Summary
Neonatal exposure to monosodium glutamate (MSG) induces circadian disorders in several physiological and behavioural processes regulated by the suprachiasmatic nucleus (SCN). The objective of this study was to evaluate the effects of neonatal exposure to MSG on locomotor activity, and on morphology, cellular density and expression of proteins, as evaluated by optical density (OD), of vasopressin (VP)‐, vasoactive intestinal polypeptide (VIP)‐ and glial fibrillary acidic protein (GFAP)‐immunoreactive cells in the SCN. Male Wistar rats were used: the MSG group was subcutaneously treated from 3 to 10 days of age with 3.5 mg/g/day. Locomotor activity was evaluated at 90 days of age using ‘open‐field’ test, and the brains were processed for immunohistochemical studies. MSG exposure induced a significant decrease in locomotor activity. VP‐ and VIP‐immunoreactive neuronal densities showed a significant decrease, while the somatic OD showed an increase. Major axes and somatic area were significantly increased in VIP neurons. The cellular and optical densities of GFAP‐immunoreactive sections of SCN were significantly increased. These results demonstrated that newborn exposure to MSG induced morphological alterations in SCN cells, an alteration that could be the basis for behavioural disorders observed in the animals.
Keywords: glial fibrillary acidic protein, locomotor activity, monosodium glutamate, suprachiasmatic nucleus, vasoactive intestinal polypeptide, vasopressin
In the central nervous system of mammals, the most abundant excitatory neurotransmitter is glutamate. In particular, this amino acid plays an important role not only in neuronal proliferation, survival, migration and differentiation (Jansson & Akerman 2014), but also in synaptic plasticity, learning and memory (Riedel et al. 2003) as well as in circadian rhythms (Silver & Kriegsfeld 2014; Ramkisoeensing & Meijer 2015).
Monosodium glutamate (MSG) is the salt of sodium glutamate used to increase the taste of different foods. The consumption of MSG has been reported to be capable of inducing adverse reaction in an unknown percentage of the population and as a key factor in the development of the MSG symptom complex characterized by headache, burning sensation in the lower arm and chest, nausea, tachycardia, drowsiness, bronchospasm, weakness and sweating (U. S. Department of Health and Human Services, U. S. Food and Drug Administration 1995). Studies in rodents have shown that the administration of MSG at the neonatal stage produces neuronal decrease in the retina (Lucas & Newhouse 1957), arcuate nucleus (Olney 1969; Holzwarth‐McBride et al. 1976; Bloch et al. 1984), ventromedial nucleus, hippocampus and cerebral cortex as well as neuronal morphology alterations in the last two regions (Ureña‐Guerrero et al. 2009). Moreover, disorders in the secretion of different hormones (Ferry et al. 1981; Fernandes et al. 2012) and alterations in locomotor activity (Dubovicky et al. 1997; Hlinak et al. 2005) have been shown.
Some studies in rodents have shown that neonatal exposure to MSG induces alterations in the circadian rhythms of corticosterone secretion (Miyabo et al. 1982), locomotor activity (Kiss et al. 2007) and sleep–wake cycle (Olivo et al. 1986) where the suprachiasmatic nucleus (SCN) of the anterior hypothalamus is found to be deeply involved in the alterations (Prendergast et al. 2013; Guimarães et al. 2015). Using haematoxylin–eosin technique, Tanaka et al. (1978) showed that rodent pups exposed to MSG had SCN neuronal degeneration, while Sun et al. (1991) demonstrated a significant decrease in neuronal density in cresyl violet‐stained histological sections. In mammals, SCN is the circadian pacemaker that functions in self‐sustained form in a scale of 24 h for the generation and maintenance of temporal order in different physiological and behavioural processes of the organism (Klein et al. 1991; Reppert & Weaver 2002; Reale et al. 2013). SCN has anatomically been divided into two regions. The ventrolateral region or the core receives photic information directly from the retina through retinohypothalamic tract that uses glutamate as the principal neurotransmitter. Moreover, it receives non‐photic information from geniculohypothalamic tract and from dorsal and median raphe nuclei. Its neurons are vasoactive intestinal polypeptide (VIP)‐immunoreactive and process‐synchronizing signals which are relayed to the dorsomedial region or shell that contains vasopressin (VP)‐immunoreactive cells, in charge of processing information from hypothalamus and limbic areas. These cells function as pacemakers that relay circadian signals to the rest of the organism (Klein et al. 1991; Golombek & Rosenstein 2010). The levels of VIP and VP show a circadian fluctuation in SCN and could be considered as indicators of its function (Inouye & Shibata 1994). VP and its receptor (VIa) could play an important role in the synchronization of SCN cellular oscillation (Bittman 2009). Moreover, the immunoreactive cells to VP are directly involved in the regulation of the circadian rhythm of locomotor activity as this behaviour is inhibited with systemic injection or microinjection of VP within the SCN (Cormier et al. 2015). On the other hand, several studies have shown that astrocytes in SCN play a crucial role in circadian pacemaker function (Prosser et al. 1994; Shinohara et al. 1995). However, reports analysing the effects of MSG administration in neonatal stage on SCN‐specific cell types are non‐existent. On this basis, the objectives of this study were to determine whether neonatal exposure to MSG induces alterations in locomotor activity; VP‐ and VIP‐immunoreactive neuronal morphologies; and cellular density of VP‐, VIP‐, and glial fibrillary acidic protein (GFAP)‐immunoreactive cells as well as in the expression of these proteins in SCN of adult male rats.
Materials and methods
Animals
Pregnant Wistar rats bred at Instituto Nacional de Pediatría, Mexico City, were used. On the delivery day (day 1 of postnatal life), and for the purposes of ensuring adequate nutritional state until weaning, the size of the litters was adjusted to 8 pups (4 males and 4 females). The pups were weaned at 21 days of age with all animals having access to water and standard laboratory food ad libitum (Teklad Global 2018S; Harlan Laboratories, Inc, Madison, WI, USA) and were maintained under light/dark cycle of 12:12 h (light on at 06:00 h), temperature of 21°C ± 1°C and relative humidity of 52 ± 10%.
The study was approved by the Committee of research of Instituto Nacional de Pediatria, Mexico City. All animals were treated in accordance with the ethical principles and specified regulations which conforms to international guidelines for laboratory animal handling.
MSG administration
The male pups of the group exposed to MSG received 3.5 mg/g/day of MSG (Sigma‐Aldrich, St. Louis, MO, USA), while the male control group was given only saline solution. Because sodium chloride equimolar treatment did not affect locomotor activity (Dubovicky et al. 1997), morphology and cellular density, and [3H] GABA uptake, compared to intact group (Ureña‐Guerrero et al. 2009), we decided not to use an equimolar group. The amount of MSG consumption varies in each country, and even among their populations. The mean daily intake of MSG reported for the United States is 550 mg/d; United Kingdom, 580 mg/day–4.68 g/day; Japan and Korea, 1.2–1.7 g/day. However, the daily consumption of MSG may be up to 10 g/day (He et al. 2011). Therefore, the amount of MSG used in this study reflects 25 times the consumption levels of extreme users. Both solutions were subcutaneously administered from 3rd to 10th day of postnatal life (Miyabo et al. 1985). Each of the litters contains the same number of pups treated with MSG or with the vehicle.
Locomotor activity
At 90 days of life, five male rats from each group were randomly selected to evaluate locomotor activity using ‘open‐field’ test with some modifications in what was reported by Sansar et al. (2011). In the beginning of the dark phase (18:00), each of the animals was individually placed in the centre of the field (always facing the same direction). Then, the ambulation (number of squares traversed by animal) of the animals for a period of 30 min was registered. After each test, the field was cleaned with a solution of alcohol at 70%.
Obtention of samples and tissue processing
A day after the evaluation of locomotor activity, the animals were euthanized between 12:00 and 13:00 h with an overdose of sodium pentobarbital (40 mg/kg, ip; Pfizer, Toluca, Estado de Mexico, Mexico) and were intracardially perfused with physiological saline solution (0.9%) followed by paraformaldehyde at 4% in phosphate‐buffered saline (PBS; 0.1 M, pH 7.4). The brains were dissected, postfixed for 5 h in fresh fixative and were cryoprotected in solutions of 10%, 20% and 30% sucrose in PBS. The brains were sectioned in coronal plane at a thickness of 40 μm with a cryostat (CM1850; Leica Microsystems Nussloch GmbH, Nussloch, Germany). To correctly identify the tissue sections containing SCN which were selected for immunostaining, some tissue sections of each brain were stained with cresyl violet and were observed in a microscope at ×10. These were identified with stereotaxic atlas (Paxinos & Watson 1998).
Tissue sections containing SCN were separately collected in an alternate form to obtain 3 independent series of each brain. Each of the series was then processed for immunohistochemical detection of VIP, VP or GFAP.
Immunohistochemistry
The tissue sections were washed in PBS to remove excess of aldehydes and then exposed in 0.3% hydrogen peroxide (Merck KGaA, Darmstadt, Germany) solution for 10 min. The free‐floating sections were subjected to incubation with rabbit polyclonal antibodies against VIP, raised against amino acids 1‐95 mapping at the N‐terminus of VIP that detects a band of 20 kDa on Western blots of mouse brain (manufacturer's data sheets) (sc‐20727; Santa Cruz Biotechnology, Santa Cruz, CA, USA), VP, <1% cross‐reactivity with oxytocin (manufacturer's data sheets) (AB1565; Chemicon International/Millipore, Billerica, MA, USA), or GFAP, raised against amino acids 1‐50 of GFAP that detects a 50‐kDa band on Western blots of mouse brain (manufacturer's data sheets) (sc‐9065; Santa Cruz Biotechnology) at a dilution of 1:500 in 5% bovine serum albumin (Amersham Biosciences, Buckinghamshire, UK) and 0.1% Tween‐20 (Sigma‐Aldrich) in PBS for 72 h at 4°C. This was followed by 1‐h incubation at room temperature with biotinylated anti‐rabbit IgG (Dako, Carpinteria, CA, USA). Subsequently, the sections were incubated with streptavidin–HRP (Dako) for 1 h at room temperature. The antigen–antibody–peroxidase complex was stained with diaminobenzidine (Dako) in accordance with the manufacturer's instruction. The sections were rinsed three times, each for 10 min, with PBS between incubations and mounted on gelatin‐subbed slides, dehydrated with graded ethanol solutions and coverslipped with Entellan (Merck). All tissue sections from control group and MSG‐exposed group were processed at the same time to minimize any variation in immunostaining procedure. To confirm that immunostaining was specific to the antigen of interest, negative controls were incubated omitting the primary antibody, but all other procedures were maintained. No immunoreactive cells were observed when the primary antibody was absent in tissue sections.
Morphological quantification
Slides containing middle level of SCN tissue sections of both groups were randomized and coded to ensure that further analysis is blindly performed. Unilateral images of two tissue sections from the middle level of SCN (anteroposterior, 1.3 mm of bregma, stereotaxic atlas of Paxinos & Watson 1998) were captured with an image‐analysing system (MetaMorph, version 4.5; Molecular Devices, Downingtown, PA, USA) attached to a light microscope (DMLS; Leica Microsystems GMBH, Wetzlar, Germany). The images were manually analysed using a previously established quantification protocol in our laboratory (Rojas‐Castañeda et al. 2011) with some modifications. Briefly, SCN cellular density was determined at ×60 in VP‐, VIP‐, or GFAP‐immunoreactive sections. The immune‐stained cells that were counted had a minimum immunoreactivity rate of 3:1 in relation to the background optical density (OD).
To determine whether VP‐ or VIP‐immunoreactive cell morphology was altered by neonatal exposure to MSG, the major axis (length of the longest chord through the neuronal soma), minor axis (maximum width of the neuronal soma perpendicular to the longest chord) and the soma area (cell body area) were measured delimiting manually the outline of neuronal soma of 10 well‐delineated neuronal bodies with prominent nuclei. They were randomly chosen of each animal without knowledge of the experimental group and were evaluated at ×100. Measurements were calculated automatically by the imaging system. Based on the fact that OD has been used as a tool for indirect determination of the quantity of proteins in the histological sections, and for the fact that similar results are obtained by biochemical techniques (Mufson et al. 1997), in the same cells, cellular OD was determined at ×100 and was expressed in 100 μm2. Furthermore, in VIP‐, VP‐ or GFAP‐immunoreactive sections, the OD in the area occupied by SCN was determined at ×20 and expressed in 10,000 μm2. The purpose was to determine variations in the protein expression at tissue level.
For quantitative OD analysis, images of tissue sections stained for VIP, VP or GFAP were digitized at a magnification of ×20 and ×100. Lighting conditions and magnifications were held constant for all measurements performed in each of the microscope objectives used. All images were captured at 1392 × 1040 pixels in RGB colours and saved in 24‐bit colour TIFF format. The detection of immunoreactive structures was achieved by image threshold considering only the green channel (500 nm, a wavelength absorbed by DAB) of the RGB image acquired. Then, the cells or regions were outlined manually and the images were converted to eight‐bit greyscale for analysis, represented by an array of pixels with intensity values between 0 and 255 (255 represent the maximum brightness). The brightness of the pixels in the image is inversely correlated with the staining intensity. The OD measurements were automatically analysed by the image system. To account for differences in background staining intensity, two background OD measurements were taken in nearby regions lacking immunoreactive profiles, for each of the cells or regions evaluated. The mean background OD value obtained was subtracted from cellular OD value or at the tissue level measured to obtain the final OD value. OD and OD background values were determined for each of the parameters evaluated and for each of the sections.
Statistical analysis
The data were analysed using Student's t‐test, and the values of P < 0.05 were considered significant and were expressed as mean ± SEM.
Ethical approval
This work was approved (in accordance with the Official Mexican Norm, NOM‐062‐200‐1999) by the Committee for the Use and Care of Laboratory Animals of the Instituto Nacional de Pediatría.
Results
Locomotor activity
The rats belonging to the MSG‐treated group showed a significant reduction in locomotor activity (P < 0.05) when compared with the control group. There was no significant difference in the body and brain weights of both groups (Table 1).
Table 1.
Effects of neonatal exposure to monosodium glutamate on locomotor activity and on body and brain weights of adult rats
| Group | Locomotor activity (Number of squares traversed) | Body weight (g) | Brain weight (g) |
|---|---|---|---|
| Control (n = 5) | 256.00 ± 15.2 | 437.40 ± 13.12 | 1.91 ± 0.02 |
| MSG (n = 5) | a183.4 ± 20.00 | 403.80 ± 31.25 | 1.88 ± 0.16 |
Values are expressed as mean ± SEM of 5 animals per group.
P < 0.05. MSG: monosodium glutamate.
Morphological evaluation
The cellular organization pattern in the MSG‐exposed group and control was similar. In both groups, a higher number of VIP‐immunoreactive cells were distributed in the ventral region of SCN, while a higher concentration of VP‐immunoreactive neurons were observed in the dorsomedial subdivision of SCN (Figure 1a–d). Furthermore, scattered cells of both types of neurons were observed throughout SCN, while some neurons were entirely embedded within the optic chiasm (Figures 1a–d, 2a and b and 3a and b). GFAP‐immunoreactive cells were observed throughout SCN (Figures 1e and f and 4a and b).
Figure 1.
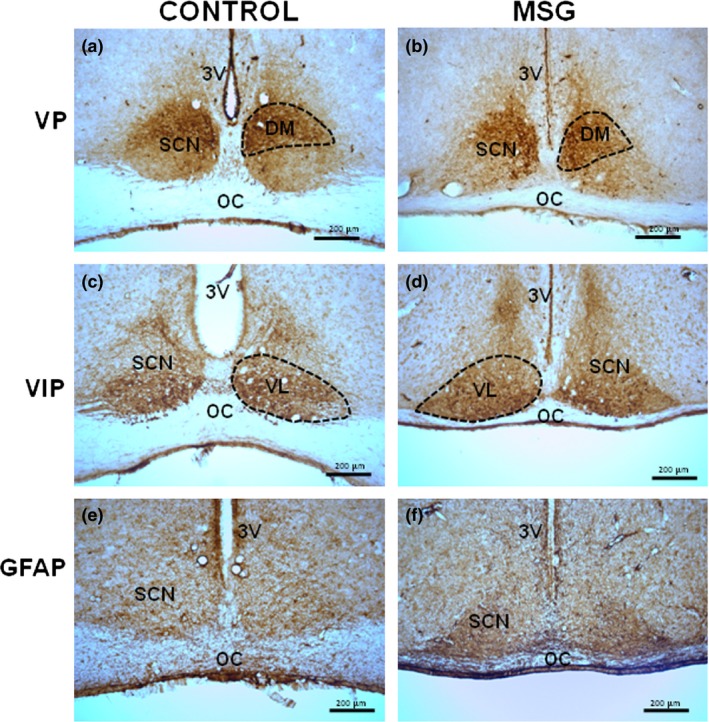
The expression pattern of immunoreactive cells to vasopressin (VP), vasoactive intestinal polypeptide (VIP) and glial fibrillary acidic protein (GFAP) in the suprachiasmatic nucleus (SCN) showed no alterations between control (a, c and e) and neonatal MSG‐exposed (b, d and f) groups. The dorsomedial (DM) and ventrolateral (VL) regions are shown with dotted lines (a–d). Coronal sections of 40 μm thickness. 3V: third ventricle; OC: optic chiasm.
Figure 2.
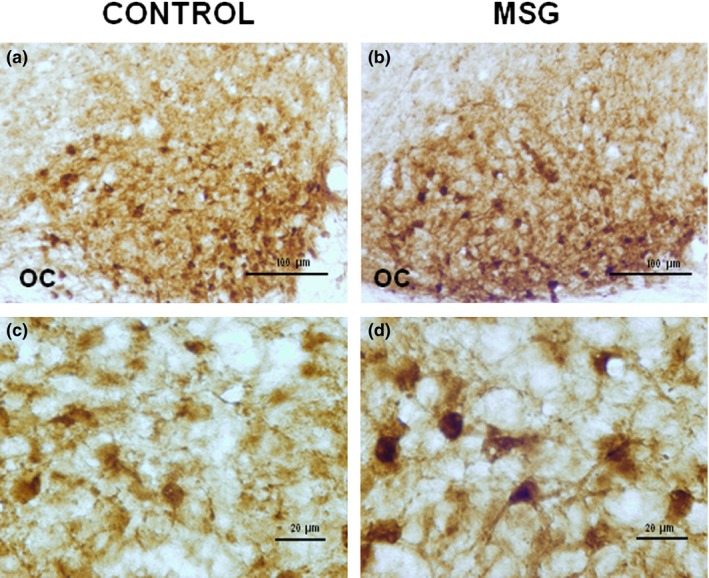
Morphological changes in neurons immunoreactive to vasoactive intestinal polypeptide of the suprachiasmatic nucleus of rats induced by neonatal exposure to monosodium glutamate (MSG). MSG group showed a decrease in the neuronal population as well as an increase in the intensity of reaction in the soma (b and d), compared with the control group (a and c). Histological sections of 40 μm thickness. OC: optic chiasm.
Figure 3.
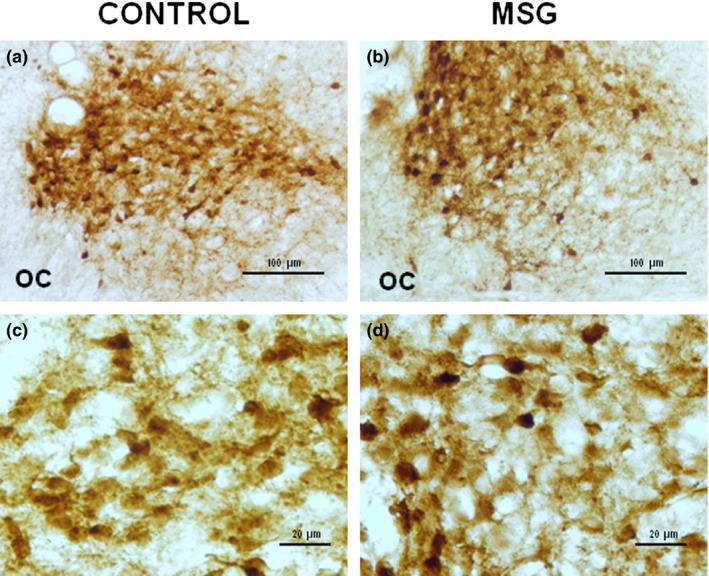
Immunohistochemistry for the detection of vasopressin in neurons of the suprachiasmatic nucleus of control group (a and c) and neonatal MSG‐exposed (b and d) group. The number of cells was decreased, while the immunostaining intensity showed an increase in animals exposed to MSG. Coronal sections of 40 μm thickness. OC: optic chiasm.
Figure 4.
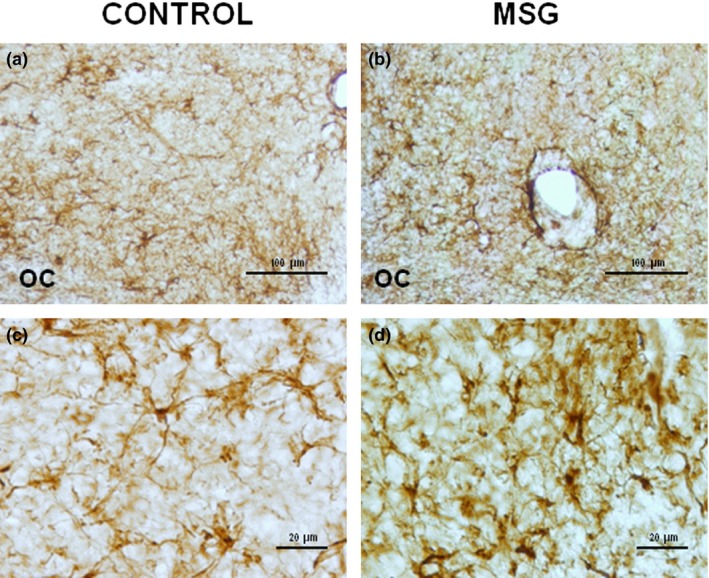
Glial fibrillary acidic protein immunostaining in suprachiasmatic nucleus of control group (a and c) and neonatal MSG‐exposed (b and d) group. The higher number of astrocytes with a more intense GFAP immunoreactivity was observed in animals exposed to MSG. Histological sections of 40 μm thickness. OC: optic chiasm.
Neonatal exposure to MSG induced a significant increase (P < 0.05) in the major axis and somatic area of VIP‐immunoreactive cells without a significant effect on the minor axis. In VP‐immunoreactive neurons, significant alterations in the major and minor axes or in the somatic area were not observed. The cellular density of VIP‐ and VP‐immunoreactive cells in MSG‐exposed animals was significantly low (P < 0.05) when compared with the controls (Figures 2c and d, 3c and d, and Table 2). In contrast, the density of GFAP‐immunoreactive cells increased significantly (P < 0.05) in MSG‐exposed animals compared with the control group (Figure 4c and d).
Table 2.
Effects of neonatal exposure to monosodium glutamate on the morphology of the cells in the suprachiasmatic nucleus of adult rats
| Cellular type | Group | Minor axis (μm) | Major axis (μm) | Area of soma (μm2) | Cellular density (1000 μm2) |
|---|---|---|---|---|---|
| VP | Control | 5.90 ± 0.19 | 9.31 ± 0.25 | 32.55 ± 1.54 | 5.85 ± 0.15 |
| MSG | 6.23 ± 0.23 | 8.85 ± 0.27 | 34.19 ± 2.05 | a3.04 ± 0.11 | |
| VIP | Control | 6.63 ± 0.19 | 9.21 ± 0.32 | 38.29 ± 2.09 | 5.84 ± 0.23 |
| MSG | 7.12 ± 0.31 | a10.78 ± 0.31 | a49.82 ± 3.45 | a3.17 ± 0.47 | |
| GFAP | Control | 2.74 ± 0.10 | |||
| MSG | a3.75 ± 0.18 |
Values are expressed as mean ± SEM of 5 animals per group.
P < 0.05.
GFAP, glial fibrillary acidic protein‐immunoreactive cells; MSG, monosodium glutamate; VP, vasopressin‐immunoreactive cells; VIP, vasoactive intestinal polypeptide‐immunoreactive cells.
MSG‐exposed rats showed a significant increase (P < 0.05) in somatic OD of VIP (Figure 2d and Table 3)‐ and VP (Figure 3d and Table 3)‐immunoreactive neurons, compared with the control group (Figures 2c and 3c respectively, and Table 3). No significant difference was found in OD of SCN in immunostained tissue sections for VIP or VP (Figures 2a and b and 3a and b, and Table 3). However, OD of SCN in immunostained sections for GFAP of MSG‐exposed animals increased significantly (P < 0.05) when compared with the controls (Figure 4a–d and Table 3).
Table 3.
Effects of neonatal exposure to monosodium glutamate on cellular optical density and on suprachiasmatic nucleus area of adult rats
| Group | Cellular OD (100 μm2) (arbitrary units) | OD in the SCN (10,000 μm2) (arbitrary units) | |
|---|---|---|---|
| VP | Control | 0.304 ± 0.036 | 0.035 ± 0.001 |
| MSG | a1.051 ± 0.056 | 0.033 ± 0.001 | |
| VIP | Control | 0.289 ± 0.033 | 0.035 ± 0.002 |
| MSG | a0.720 ± 0.062 | 0.036 ± 0.002 | |
| GFAP | Control | 0.021 ± 0.001 | |
| MSG | a0.025 ± 0.001 |
Values are expressed as median ± SEM of 5 animals per group.
P < 0.05.
GFAP, glial fibrillary acidic protein‐immunoreactive cells; MSG, monosodium glutamate; OD, optical density; SCN, suprachiasmatic nucleus; VIP, vasoactive intestinal polypeptide‐immunoreactive cells; VP, vasopressin‐immunoreactive cells.
Discussion
Our results show that the neonatal MSG exposure protocol used in this study induced a decrease in the locomotor activity at the onset of dark phase, which is a time point at which rats, without any kind of treatment, begin marked intensive activity. Locomotor activity circadian rhythm is regulated by the circadian pacemaker (Saper 2013). Therefore, it is possible that SCN and/or the structures that regulate the circadian rhythm of locomotor activity through SCN, such as subparaventricular zone of the hypothalamus and ventromedial hypothalamic nucleus (Saper 2013; Vujovic et al. 2015), show disorders in the mechanisms of regulation of locomotor activity induced by MSG exposure. In this context, it was reported that neonatal MSG exposure induced a decrease in the number of neurons in the ventromedial nucleus (Tanaka et al. 1978; Sun et al. 1991; Xue et al. 1997).
On the other hand, prokineticin 2 (PK2), cardiotrophin‐like cytokine (CLC) and transforming growth factor‐alpha (TGF‐α) have been proposed as factors that are involved in circadian regulation of locomotor activity. These factors are produced by SCN, and its intracerebroventricular injection inhibits locomotor activity (Bittman 2009; Li et al. 2009). PK2 is present in more than 50% of the cells that contain VP or VIP (Bittman et al., 2009) and CLC is colocalized in VP neurons (Li et al. 2009), while TGF‐α is expressed in the astrocytes, and therefore, its role continued to be controversial (Li et al. 2009). However, it has been recently shown that locomotor activity is inhibited with systemic injection or microinjection of VP inside SCN (Cormier et al. 2015). Moreover, it has been reported that there is an association between the increase in locomotor activity and the increase in the number of VP neurons (Bult et al. 1993). This suggests that immunoreactive neurons to VP in SCN play an important role in the regulation of circadian locomotor activity.
In this study, we observed that neonatal MSG exposure resulted in a significant decrease in the cellular density of VIP‐ and VP‐immunoreactive neurons. It is probably induced by prolonged overexcitation of glutamate receptors (Bawari et al. 1995; Swamy et al. 2013). This event could be attributed to lack of blood–brain barrier development in the period of MSG application (Ribatti et al. 2006), to the anatomical location of SCN (hypothalamus and periventricular areas are particularly vulnerable to systemic glutamate, Olney & Sharpe 1969), and to the presence of glutamate receptors in SCN neurons (Mick et al. 1995; Ghosh et al. 1997; Ikeda et al. 2003; Sollars & Pickard 2015). Furthermore, significant alterations in the morphology of VIP‐immunoreactive neurons were observed. This shows that neonatal MSG exposure affects, in different ways, the various SCN neuronal populations. These possible alterations could put at risk adequate functionality of SCN because it is known that the morphology and neuronal number are crucial parameters for adequate function of different central nervous system regions (Kobe et al. 2012; Kida et al. 2013).
In the present study, we observed a significant increase in the OD of VP‐ and VIP‐immunoreactive neurons in the MSG group, while OD in the SCN of immunostained sections with VP and VIP was similar in experimental and control animals. This suggests that these two kinds of neurons present similar compensatory mechanisms for increasing protein production in the SCN in response to neonatal MSG exposure‐induced cell density reduction as has been reported in neonatal MSG‐exposed hippocampal GABAergic cells (Ureña‐Guerrero et al. 2009). Moreover, Beas‐Zárate et al. (1998) reported a decrease in GABA‐stimulated release induced by neonatal MSG exposure. Therefore, the fact that the levels of VP and VIP expression in SCN medial sections of MSG‐exposed animals are similar to those of the control group does not imply that the release of these proteins is normal.
The results of the present study show that MSG exposure induced a significant increase not only in the number of astrocytes but also in OD of SCN in immunostained sections with GFAP. This could be considered as a permanent consequence of MSG neuro‐excitotoxic effects and could represent reactive gliosis (Zhao & Schwartz 1998) as was observed in the cerebral cortex of neonate animals exposed to MSG (Martínez‐Contreras et al. 2002) and both alterations are characteristics of neurodegenerative diseases (Middeldrop & Hol 2011; Nguyen et al. 2015). On the other hand, it has been reported that astrocytes are able to protect neurons from the neurotoxicity induced by MSG (possibly by a mechanism that involves glutathione). However, astrocytes are also affected by MSG; therefore, the deleterious effects of MSG on the nervous system would be increased (Hashem et al. 2012).
In this study, a potential limitation was the lack in the continuous acquisition of behavioural data during the activity phase of the animal. However, in our study was quantified the behaviour from the beginning of the active phase and for a period of 30 min. In this regard, Hlinak et al. (2005) and Kiss et al. (2007) quantified locomotor activity in control and neonatal MSG‐exposed animals during the resting phase for a period of 5 min. Furthermore, a potential limitation is the lack of direct measurements of the levels of proteins. In this regard, we use the indirect technique of OD which has been validated for this purpose, and with it, we were able to indirectly measure the protein levels in tissue sections and even at the level of individual cells.
In conclusion, the results of this study show that neonatal MSG exposure in rats induced alterations in the morphology, cellular density and expression of proteins in specific types of cells in SCN that are important for its function and could be related to the changes in locomotor activity that was determined in this study.
It is suggested that special attention needs to be paid to the constituents of the food products especially at young ages.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The authors are grateful to Mr. Pedro Medina (Tech) for his technical assistance. This work was supported by a grant (27/2008) from Federal Resources of the Instituto Nacional de Pediatría, México D.F., México.
References
- Bawari M., Babu G.N., Ali M.M. et al (1995) Effect of neonatal monosodium glutamate on lipid peroxidation in adult rat brain. NeuroReport 6, 650–652. [DOI] [PubMed] [Google Scholar]
- Beas‐Zárate C., Sánchez‐Ruíz M.Y., Ureña‐Guerrero M.E. et al (1998) Effect of neonatal exposure to monosodium L‐glutamate on regional GABA release during postnatal development. Neurochem. Int. 33, 217–232. [DOI] [PubMed] [Google Scholar]
- Bittman E.L. (2009) Vasopressin: more than just an output of the circadian pacemaker? Focus on “Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock‐controlled genes in the suprachiasmatic nuclei”. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R821–R823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch B., Ling N., Benoit R. et al (1984) Specific depletion of immunoreactive growth hormone‐releasing factor by monosodium glutamate in rat median eminence. Nature 307, 272–273. [DOI] [PubMed] [Google Scholar]
- Bult A., Hiestand L., Van der Zee E.A. et al (1993) Circadian rhythms differ between selected mouse lines: a model to study the role of vasopressin neurons in the suprachiasmatic nuclei. Brain Res. Bull. 32, 623–627. [DOI] [PubMed] [Google Scholar]
- Cormier H.C., Della‐Maggiore V., Karatsoreos I.N. et al (2015) Suprachiasmatic vasopressin and the circadian regulation of voluntary locomotor behavior. Eur. J. Neurosci. 41, 79–88. [DOI] [PubMed] [Google Scholar]
- Dubovicky M., Tokarev D., Skultetyova I. et al (1997) Changes in exploratory behavior and its habituation in rats neonatally treated with monosodium glutamate. Pharmacol. Biochem. Behav. 56, 565–569. [DOI] [PubMed] [Google Scholar]
- Fernandes G.S., Arena A.C., Campos K.E. et al (2012) Glutamate‐induced obesity leads to decreased sperm reserves and acceleration of transit time in the epididymis of adult male rats. Reprod. Biol. Endocrinol. 10, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry L.C., Epelbaum J. & Martin J.B. (1981) Monosodium glutamate: acute and chronic effects on rhythmic growth hormone and prolactin secretion, and somatostatin in the undisturbed male rat. Brain Res. 217, 129–142. [DOI] [PubMed] [Google Scholar]
- Ghosh P.K., Baskaran N. & van den Pol A.N. (1997) Developmentally regulated gene expression of all eight metabotropic glutamate receptors in hypothalamic suprachiasmatic and arcuate nuclei ‐ a PCR analysis. Brain Res. Dev. Brain Res. 2, 1–12. [DOI] [PubMed] [Google Scholar]
- Golombek D.A. & Rosenstein R.E. (2010) Physiology of circadian entrainment. Physiol. Rev. 90, 1063–1102. [DOI] [PubMed] [Google Scholar]
- Guimarães E.D., de Caires Júnior L.C., Musso C.M. et al (2015) Altered behavior of adult obese rats by monosodium l‐glutamate neonatal treatment is related to hypercorticosteronemia and activation of hypothalamic ERK1 and ERK2. Nutr. Neurosci. in press. doi.org/10.1179/1476830515Y.0000000004 [DOI] [PubMed] [Google Scholar]
- Hashem H.E., El‐Din Safwat M.D. & Algaidi S. (2012) The effect of monosodium glutamate on the cerebellar cortex of male albino rats and the protective role of vitamin C (histological and immunohistochemical study). J. Mol. Histol. 43, 179–186. [DOI] [PubMed] [Google Scholar]
- He K., Du S., Xun P. et al (2011) Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS). Am. J. Clin. Nutr. 93, 1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlinak Z., Gandalovicova D. & Krejci I. (2005) Behavioral deficits in adult rats treated neonatally with glutamate. Neurotoxicol. Teratol. 27, 465–473. [DOI] [PubMed] [Google Scholar]
- Holzwarth‐McBride M.A., Sladek J.R. & Knigge K.M. (1976) Monosodium glutamate induced lesion of the arcuate nucleus. II Fluorescence histochemistry of catecholamines. Anat. Rec. 186, 197–205. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Yoshioka T. & Allen C.N. (2003) Developmental and circadian changes in Ca2+ mobilization mediated by GABAA and NMDA receptors in the suprachiasmatic nucleus. Eur. J. Neurosci. 17, 58–70. [DOI] [PubMed] [Google Scholar]
- Inouye S.T. & Shibata S. (1994) Neurochemical organization of circadian rhythm in the suprachiasmatic nucleus. Neurosci. Res. 20, 109–130. [DOI] [PubMed] [Google Scholar]
- Jansson L.C. & Akerman K.E. (2014) The role of glutamate and its receptors in the proliferation, migration, differentiation and survival of neural progenitor cells. J. Neural. Transm. 121, 819–836. [DOI] [PubMed] [Google Scholar]
- Kida H., Nomura S., Shinoyama M. et al (2013) The effect of hypothermia therapy on cortical laminar disruption following ischemic injury in neonatal mice. PLoS ONE 8, e68877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss P., Hauser D., Tamás A. et al (2007) Changes in open‐field activity and novelty‐seeking behavior in periadolescent rats neonatally treated with monosodium glutamate. Neurotox. Res. 12, 85–93. [DOI] [PubMed] [Google Scholar]
- Klein D.C., Moore R.Y. & Reppert S.M. (1991) Suprachiasmatic Nucleus: The Mind′s Clock. New York, NY: Oxford University Press. [Google Scholar]
- Kobe F., Guseva D., Jensen T.P. et al (2012) 5‐HT7R/G12 signaling regulates neuronal morphology and function in an age‐dependent manner. J. Neurosci. 32, 2915–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.D., Burton K.J., Zhang C. et al (2009) Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock‐controlled genes in the suprachiasmatic nuclei. Am. J. Physiol. Regul. Integr. Comp. Physiol. 3, R824–R830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas D.R. & Newhouse J.P. (1957) The toxic effect of sodium L‐glutamate on the inner layers of the retina. A. M. A. Arch. Opthalmol. 58, 193–201. [DOI] [PubMed] [Google Scholar]
- Martínez‐Contreras A., Huerta M., López‐Pérez S. et al (2002) Astrocytic and microglia cells reactivity induced by neonatal administration of glutamate in cerebral cortex of the adult rats. J. Neurosci. Res. 67, 200–210. [DOI] [PubMed] [Google Scholar]
- Mick G., Yoshimura R., Ohno K. et al (1995) The messenger RNAs encoding metabotropic glutamate receptor subtypes are expressed in different neuronal subpopulations of the rat suprachiasmatic nucleus. Neuroscience 66, 161–173. [DOI] [PubMed] [Google Scholar]
- Middeldrop J. & Hol E.M. (2011) GFAP in health and disease. Prog. Neurobiol. 3, 421–443. [DOI] [PubMed] [Google Scholar]
- Miyabo S., Ooya E., Yamamura I. et al (1982) Ontogeny of circadian corticosterone rhythm in rats treated with monosodium glutamate neonatally. Brain Res. 248, 341–345. [DOI] [PubMed] [Google Scholar]
- Miyabo S., Yamamura I., Ooya E. et al (1985) Effects of neonatal treatment with monosodium glutamate on circadian locomotor rhythm in the rat. Brain Res. 339, 201–208. [DOI] [PubMed] [Google Scholar]
- Mufson E.J., Lavine N., Jaffar S. et al (1997) Reduction in p140‐TrkA receptor protein within the nucleus basalis and cortex in Alzheimer's disease. Exp. Neurol. 146, 91–103. [DOI] [PubMed] [Google Scholar]
- Nguyen L., Lucke‐Wold B.P., Mookerjee S.A. et al (2015) Role of sigma‐1 receptors in neurodegenerative diseases. J. Pharmacol. Sci. 127, 17–29. [DOI] [PubMed] [Google Scholar]
- Olivo M., Kitahama K., Valatx J.L. et al (1986) Neonatal monosodium glutamate dosing alters the sleep‐wake cycle of the mature rat. Neurosci. Lett. 67, 186–190. [DOI] [PubMed] [Google Scholar]
- Olney J. (1969) Brain lesions, obesity and other disturbances in mice treated with monosodium glutamate. Science 164, 719–721. [DOI] [PubMed] [Google Scholar]
- Olney J.W. & Sharpe L.G. (1969) Brain lesions in an infant rhesus monkey treated with monosodium glutamate. Science 166, 386–388. [DOI] [PubMed] [Google Scholar]
- Paxinos G. & Watson C. (1998) The Rat Brain in Stereotaxic Coordinates, 4th edn San Diego, CA: Academic Press. [Google Scholar]
- Prendergast B.J., Onishi K.G. & Zucker I. (2013) Neonatal monosodium glutamate treatment counteracts circadian arrhythmicity induced by phase shifts of the light‐dark cycle in female and male Siberian hamsters. Brain Res. 1521, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser R.A., Edgar D.M., Heller H.C. et al (1994) A possible glial role in the mammalian circadian clock. Brain Res. 643, 296–301. [DOI] [PubMed] [Google Scholar]
- Ramkisoeensing A. & Meijer J.H. (2015) Synchronization of biological clock neurons by light and peripheral feedback systems promotes circadian rhythms and health. Front. Neurol. 6, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M.E., Webb I.C., Wang X. et al (2013) The transcription factor Runx2 is under circadian control in the suprachiasmatic nucleus and functions in the control of rhythmic behavior. PLoS ONE 8, e54317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert S.M. & Weaver D.R. (2002) Coordination of circadian timing in mammals. Nature 418, 935–941. [DOI] [PubMed] [Google Scholar]
- Ribatti D., Nico B., Crivellato E. et al (2006) Development of the blood‐brain barrier: a historical point of view. Anat. Rec. B New Anat. 289, 3–8. [DOI] [PubMed] [Google Scholar]
- Riedel G., Platt B. & Micheau J. (2003) Glutamate receptor function in learning and memory. Behav. Brain Res. 140, 1–47. [DOI] [PubMed] [Google Scholar]
- Rojas‐Castañeda J.C., Vigueras‐Villaseñor R.M., Rojas P. et al (2011) Alterations induced by chronic lead exposure on the cells of circadian pacemaker of developing rats. Int. J. Exp. Pathol. 92, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansar W., Ahboucha S. & Gamrani H. (2011) Chronic lead intoxication affects glial and neural systems and induces hypoactivity in adult rat. Acta Histochem. 113, 601–607. [DOI] [PubMed] [Google Scholar]
- Saper C.B. (2013) The central circadian timing system. Curr. Opin. Neurobiol. 23, 747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K., Honma S., Katsumo Y. et al (1995) Two distinct oscillators in the rat suprachiasmatic nucleus in vitro . Proc. Natl Acad. Sci. 92, 7396–7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. & Kriegsfeld L.J. (2014) Circadian rhythms have broad implications for understanding brain and behavior. Eur. J. Neurosci. 39, 1866–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollars P.J. & Pickard G.E. (2015) The neurobiology of circadian rhythms. Psychiatr. Clin N. Am. in press. doi.org/10.1016/j.psc.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.M., Hsu H.K., Lue S.I. et al (1991) Sex‐specific impairment in sexual and ingestive behaviors of monosodium glutamate‐treated rats. Physiol. Behav. 50, 873–880. [DOI] [PubMed] [Google Scholar]
- Swamy A.V., Patel N.L., Gadad P.C. et al (2013) Neuroprotective activity of pongamia pinnata in monosodium glutamate‐induced neurotoxicity in rats. Indian. J. Pharm. Sci. 75, 657–663. [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Shimada M., Nakao K. et al (1978) Hypothalamic lesion induced by injection of monosodium glutamate in suckling period and subsequent development of obesity. Exp. Neurol. 62, 191–199. [DOI] [PubMed] [Google Scholar]
- U. S. Department of Health and Human Services, U. S. Food and Drug Administration (1995) FDA and monosodium glutamate (MSG) August 31.
- Ureña‐Guerrero M.E., Orozco‐Suárez S., López‐Pérez S.J. et al (2009) Excitotoxic neonatal damage induced by monosodium glutamate reduces several GABAergic markers in the cerebral cortex and hippocampus in adulthood. Int. J. Dev. Neurosci. 27, 845–855. [DOI] [PubMed] [Google Scholar]
- Vujovic N., Gooley J.J., Jhou T.C. et al (2015) Projections from the subparaventricular zone define four channels of output from the circadian timing system. J. Comp. Neurol. doi:10.1002/cne.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y.D., Wong P.T. & Leong S. (1997) Nitric oxide synthase‐, N‐methyl‐D‐aspartate receptor‐, glutamate‐ and aspartate‐immunoreactive neurons in the mouse arcuate nucleus: effects of neonatal treatment with monosodium glutamate. Acta Neuropathol. 94, 572–582. [DOI] [PubMed] [Google Scholar]
- Zhao B. & Schwartz J.P. (1998) Involvement of cytokines in normal CNS development and neurological diseases: recent progress and perspectives. J. Neurosci. Res. 52, 7–16. [DOI] [PubMed] [Google Scholar]


