacy of silymarin and naringenin in reducing arsenic‐induced hepatic damage in young rats. Ecotoxicol. Environ. Saf. 74, 607–614. [DOI] [PubMed] [Google Scholar]
- Jana K., Jana S. & Samanta P.K. (2006) Effects of chronic exposure to sodium arsenite on hypothalamo‐pituitary testicular activities in adult rats; possible estrogenic mode of action. Reprod. Biol. Endocrinol. 16, 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Liu J., Wang L. et al (2012) Permethrin exposure during puberty has the potential to enantioselectively induce reproductive toxicity in mice. Environ. Int. 42, 144–151. [DOI] [PubMed] [Google Scholar]
- Kannappan S., Palanisamy N. & Anuradha C.V. (2010) Suppression of hepatic oxidative events and regulation of eNOS expression in the liver by naringenin in fructose‐administered rats. Eur. J. Pharmacol. 645, 177–184. [DOI] [PubMed] [Google Scholar]
- Kapoor R. & Kakkar P. (2014) Naringenin accords hepatoprotection from streptozotocin induced diabetes in vivo by modulating mitochondrial dysfunction and apoptotic signaling cascade. Toxicol. Rep. 1, 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbalay‐Doust S., Noorafshan A., Ardekani F.M. et al (2007) The reversibility of sperm quality after discontinuing nandrolone decanoate in adult male rats. Asian J. Androl. 9, 235–239. [DOI] [PubMed] [Google Scholar]
- Kiernan J.A. (2000) Histological and Histochemical Methods: Theory and Practice, 3rd edn London, New York & New Delhi: Hodder Arnold Publishers, pp. 175–180. [Google Scholar]
- Kim S., Kwon H., Park J.H. et al (2012) A low level of serum total testosterone is independently associated with nonalcoholic fatty liver disease. BMC Gastroenterol. 12, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen L.W., Lawrence K.L. & Coleman R.E. (2009) The role of the United States military in the development of vector control products, including insect repellents, insecticides, and bed nets. J. Vector. Ecol. 34, 50–61. [DOI] [PubMed] [Google Scholar]
- Koutros S., Beane Freeman L.E., Berndt S.I. et al (2010) Pesticide use modifies the association between genetic variants on chromosome 8q24 and prostate cancer. Cancer Res. 70, 9224–9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartsson J., Voytyuk O., Heiss E. et al (2005) C‐KIT signal transduction and involvement in cancer. Cancer Ther. 3, 5–28. [Google Scholar]
- Leon J., Acuna‐Castroviejo D., Escames G. et al (2005) Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 2005, 38. [DOI] [PubMed] [Google Scholar]
- Li Y.F., Pan C., Hu J.X. et al (2013) Effects of cypermethrin on male reproductive system in adult rats. Biomed. Environ. Sci. 26, 201–208. [DOI] [PubMed] [Google Scholar]
- Mershiba S.D., Dassprakash M.V. & Saraswathy S.D. (2013) Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol. Biol. Rep. 40, 3681–3691. [DOI] [PubMed] [Google Scholar]
- Morgan M.K., Sheldon L.S., Croghan C.W. et al (2007) An observational study of 127 preschool children at their homes and day care centers in Ohio: environmental pathways to cis‐ and trans‐permethrin exposure. Environ. Res. 104, 266–274. [DOI] [PubMed] [Google Scholar]
- Muciaccia B., Sette C., Paronetto M.P. et al (2010) Expression of a truncated form of KIT tyrosine kinase in human spermatozoa correlates with sperm DNA integrity. Hum. Reprod. 25, 2188–2202. [DOI] [PubMed] [Google Scholar]
- Nasuti C., Falcioni M.L., Nwankwo I.E. et al (2008) Effect of permethrin plus antioxidants on locomotor activity and striatum in adolescent rats. Toxicology 251, 45–50. [DOI] [PubMed] [Google Scholar]
- Nasuti C., Fattoretti P., Carloni M. et al (2014) Neonatal exposure to permethrin pesticide causes lifelong fear and spatial learning deficits and alters hippocampal morphology of synapses. J. Neurodev. Disord. 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otitoju O., Onwurah I.N., Otitoju G.T. et al (2008) Oxidative stress and superoxide dismutase activity in brain of rats fed with diet containing permethrin. Biokemistri 20, 93–98. [Google Scholar]
- Paniagua R., Nistal M., Saez F.J. et al (1991) Ultrastructure of the aging human testis. J. Electron Microsc. Tech. 19, 241–260. [DOI] [PubMed] [Google Scholar]
- Petrie A., Sabin C. (2005) Basic techniques for analysing data In: Medical Statistics at a Glance, 2nd edn, pp. 55–56 (eds Sugden M., Moore K.). Malden, Massachusetts, USA: Blackwell Publishing Ltd. [Google Scholar]
- Podder B., Song H.Y. & Kim Y.S. (2014) Naringenin exerts cytoprotective effect against paraquat‐induced toxicity in human bronchial epithelial BEAS‐2B cells through NRF2 Activation. J. Microbiol. Biotechnol. 24, 605–613. [DOI] [PubMed] [Google Scholar]
- Pogribny I.P. & Rusyn I. (2013) Environmental toxicants, epigenetics, and cancer. Adv. Exp. Med. Biol. 754, 215–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabu S.M., Shagirtha K. & Renugadevi J. (2011) Reno‐protective effect of Naringenin in combination with vitamins C and E on cadmium induced oxidative nephrotoxicity in rats. J. Pharm. Res. 4, 1921–1926. [Google Scholar]
- Ramos‐Vara J.A., Kiupel M., Baszler T. et al (2008) American association of veterinary laboratory diagnosticians subcommittee on standardization of immunohistochemistry: suggested guidelines for immunohistochemical techniques in veterinary diagnostic laboratories. J. Vet. Diagn. Invest. 20, 393–413. [DOI] [PubMed] [Google Scholar]
- Renugadevi J. & Prabu S.M. (2009) Naringenin protects against cadmium‐induced oxidative renal dysfunction in rats. Toxicology 256, 128–134. [DOI] [PubMed] [Google Scholar]
- Renugadevi J. & Prabu S.M. (2010) Cadmium‐induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 62, 171–181. [DOI] [PubMed] [Google Scholar]
- Roma G.C., De Oliveira P.R., Bechara G.H. et al (2012) Cytotoxic effects of permethrin on mouse liver and spleen cells. Microsc. Res. Tech. 75, 229–238. [DOI] [PubMed] [Google Scholar]
- Ronnstrand L. (2004) Signal transduction via the stem cell factor receptor/c‐Kit. Cell. Mol. Life Sci. 61, 2535–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R.L., Tang J., Choi J. et al (2005) Pesticide metabolism in humans, including polymorphisms. Scand. J. Work Environ. Health 31, 156–163. [PubMed] [Google Scholar]
- Rosita R., Cinzia N., Giancarlo F. et al (2004) Lymphocyte DNA damage in rats exposed to pyrethroids: effect of supplementation with vitamins E and C. Toxicology 203, 17–26. [DOI] [PubMed] [Google Scholar]
- Rossbach B., Appel K.E., Mross K.G. et al (2010) Uptake of permethrin from impregnated clothing. Toxicol. Lett. 192, 50–55. [DOI] [PubMed] [Google Scholar]
- Rusiecki J.A., Patel R., Koutros S. et al (2009) Cancer incidence among pesticide applicators exposed to permethrin in the Agricultural Health Study. Environ. Health Perspect. 117, 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa R., Miranda C., Carvalho F. et al (2013) Expression of stem cell markers: OCT4, KIT, ITGA6, and ITGB1 in the male germinal epithelium. Syst. Biol. Reprod. Med. 59, 233–243. [DOI] [PubMed] [Google Scholar]
- Salama N., Bergh A. & Damber J.E. (2003) The changes in testicular vascular permeability during progression of the experimental varicocele. Eur. Urol. 43, 84–91. [DOI] [PubMed] [Google Scholar]
- Santoro G., Romeo C., Impellizzeri P. et al (1999) A morphometric and ultrastructural study of the changes in the lamina propria in adolescents with varicocele. BJU Int. 83, 828–832. [DOI] [PubMed] [Google Scholar]
- Sato T., Yokonishi T., Komeya M. et al (2012) Testis tissue explantation cures spermatogenic failure in c‐Kit ligand mutant mice. Proc. Natl Acad. Sci. USA 109, 16934–16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollon E.J., Starr J.M., Godin S.J. et al (2009) In vitro metabolism of pyrethroid pesticides by rat and human hepatic microsomes and cytochrome P450 isoforms. Drug Metab. Dispos. 37, 221–228. [DOI] [PubMed] [Google Scholar]
- Shafer T.J. & Meyer D.A. (2004) Effects of pyrethroids on voltage sensitive calcium channels: a critical evaluation of strengths, weaknesses, data needs, and relationship to assessment of cumulative neurotoxicity. Toxicol. Appl. Pharmacol. 196, 303–318. [DOI] [PubMed] [Google Scholar]
- Sharma P., Huq A.U. & Singh R. (2014) Cypermethrin‐induced reproductive toxicity in the rat is prevented by resveratrol. J. Hum. Reprod. Sci. 7, 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikarwar A.P. & Reddy K.V.R. (2008) SiRNA‐mediated silencing of c‐kit in mouse primary spermatogonial cells induces cell cycle arrest. Oligonucleotides 18, 145–160. [DOI] [PubMed] [Google Scholar]
- Sun H., Xu X.L., Xu L.C. et al (2006) Antiandrogenic activity of pyrethroid pesticides and their metabolite in reporter gene assay. Chemosphere 66, 474–479. [DOI] [PubMed] [Google Scholar]
- Tornero‐Velez R., Davis J., Scollon E.J. et al (2012) A pharmacokinetic model of cis‐ and trans‐permethrin disposition in rats and humans with aggregate exposure application. Toxicol. Sci. 130, 33–47. [DOI] [PubMed] [Google Scholar]
- Türkez H. & Aydin E. (2013) The genoprotective activity of resveratrol on permethrin‐induced genotoxic damage in cultured human lymphocytes. Braz. Arch. Boil. Technol. 56, 405–411. [Google Scholar]
- Unni S.K., Modi D.N., Pathak S.G. et al (2009) Stage‐specific localization and expression of c‐kit in the adult human testis. J. Histochem. Cytochem. 57, 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadhana M.D., Nasuti C. & Gabbianelli R. (2010) Purine bases oxidation and repair following permethrin insecticide treatment in rat heart cells. Cardiovasc. Toxicol. 10, 199–207. [DOI] [PubMed] [Google Scholar]
- Vadhana M.D., Carloni M., Nasuti C. et al (2011) Early life permethrin insecticide treatment leads to heart damage in adult rats. Exp. Gerontol. 46, 731–738. [DOI] [PubMed] [Google Scholar]
- Vadhana M.D., Arumugam S.S., Carloni M. et al (2013) Early life permethrin treatment leads to long‐term cardiotoxicity. Chemosphere 93, 1029–1034. [DOI] [PubMed] [Google Scholar]
- Wang J., Yang Z., Lin L. et al (2012) Protective effect of naringenin against lead‐induced oxidative stress in rats. Biol. Trace Elem. Res. 146, 354–359. [DOI] [PubMed] [Google Scholar]
- Wolansky M.J. & Harrill J.A. (2008) Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol. Teratol. 30, 55–78. [DOI] [PubMed] [Google Scholar]
- Yan W., Kero J. & Huhtaniemi I. (2000a) Stem cell factor functions as a survival factor for mature Leydig cells and a growth factor for precursor Leydig cells after ethylene dimethane sulfonate treatment: implication of a role of the stem cell factor/c‐Kit system in Leydig cell development. Dev. Biol. 227, 169–182. [DOI] [PubMed] [Google Scholar]
- Yan W., Suominen J. & Toppari J. (2000b) Stem cell factor protects germ cells from apoptosis in vitro. J. Cell Sci. 113, 161–168. [DOI] [PubMed] [Google Scholar]
- Yuan C., Wang C., Gao S.Q. et al (2010) Effects of permethrin, cypermethrin and 3‐phenoxybenzoic acid on rat sperm motility in vitro evaluated with computer‐assisted sperm analysis. Toxicol. In Vitro 24, 382–386. [DOI] [PubMed] [Google Scholar]
- Zhang S.Y., Ito Y., Yamanoshita O. et al (2007) Permethrin may disrupt testosterone biosynthesis via mitochondrial membrane damage of Leydig cells in adult male mouse. Endocrinology 148, 3941–3949. [DOI] [PubMed] [Google Scholar]
- Zhang S.Y., Ueyama J., Ito Y. et al (2008) Permethrin may induce adult male mouse reproductive toxicity due to cis isomer not trans isomer. Toxicology 248, 136–141. [DOI] [PubMed] [Google Scholar]
- Zhang L., Tang J., Haines C.J. et al (2013) c‐kit expression profile and regulatory factors during spermatogonial stem cell differentiation. BMC Dev. Biol. 13, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]


