Abstract
Dense phytoplankton blooms in eutrophic waters often experience large daily fluctuations in environmental conditions. We investigated how this diel variation affects in situ gene expression of the CO2-concentrating mechanism (CCM) and other selected genes of the harmful cyanobacterium Microcystis aeruginosa. Photosynthetic activity of the cyanobacterial bloom depleted the dissolved CO2 concentration, raised pH to 10, and caused large diel fluctuations in the bicarbonate and O2 concentration. The Microcystis population consisted of three Ci uptake genotypes that differed in the presence of the low-affinity and high-affinity bicarbonate uptake genes bicA and sbtA. Expression of the bicarbonate uptake genes bicA, sbtA, and cmpA (encoding a subunit of the high-affinity bicarbonate uptake system BCT1), the CCM transcriptional regulator gene ccmR and the photoprotection gene flv4 increased at first daylight and was negatively correlated with the bicarbonate concentration. In contrast, genes of the two CO2 uptake systems were constitutively expressed, whereas expression of the RuBisCO chaperone gene rbcX, the carboxysome gene ccmM, and the photoprotection gene isiA was highest at night and down-regulated during daytime. In total, our results show that the harmful cyanobacterium Microcystis is very responsive to the large diel variations in carbon and light availability often encountered in dense cyanobacterial blooms.
Keywords: carbon dioxide, climate change, CO2-concentrating mechanism, gene expression, harmful algal blooms, lakes, Microcystis aeruginosa
Introduction
Harmful cyanobacterial blooms are a recurring problem in many eutrophic lakes worldwide (Chorus and Bartram, 1999; Huisman et al., 2005; Michalak et al., 2013). Cyanobacteria can produce a variety of hepatotoxins, gastrointestinal toxins, and neurotoxins causing liver, digestive and neurological disease in birds and mammals, including humans (Carmichael, 2001; Codd et al., 2005). This may lead to the closure of water bodies for recreational use, drinking or irrigation water, and aquaculture (Verspagen et al., 2006; Qin et al., 2010; Steffen et al., 2014). Cyanobacterial blooms are often favored by excessive nutrients and high temperatures (Jöhnk et al., 2008; Kosten et al., 2012), and it is foreseen that rising CO2 concentrations and global warming will further promote harmful cyanobacterial blooms (Paerl and Huisman, 2008; O’Neil et al., 2012; Verspagen et al., 2014; Visser et al., 2016).
Yet, effects of daily fluctuations in environmental conditions on harmful cyanobacterial blooms remain poorly investigated. The day–night cycle leads to large changes in the activity of phototrophic organisms. The high photosynthetic activity of cyanobacterial blooms during daytime can deplete the dissolved CO2 (CO2(aq)) concentration, causing a concomitant increase in pH (Ibelings and Maberly, 1998; Balmer and Downing, 2011; Gu et al., 2011). Conversely, at night, cyanobacteria obtain their energy from respiration of the stored carbon compounds, releasing CO2 back in the water column. Cyanobacteria adjust to this daily variation by changes in gene expression and the built-up or break-down of specific enzymes and cellular components (Kucho et al., 2005; Straub et al., 2011; Brauer et al., 2013). In particular, the daily excursions in the availability of both light and inorganic carbon (Ci) imply that cyanobacteria may not only acclimate their light reactions of photosynthesis, but may also adjust their Ci uptake machinery to the diel cycle.
Cyanobacteria have evolved a CO2-concentrating mechanism (CCM) to efficiently fix CO2 at a wide range of Ci conditions (Kaplan and Reinhold, 1999; Price et al., 2008; Raven et al., 2012; Burnap et al., 2015). The cyanobacterial CCM is based on the uptake of dissolved CO2 and bicarbonate by different uptake systems and subsequent concentration of Ci in specialized compartments, called carboxysomes, that also contain the CO2 fixing enzyme RuBisCO. Five different Ci uptake systems are known in cyanobacteria, three for bicarbonate uptake and two for CO2 uptake (Price, 2011). The bicarbonate transporter BCT1 and the two CO2 uptake systems are present in most freshwater cyanobacteria (Badger et al., 2006; Rae et al., 2011; Sandrini et al., 2014). BCT1 is directly ATP-dependent, and combines a high affinity with a low flux rate (Omata et al., 1999). The two redox-dependent CO2 uptake systems, NDH-I3 and NDH-I4, have contrasting properties: NDH-I3 has a high affinity and low flux rate, while NDH-I4 has a low affinity and high flux rate (Maeda et al., 2002; Price et al., 2002). The presence of the other two bicarbonate uptake systems, BicA and SbtA, varies among freshwater cyanobacteria (Badger et al., 2006; Rae et al., 2011; Sandrini et al., 2014). Both BicA and SbtA are sodium-dependent symporters, where SbtA usually has a high affinity but low flux rate, and BicA has a low affinity but high flux rate (Price et al., 2004; Du et al., 2014). We recently compared CCM gene sequences of 20 strains of the harmful cyanobacterium Microcystis aeruginosa (Kützing; Sandrini et al., 2014). Interestingly, some strains lacked the high-flux bicarbonate uptake gene bicA, whereas others lacked the high-affinity bicarbonate uptake gene sbtA. Hence, in Microcystis, three different Ci uptake genotypes can be distinguished: bicA strains (with bicA but no sbtA), sbtA strains (with sbtA but no or incomplete bicA), and bicA + sbtA strains.
In laboratory studies, several cyanobacterial CCM genes were shown to be responsive to changes in both Ci availability and light intensity (Hihara et al., 2001; Wang et al., 2004; Schwarz et al., 2011; Straub et al., 2011; Burnap et al., 2013; Sandrini et al., 2015a). However, whereas lab studies usually focus on a single strain, cyanobacterial blooms often consist of multiple strains (Saker et al., 2005; Kardinaal et al., 2007). Recent work shows that different strains may respond differently to changes in CO2 levels (Van de Waal et al., 2011; Sandrini et al., 2014, 2015b), which may complicate how daily fluctuations in Ci availability affect gene expression and cellular physiology of cyanobacterial communities in lakes. Two lake studies used metatranscriptomics to study diel variation in cyanobacterial gene expression (Penn et al., 2014) or to compare gene expression between different locations (Steffen et al., 2015), yet detailed information on Ci concentrations and differences in expression of, for example, the Ci uptake genes were missing. Another interesting field study used RT-qPCR to investigate diel variation in the expression of CCM and other selected genes of a Synechococcus strain in the hot spring microbial mats from Yellowstone National Park (Jensen et al., 2011). However, detailed data about the Ci conditions in the mats were not presented.
In this study, we investigate diel variation in gene expression patterns of Microcystis in a dense cyanobacterial bloom of a eutrophic lake. Microcystis is a ubiquitous harmful cyanobacterium that often threatens water quality by the production of dense blooms and hepatotoxins called microcystins (Carmichael, 2001; Huisman et al., 2005; Qin et al., 2010; Dittmann et al., 2013). We quantified expression of the CCM genes as well as a few other selected genes of Microcystis using RT-qPCR. Furthermore, we monitored diel changes in environmental conditions, to link observed gene expression patterns to fluctuations in Ci and light availability. Our study provides a better understanding of the daily variations in the CCM during dense cyanobacterial blooms.
Results
Community Composition
Changes in gene expression and environmental conditions were monitored in Lake Kennemermeer (Figure 1) for 24 h, from 13:00 to 13:00 the next day on July 17 and 18, 2013. At this time, the lake was dominated by cyanobacteria comprising >97% of the total phytoplankton biovolume. The three most abundant cyanobacterial groups included Pseudanabaenaceae (biovolume: 380 mm3 L-1; cell counts: 10.9 × 109 cells L-1), Anabaenopsis hungarica (67 mm3 L-1; 53 × 107 cells L-1) and small Chroococcales (40 mm3 L-1; 24.6 × 109 cells L-1), whereas Microcystis spp. represented a smaller portion of the cyanobacterial community (1 mm3 L-1; 70 × 106 cells L-1). We detected two morphotypes of Microcystis, known as M. aeruginosa and M. flos-aquae (Komárek and Anagnostidis, 1999). These two morphotypes differ in morphology of the Microcystis colonies, but belong to the same genetic species M. aeruginosa (Kützing) according to the Bacterial Code (Otsuka et al., 2001).
FIGURE 1.
Map of Lake Kennemermeer with the sampling station.
Quantification of the Ci uptake genotypes of Microcystis was based on qPCR using a series of primers targeting the bicA and sbtA genes in the sampled gDNA. The results revealed that the total Microcystis population consisted of bicA strains (23 ± 1%), sbtA strains (15 ± 1%) and bicA + sbtA strains (62 ± 2%). Hence, all three Ci uptake genotypes of Microcystis were present in the lake. Quantification of the microcystin synthetase gene mcyB showed that 82 ± 5% of the Microcystis population was potentially toxic.
Diel Variation in Environmental Conditions
During the study period it was sunny and dry at daytime, and the photosynthetically active radiation (PAR) increased above 2,000 μmol photons m-2 s-1 in the early afternoon (Figure 2A). Sunset was at 21:51, the night was dry with clear skies, with sunrise at 5:42. Water temperature was uniformly distributed over the depth of the water column, peaked at 22.9°C during early evening, and dropped to 20.6°C in the early morning of the next day (Figure 2A). The lake is only ~1 m deep, and depth profiles of chlorophyll fluorescence indicated that the cyanobacterial community was uniformly distributed over the water column. We did not observe formation of scum layers at the water surface.
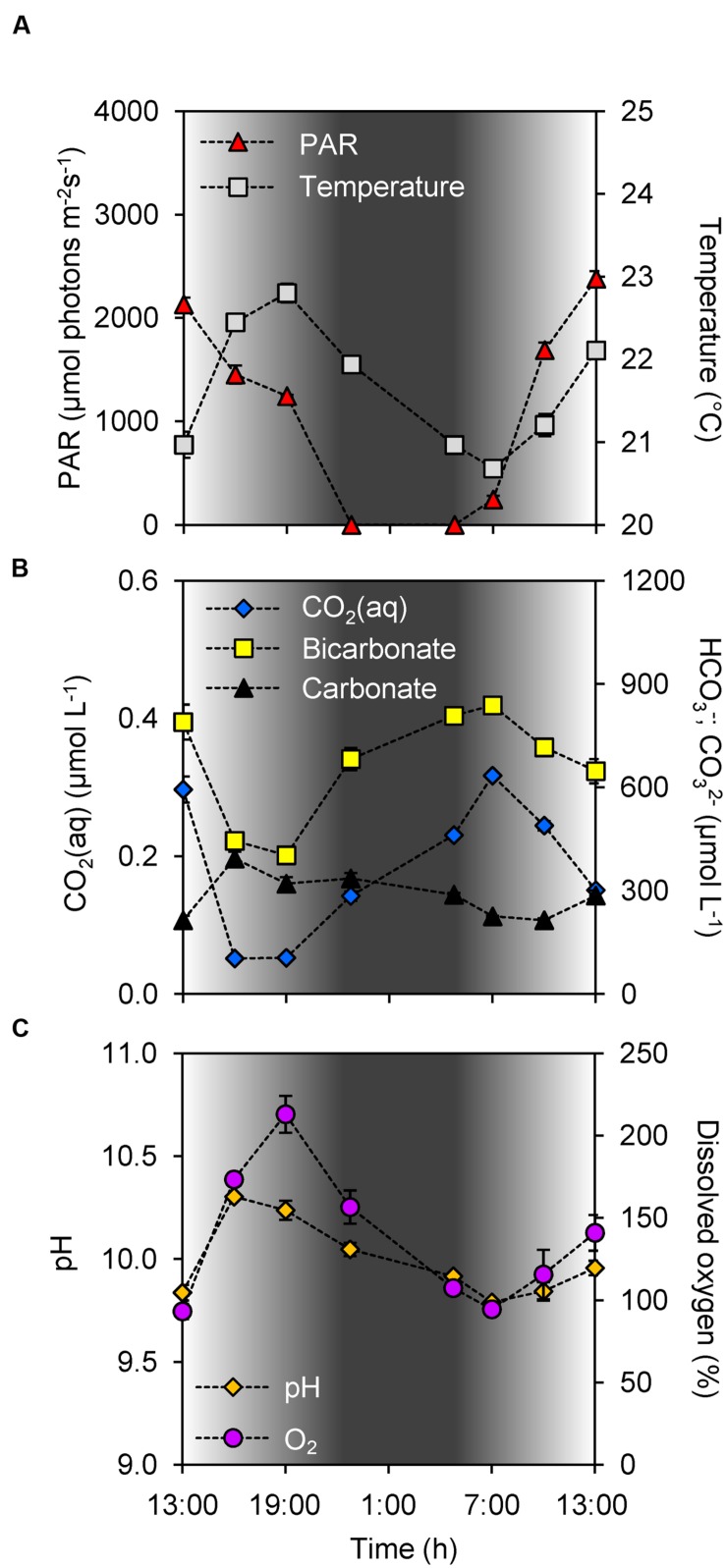
FIGURE 2.
Diel changes in environmental conditions. (A) Photosynthetically active radiation (PAR) and water temperature. (B) Dissolved CO2 (CO2(aq)), bicarbonate and carbonate concentrations. (C) pH and dissolved oxygen. Shading indicates the light intensity measured at the water surface, with complete darkness between 22:30 and 5:00. Each data point shows the mean ± SD of three independent measurements, for photosynthetically active radiation (PAR) at the water surface and for the other environmental parameters at 0.2 m depth.
According to Henry’s law, if the concentration of dissolved CO2 (CO2(aq)) in the lake would be in equilibrium with an atmospheric partial pressure of 390 ppm, one would expect a CO2(aq) concentration of ~15 μmol L-1. Instead, however, the CO2(aq) concentration was depleted to 0.05 μmol L-1 (which is equivalent to a partial pressure of 1 ppm) during daytime, and increased during the night to a maximum of only 0.32 μmol L-1 (8 ppm) at the early morning of the next day (Figure 2B). Hence, the lake was strongly CO2 undersaturated, even at night. Concomitant with the diel CO2(aq) fluctuations, the bicarbonate concentration showed large diel variation, from ~400 μmol L-1 in the early evening to ~850 μmol L-1 in the early morning of the next day (Pearson correlation of CO2(aq) vs. bicarbonate: ρ = 0.94, n = 8, p < 0.01; Figure 2B).
CO2 depletion by the cyanobacterial bloom led to an increase in pH with values up to 10.3 in the afternoon [Pearson correlation of pH vs log CO2(aq): ρ = -0.99, n = 8, p < 0.001; Figures 2B,C). The pH decreased <0.2 units over depth, indicating that this shallow lake was well mixed with nearly uniform Ci concentrations over the depth of the lake. CO2 depletion was correlated with oxygen production by the bloom (Pearson correlation of CO2(aq) vs. dissolved oxygen: ρ = -0.95, n = 8, p < 0.001; Figures 2B,C), with dissolved oxygen peaking at ~200% during the early evening. During most of the day the lake was supersaturated with oxygen, except for the early morning when it was slightly undersaturated. The sodium concentration in the lake was 12.7 ± 0.4 mmol L-1.
Diel Variation in Gene Expression
Most of the investigated genes of Microcystis showed significant diel variation in gene expression (Figure 3; Table 1). Hierarchical cluster analysis revealed that the expression patterns of the studied genes could be grouped into four distinct clusters (Figure 4).
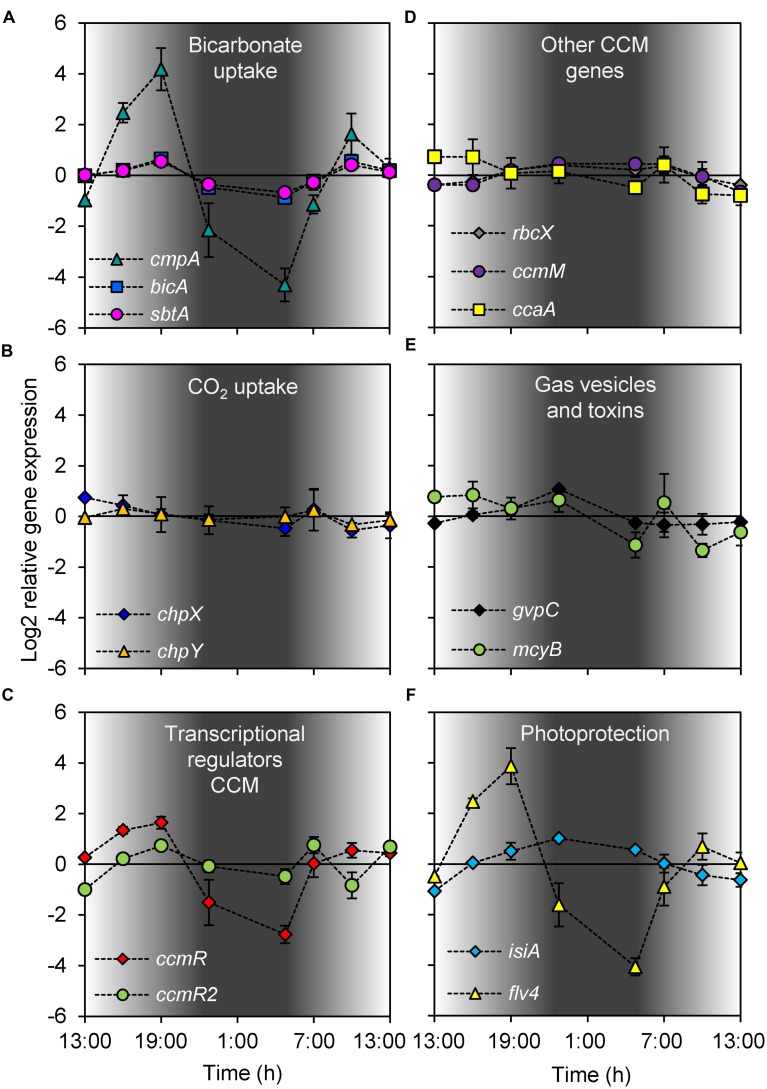
FIGURE 3.
Diel changes in gene expression. (A) Bicarbonate uptake genes cmpA, bicA, and sbtA. (B) CO2 uptake genes chpX and chpY. (C) CCM transcriptional regulator genes ccmR and ccmR2. (D) RuBisCO chaperone gene rbcX, carboxysome gene ccmM and carbonic anhydrase gene ccaA. (E) Gas vesicle gene gvpC and microcystin gene mcyB. (F) Photoprotection genes isiA and flv4. Gene expression was quantified as log2 ratios of the expression at the given time point relative to the mean expression over the 24-h period. Shading indicates the light intensity measured at the water surface, with complete darkness between 22:30 and 5:00. Each data point shows the mean ± SD of three independent lake samples. Significant differences in gene expression between time points are reported in Table 1.
Table 1.
Testing for significant differences in gene expression between time points.
| Gene | df1, df2 | F | p | Time points |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 13:00 (day 1) |
16:00 (day 1) |
19:00 (day 1) |
22:45 (day 1) |
4:45 (day 2) |
7:00 (day 2) |
10:00 (day 2) |
13:00 (day 2) |
||||
| cmpA | 7, 16 | 53.808 | 0.000 | bc | ef | f | b | a | bc | de | cd |
| bicA | 7, 16 | 34.031 | 0.000 | cd | de | f | ab | a | bc | ef | cde |
| sbtA | 7, 16 | 23.886 | 0.000 | bcd | de | e | ab | a | abc | de | cd |
| chpX | 7, 16 | 1.620 | 0.200 | a | a | a | a | a | a | a | a |
| chpY | 7, 16 | 0.990 | 0.473 | a | a | a | a | a | a | a | a |
| ccmR | 7, 16 | 36.693 | 0.000 | cd | de | e | b | a | c | cde | cd |
| ccmR2 | 7, 16 | 22.693 | 0.000 | a | bc | c | b | ab | c | a | c |
| rbcX | 7, 16 | 7.987 | 0.000 | a | ab | bc | c | bc | c | abc | a |
| ccmM | 7, 16 | 7.940 | 0.000 | ab | ab | bc | c | c | c | abc | a |
| ccaA | 7, 16 | 4.870 | 0.004 | b | b | ab | ab | ab | ab | a | a |
| gvpC | 7, 16 | 10.850 | 0.000 | a | a | a | b | a | a | a | a |
| mcyB | 7, 16 | 7.317 | 0.001 | c | c | bc | c | ab | c | a | abc |
| flv4 | 7, 16 | 60.401 | 0.000 | bcd | e | e | b | a | bc | d | cd |
| isiA | 7, 16 | 22.980 | 0.000 | a | bc | cd | d | cd | bc | ab | ab |
This table tests differences in gene expression between time points, for the data shown in Figure 3. The first four columns in the table indicate the genes, the degrees of freedom (df1 and df2), the value of the F-statistic (F), and the corresponding probability (p) of a one-way analysis of variance. The subsequent columns indicate significant differences in gene expression between time points based on Tukey’s HSD post hoc test (α = 0.05). Time points that do not share the same letter are significantly different from each other.
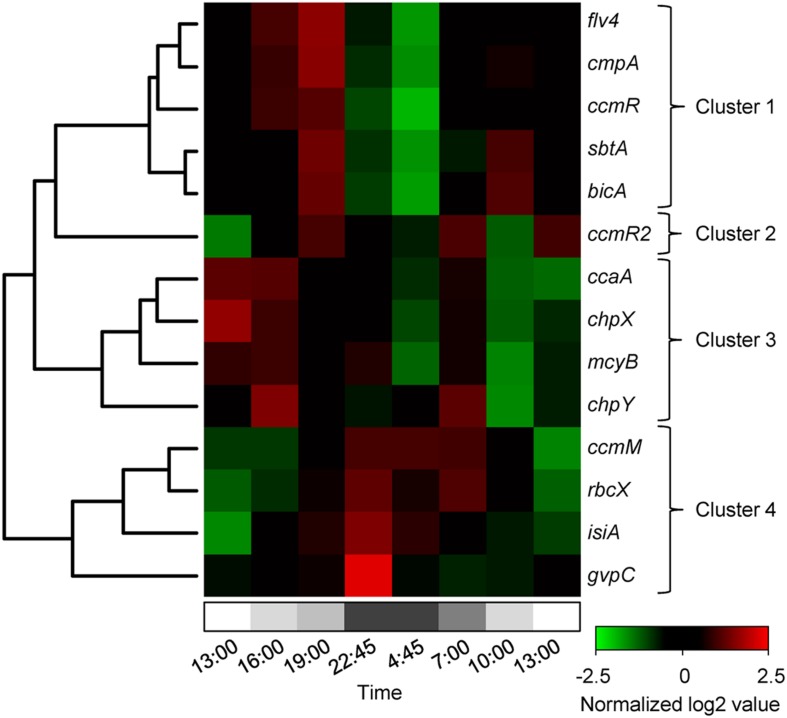
FIGURE 4.
Hierarchical clustering of the diel changes in gene expression. The heatmap shows normalized expression data. The log2 expression values of each gene were normalized with respect to the mean and standard deviation for that gene over the 24-h period. Upregulated genes are in red and downregulated genes in green. Gray shading at the bottom indicates the light intensity at the water surface, with complete darkness between 22:30 and 5:00. Hierarchical clustering resulted in four distinct gene clusters.
Expression of the genes in cluster 1 increased during daytime and decreased at night (Figure 4). This cluster comprised the three bicarbonate uptake genes cmpA, bicA, and sbtA, the transcriptional regulator gene ccmR and the flavodiiron protein gene flv4. Expression of cmpA, encoding for one of the subunits of the bicarbonate uptake system BCT1, showed the largest diel variation in this cluster. Its maximum expression during daytime was almost 360-fold higher (log2 difference of 8.5) than its minimum expression at night (Figure 3A). Expression of flv4 and ccmR, which is most likely a transcriptional regulator of the cmpABCD operon of Microcystis (Sandrini et al., 2015b), also showed large diel variation (Figures 3C,F). Expression of bicA and sbtA, encoding for the two sodium-dependent bicarbonate uptake systems, varied in tandem but at a lower amplitude than the other genes in this cluster (Figure 3A).
Cluster 2 consisted only of ccmR2 (Figure 4), a transcriptional regulator of the bicA-sbtA operon of Microcystis (Sandrini et al., 2014). Similar to the genes in cluster 1, the expression of ccmR2 increased during daytime and decreased at night, although at a lower amplitude than ccmR (Figure 3C). Its expression pattern differed from the genes in cluster 1 by a dip during the morning of the 2nd day.
Cluster 3 comprised the genes chpX and chpY (encoding the hydration subunits of the low-affinity and high-affinity CO2 uptake system, respectively), ccaA (encoding the carboxysomal carbonic anhydrase) and mcyB (encoding a microcystin synthetase; Figure 4). The cluster analysis suggests that these genes displayed a higher expression during the first daytime than during the second daytime period. However, diel variation of these genes was only minor and in most cases not significant, except for mcyB which decreased at night and increased briefly but significantly at first daylight of the 2nd day (Figure 3; Table 1).
Expression of the genes in cluster 4 was lowest during daytime and highest at night (Figures 3 and 4). This cluster comprised the CCM genes rbcX located in the RuBisCO operon and ccmM encoding for a carboxysomal shell protein, the gas vesicle protein gene gvpC and the gene isiA encoding for the iron-starvation induced protein.
Comparison of Gene Expression Patterns with Environmental Conditions
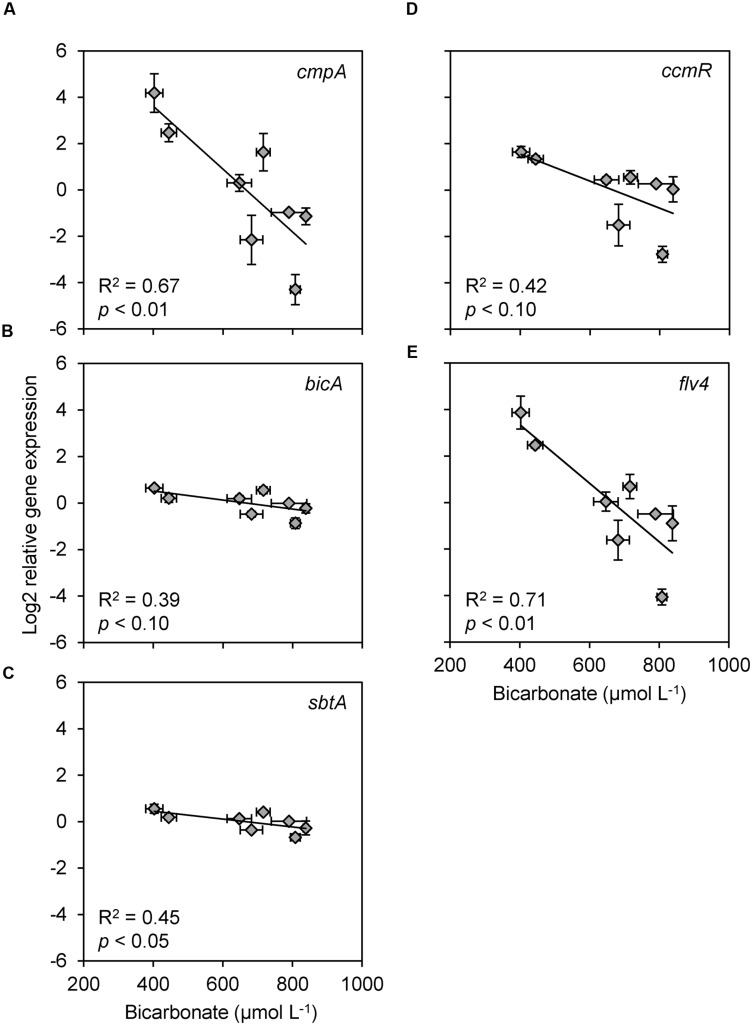
Several of the gene expression patterns were associated with diel changes in environmental conditions (Table 2). Correlations with the bicarbonate concentration and incident light intensity showed the most consistent response across the gene clusters. The expression of all five genes in cluster 1 showed a significant (p < 0.05) or marginally significant (p < 0.10) negative correlation with the bicarbonate concentration, whereas none of the other gene expression patterns were correlated with bicarbonate (Figure 5; Table 2). Of these five gene expression patterns, cmpA and flv4 were strongly correlated with the bicarbonate concentration, whereas bicA, sbtA, and ccmR showed a weaker correlation.
Table 2.
Pearson correlation coefficients between gene expression patterns and environmental variables (n = 8).
| Gene | CO2(aq) | Bicarbonate§ | pH | Temperature | Light§ | Dissolved oxygen |
|---|---|---|---|---|---|---|
| Cluster 1: | ||||||
| cmpA | ns | -0.82** | ns | 0.69* | ns | 0.68* |
| bicA | ns | -0.62# | ns | ns | 0.69* | ns |
| sbtA | ns | -0.67* | ns | ns | 0.70* | ns |
| ccmR | ns | -0.65# | ns | ns | 0.67* | ns |
| flv4 | -0.62# | -0.84** | ns | 0.72* | ns | 0.70* |
| Cluster 2: | ||||||
| ccmR2 | ns | ns | ns | ns | ns | ns |
| Cluster 3: | ||||||
| chpX | ns | ns | ns | ns | ns | ns |
| chpY | ns | ns | ns | ns | ns | ns |
| ccaA | ns | ns | ns | ns | ns | ns |
| mcyB | ns | ns | ns | ns | ns | ns |
| Cluster 4: | ||||||
| rbcX | ns | ns | ns | ns | -0.92** | ns |
| ccmM | ns | ns | ns | ns | -0.94** | ns |
| isiA | ns | ns | ns | ns | -0.83** | ns |
| gvpC | ns | ns | ns | ns | ns | ns |
FIGURE 5.
Scatter plots of relative gene expression versus bicarbonate concentration, for all genes that showed a significant or marginally significant relationship with bicarbonate. (A–C) Bicarbonate uptake genes (A) cmpA, (B) bicA, and (C) sbtA. (D) CCM transcriptional regulator gene ccmR. (E) Photoprotection gene flv4. Each data point shows the mean ± SD of three independent measurements. The trend lines are based on linear regression (n = 8).
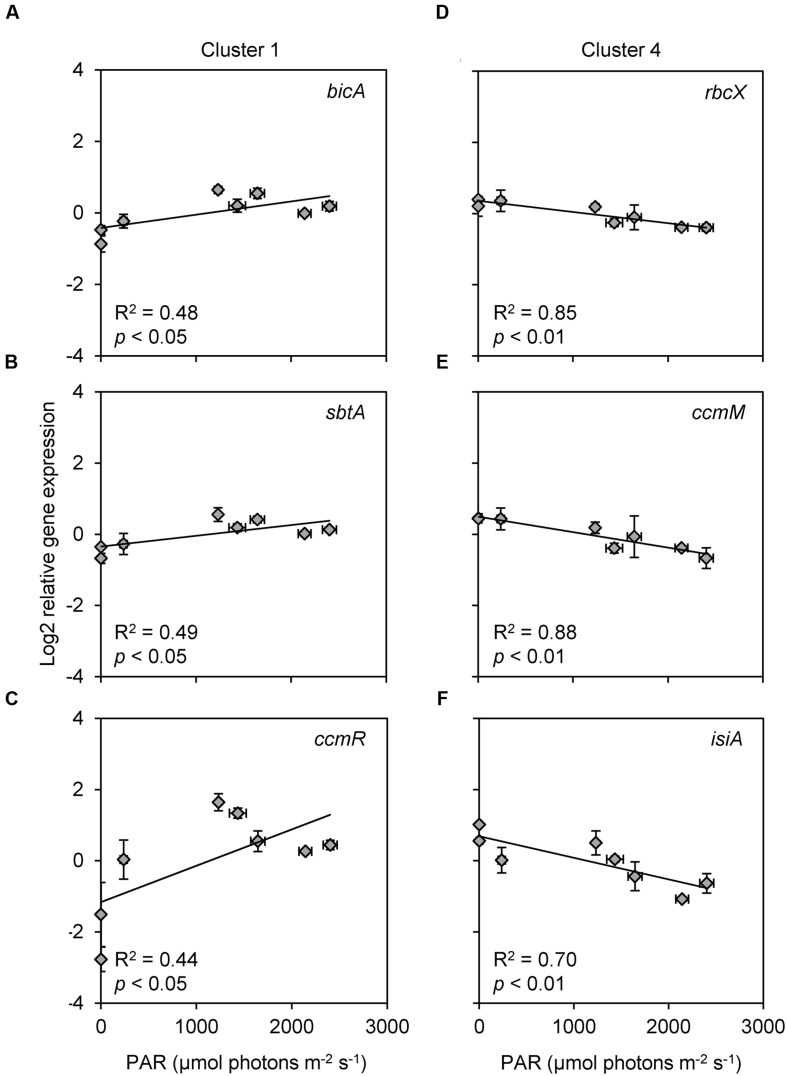
Expression of bicA, sbtA, and ccmR (cluster 1) showed a weak but significant positive correlation with the incident light intensity, whereas expression of rbcX, ccmM, and isiA (cluster 4) showed a significant negative correlation with the incident light intensity (Figure 6; Table 2). Furthermore, the expression of several genes (cmpA, bicA, ccmR, ccmR2, mcyB, and flv4) increased significantly from 4:45 to 7:00 AM, i.e., at first daylight (Figure 3; Table 1) before the Ci concentration was depleted by the bloom (Figure 2B).
FIGURE 6.
Scatter plots of relative gene expression versus light intensity, for all genes that showed a significant or marginally significant relationship with light. (A,B) Bicarbonate uptake genes (A) bicA and (B) sbtA. (C) CCM transcriptional regulator gene ccmR. (D) RuBisCO chaperone gene rbcX. (E) Carboxysomal gene ccmM. (F) Photoprotection gene isiA. Light intensity is indicated as PAR at the water surface. Each data point shows the mean ± SD of three independent measurements. The trend lines are based on linear regression (n = 8).
Discussion
Inorganic Carbon Dynamics
Most lakes worldwide are supersaturated with CO2 in the absence of phytoplankton blooms, because of, e.g., mineralization of organic carbon produced in the lake and received from the surrounding watershed (Cole et al., 1994; Sobek et al., 2005; Lazzarino et al., 2009). For instance, in a comparative study across almost 5,000 lakes, Sobek et al. (2005) found a mean pCO2 of 1,287 ppm [equivalent to a CO2(aq) concentration of ~50 μmol L-1 according to Henry’s law]. However, as our lake study illustrates, dense phytoplankton blooms in eutrophic lakes can deplete the CO2(aq) concentration and increase pH, and bring about substantial diel variation in the concentrations of dissolved inorganic carbon (DIC) and oxygen. During daytime, the CO2(aq) concentration was reduced to only 0.05 μmol L-1 (1 ppm) and pH increased above 10. At night, the CO2(aq) concentration increased, but the lake remained severely undersaturated. Similar observations have been made in other lakes with dense phytoplankton blooms. The photosynthetic activity of dense blooms can go up to 12.5–50 μmol C L-1 h-1 (Hein, 1997), which is sufficient to deplete the CO2(aq) concentration in lakes within a few hours (Talling, 1976; Maberly, 1996). Balmer and Downing (2011) reported several eutrophic lakes in which the CO2(aq) concentration was drawn down to less than 0.1 μmol L-1 during daytime, comparable to the low concentrations measured in our study. One limitation of our study is that we investigated only a single 24-h period, but other studies monitored the CO2 dynamics in lakes over longer time spans. Maberly (1996) monitored the inorganic carbon concentrations of Lake Esthwaite Water (UK) for an entire year, and found large diel fluctuations in the CO2(aq) concentration and pH (sometimes up to 1.8 units) throughout the summer. During dense phytoplankton blooms, CO2(aq) concentrations in Lake Esthwaite remained undersaturated with respect to the atmospheric CO2 level for several consecutive weeks. Verspagen et al. (2014) monitored Lake Volkerak (The Netherlands) on a biweekly basis for two consecutive years, during winter, the lake was supersaturated with CO2. In summer and early fall, however, Lake Volkerak was covered by dense blooms of the cyanobacterium Microcystis, and the lake became undersaturated with CO2 for several months.
Depletion of the CO2(aq) concentration by dense phytoplankton blooms creates a pCO2 gradient across the air-water interface, which implies that these lakes act as a sink for atmospheric CO2 (Balmer and Downing, 2011). More specifically, the CO2 influx (gCO2) from the atmosphere into a lake can be estimated from the difference between the expected concentration of CO2(aq) in equilibrium with the atmosphere (according to Henry’s law) and the measured concentration of CO2(aq) (Siegenthaler and Sarmiento, 1993; Cole et al., 2010):
| (1) |
where v is the gas transfer velocity across the air-water interface, KH is the solubility constant of CO2 gas in water (KH = 0.0375 mol L-1 atm-1 at 21.5°C; Weiss, 1974), and pCO2 is the partial pressure of CO2 in the atmosphere. Assuming a typical gas transfer velocity of v = 0.02 m h-1 (Crusius and Wanninkhof, 2003; Cole et al., 2010), an atmosphere with a pCO2 of 390 ppm and an average CO2(aq) of 0.2 μmol L-1 (Figure 2B), the daily CO2 influx into Lake Kennemermeer would amount to ~7 mmol m-2 d-1. This is a substantial CO2 influx, which will fuel further development of the cyanobacterial bloom. Moreover, the above calculation indicates that a doubling of the atmospheric CO2 concentration to 780 ppm will cause a near-doubling of the CO2 influx into the lake to ~14 mmol m-2 d-1, if we assume that dense cyanobacterial blooms still deplete the dissolved CO2 concentration. These results are in line with model predictions that rising atmospheric CO2 concentrations will stimulate cyanobacterial blooms in eutrophic lakes (Schippers et al., 2004; Verspagen et al., 2014; Visser et al., 2016).
Owing to the high pH induced by the cyanobacterial bloom, bicarbonate and carbonate were the most abundant inorganic carbon species in Lake Kennemermeer, whereas the CO2(aq) concentration was more than three orders of magnitude lower. The amplitude of the daily variation in CO2(aq) concentration was less than 0.3 μmol L-1, whereas the bicarbonate concentration varied more than 400 μmol L-1 during the 24-h period. We note that Microcystis often forms colonies. Colony morphology may affect gas transfer inside the colony, and cells deep within the colony may experience other CO2 and bicarbonate concentrations than cells at the outer edge of the colony. Nevertheless, given the low CO2(aq) concentration in the ambient lake water, it seems most likely that colonial cyanobacteria in these dense blooms will be strongly dependent on bicarbonate uptake to cover the high C demands of their photosynthetic activity, and may adapt to the large diel fluctuations in bicarbonate availability by adjusting the production of their different bicarbonate uptake systems.
Co-occurrence of Ci Uptake Genotypes
The phytoplankton bloom in Lake Kennemermeer consisted of a mixture of cyanobacterial species, including Pseudanabaenaceae, A. hungarica, small Chroococcales and Microcystis spp. For most of these species, genome sequences are not available, and we could not develop suitable primers to target their CCM genes. Therefore, we restricted our analysis to Microcystis, for which extensive sequence information is available (Kaneko et al., 2007; Frangeul et al., 2008; Humbert et al., 2013; Sandrini et al., 2014). For the interpretation of the results, however, it is important to realize that Microcystis does not grow in isolation but competes for Ci and other potentially limiting resources with other species in the lake. Changes in the relative abundances of Ci uptake genotypes and CCM gene expression of Microcystis are related to Ci drawdown and CO2 production by the entire bloom community.
Earlier we reported that Microcystis isolates from Lake Pehlitzsee (Germany) and Lake Volkerak (The Netherlands) contained both sbtA and bicA + sbtA strains, suggesting that these two Ci uptake genotypes may coexist (Sandrini et al., 2014). Our current results show that each of the three Ci uptake genotypes of Microcystis were present in Lake Kennemermeer with relative abundances of ≥15%, demonstrating that all three genotypes can co-occur in the same lake.
In a companion study, we monitored the population dynamics of the Ci uptake genotypes of Microcystis in Lake Kennemermeer from June to October 2013 (Sandrini, 2016). The results showed that bicA + sbtA strains dominated during the dense summer bloom, whereas sbtA strains and bicA strains were present at lower abundances, as reported here. However, bicA + sbtA strains were replaced by bicA strains when Ci concentrations increased during the demise of the cyanobacterial bloom in autumn. The trade-off between affinity and flux rate of the bicarbonate uptake systems provides a parsimonious explanation for these results. The SbtA enzyme has a high affinity for bicarbonate but low flux rate, whereas the BicA enzyme has a low affinity but high flux rate (Price, 2011). The dominance of bicA + sbtA strains, containing both bicarbonate uptake systems, indicates that strains that can cope with strong diel fluctuations of the bicarbonate concentration may have a selective advantage during the summer bloom. Indeed, laboratory experiments showed that bicA + sbtA strains can grow well across a wide range of Ci concentrations (Sandrini et al., 2014). The subsequent replacement of bicA + sbtA strains by bicA strains indicates that the high-affinity uptake system SbtA becomes superfluous when the Ci availability increases in autumn, providing a selective advantage for strains with only the high-flux uptake system BicA at high Ci levels.
Expression of Ci Uptake Genes
In contrast to most laboratory studies, our gene expression data reflect the average gene expression over the entire Microcystis population rather than the expression of a single strain. Nevertheless, our lake study shows distinct diel patterns of gene expression. In cyanobacteria, the expression of many genes is under the control of the circadian clock genes kaiA, kaiB, and kaiC (Ishiura et al., 1998; Iwasaki et al., 2002), and it is difficult to disentangle the role of environmental variation versus an internal circadian clock based on field observations alone. However, kai-mutants of the cyanobacterium Synechococcus PCC 7002 have shown that several CCM genes are kai-independent cycling genes (Ito et al., 2009). Therefore, it is plausible that the CCM genes of Microcystis are kai-independent as well. Our field data show that diel changes in the expression of the Ci uptake genes were associated with diel variation in Ci and/or light availability. This is in agreement with many laboratory experiments, which have demonstrated that the expression of cyanobacterial CCM genes can be strongly affected by changes in Ci and light availability (Hihara et al., 2001; McGinn et al., 2003; Woodger et al., 2003; Wang et al., 2004; Schwarz et al., 2011; Burnap et al., 2013; Sandrini et al., 2015a). We will therefore focus our interpretation of the expression data on diel variation in these environmental factors.
The expression of cmpA, encoding for a subunit of the high-affinity bicarbonate transporter BCT1, was negatively correlated with the bicarbonate concentration in the lake (Figure 5A). This pattern is in agreement with laboratory studies of Microcystis and other cyanobacteria, where cmpA expression was also strongly down-regulated at elevated Ci conditions (Sandrini et al., 2015a,b), and up-regulated under Ci-limiting conditions (Woodger et al., 2003; Wang et al., 2004; Schwarz et al., 2011). Bicarbonate concentrations in the lake decreased at daytime and increased at night, which likely caused the high cmpA expression in the late afternoon and a low cmpA expression at the end of the night. The large amplitude of the diel changes in cmpA expression suggests that the BCT1 enzyme is largely degraded at night and resynthesized each day. Expression of cmpA was not significantly correlated with light availability. However, bicarbonate uptake by BCT1 is ATP-dependent and ATP synthesis is driven by the light reactions of photosynthesis, which may explain the increase in cmpA expression at first daylight when the bicarbonate concentration was still high (Figures 2B and 3A). This explanation is also in agreement with previous laboratory studies under Ci-limited conditions, which showed increased cmpA expression at elevated light levels in several other cyanobacteria (Woodger et al., 2003; McGinn et al., 2004). Yet, ATP itself is probably not a regulator of the cmpABCD operon (Burnap et al., 2015). More likely, regulatory metabolites such as NADP+, α-ketoglutarate (α-KG), 2-phosphoglycolate (2PG) and ribulose-1,5-bisphosphate (RuBP), of which the levels can be affected by both light and Ci levels, control the activity of the CCM transcriptional regulators that in turn regulate the expression of the Ci uptake genes.
Expression of the bicarbonate uptake genes bicA and sbtA was also negatively correlated with the bicarbonate concentration (Figures 5B,C). In addition, their expression was positively correlated with the incident light intensity (Figures 6A,B). The expression of bicA and sbtA fluctuated in tandem (Figure 3A), likely because the Microcystis population was dominated by bicA + sbtA strains, in which both genes are located on the same operon (Sandrini et al., 2014). However, the diel variation in expression of bicA and sbtA had a much smaller amplitude than cmpA. The sodium concentration in the lake (12.7 ± 0.4 mmol L-1) allowed near-maximum activity of the sodium-dependent bicarbonate uptake systems BicA and SbtA, since both uptake systems have half-saturation constants of 1–2 mmol L-1 sodium (Price et al., 2004; Du et al., 2014). Therefore, the sodium concentration is unlikely to limit the amplitude of bicA and sbtA expression. Laboratory studies have shown that the response of bicA and sbtA expression to changes in Ci availability varies among Microcystis strains (Sandrini et al., 2015b). In some strains, bicA and sbtA expression is strongly down-regulated at elevated Ci conditions, whereas in other strains these genes are constitutively expressed. Hence, whether the observed low-amplitude variation in our field data reflects a generic pattern for many Microcystis blooms or a specific pattern for the Microcystis populations in our lake study remains to be investigated. Price et al. (2013) suggested that BicA and SbtA are simply inactivated by darkness and remain safeguarded for renewed activity at dawn, which may explain the low amplitude variation in gene expression, although the exact mechanisms of inactivation and re-activation remain to be revealed. Indeed, recently it was shown that SbtB is involved in the inhibition of SbtA during darkness (Du et al., 2014).
The chpX and chpY genes, encoding hydration subunits of the low-affinity and high-affinity CO2 uptake system, respectively, were constitutively expressed in our lake study (Figure 3B). Expression of these genes was not correlated with diel variation in CO2(aq) concentration or light intensity (Table 2). A possible reason could be that the diel variation in CO2(aq) concentrations was too low (from 0.05 to 0.32 μmol L-1) to affect expression of the CO2 uptake genes. However, both CO2 uptake genes were also constitutively expressed in laboratory studies with axenic Microcystis strains exposed to a much larger increase in CO2(aq) concentration (Sandrini et al., 2015a,b). In other cyanobacterial species, constitutive expression was established for the low-affinity CO2 uptake gene chpX whereas expression of the high-affinity CO2 uptake gene chpY was induced at low Ci levels (Woodger et al., 2003; Wang et al., 2004; Eisenhut et al., 2007; Schwarz et al., 2011). Furthermore, Jensen et al. (2011) found upregulation of both chpY and chpX during daytime for a Synechococcus mat. Hence, the CO2 uptake genes of different species respond differently to changing environmental conditions.
Expression of the CCM transcriptional regulator gene ccmR was negatively correlated with the bicarbonate concentration, similar to the bicarbonate uptake genes (Figure 5D). Microcystis lacks the transcriptional regulator gene cmpR, which regulates expression of the cmpABCD operon in some other cyanobacteria (Omata et al., 2001). Instead, CcmR most likely regulates expression of the cmpABCD operon in Microcystis (Sandrini et al., 2014, 2015b), which explains the similar gene expression patterns of cmpA and ccmR in our study. The ccmR2 gene is located directly upstream of the bicA/sbtA operon, and appears to be involved in the transcription of this operon (Sandrini et al., 2014). This gene formed a separate cluster in our analysis (Figure 4), probably because of a single deviant data point at 10:00 AM. Omitting this single data point, ccmR2 showed similar diel variation in expression as the bicarbonate uptake genes and ccmR (Figures 3A,C).
Diel Changes in Expression of Other Genes
The flavodiiron protein gene flv4, which is in one operon with flv2, was assigned to the same cluster as the bicarbonate uptake genes and the regulator gene ccmR (Figure 4). Flavodiiron proteins are stress proteins known to be involved in the acclimation to low Ci conditions (Zhang et al., 2009; Allahverdiyeva et al., 2011; Bersanini et al., 2014; Sandrini et al., 2015a), and can also be up-regulated by high light (Zhang et al., 2009). Similarly, in our study flv4 expression was increased at first daylight and reached high levels when Ci was depleted in the afternoon (Figure 3F). In contrast, Straub et al. (2011) found no significant diel variation in the expression of flv2 and flv4 in laboratory experiments with the axenic Microcystis strain PCC 7806, probably because their experiments were sparged with 1% CO2 and hence Ci concentrations were not limiting in their study. Both genes, flv2 and flv4, were shown to be involved in photoprotection of the PSII complex (Zhang et al., 2009). Hence, our study indicates that Flv2/4 proteins are highly active in daytime photoprotection of cyanobacterial blooms.
Interestingly, the iron-stress chlorophyll-binding protein IsiA is also involved in photoprotection (Havaux et al., 2005), but the expression pattern of isiA was very different from flv4 (Figure 3F). Expression of isiA was high at night but low during daytime (Figure 3F). In addition to its photoprotective function, IsiA is also involved in the formation of PSI antennae enhancing the light harvesting ability and in the storage of free chlorophyll molecules (Yeremenko et al., 2004; Havaux et al., 2005). Possibly, the turnover of photosystems and presence of free chlorophylls at night might stimulate isiA expression for temporary chlorophyll storage.
Expression of the rbcX, ccmM, ccaA, gvpC, and mcyB genes showed only minor diel variation. The RuBisCO gene rbcX and carboxysomal gene ccmM were negatively correlated with light intensity (Figures 6D,E), which could indicate that cells slightly increase the RuBisCO and carboxysome numbers at night to prepare for cell division, although it was previously shown that Microcystis cell division occurs both during daytime and at night (Yamamoto and Tsukada, 2009). Alternatively, the negative correlation might reflect an afternoon photosynthesis dip due to photoinhibition (Ibelings and Maberly, 1998; Wu et al., 2011) when the incident light intensity exceeded 1,500 μmol photons m-2 s-1 (Figure 2A). Expression of the other three genes (ccaA, gvpC, and mcyB) did not reveal a significant correlation with any of the environmental variables investigated (Table 2).
Conclusion
Cyanobacteria are often assumed to be favored at low Ci and high pH conditions, because of the presence of an effective CCM (e.g., Shapiro, 1997). However, recent laboratory studies revealed considerable genetic and phenotypic diversity in the CCM of the ubiquitous harmful cyanobacterium Microcystis (Sandrini et al., 2014, 2015b). Some strains perform well at low Ci levels, whereas other strains are much better competitors under high Ci conditions, suggesting that it might be difficult to foretell how natural mixtures of different Microcystis strains will respond to changes in Ci availability. Yet, our lake study showed consistent patterns in gene expression. The bicarbonate concentration in the lake showed large diel fluctuations, which may explain the predominance of bicA + sbtA strains and the concurrent diel fluctuations in expression of the bicarbonate uptake genes and their regulator genes. Hence, we conclude that the genetic and phenotypic versatility of the CCM of Microcystis enables a remarkably flexible but coherent response to the large diel fluctuations in Ci conditions often encountered in dense blooms.
Materials and Methods
Study Area and Sampling
Lake Kennemermeer (52°27′18.5′′N, 4°33′48.6′′E) is located north-west of Amsterdam, The Netherlands, near the North Sea coast (Figure 1). The man-made lake has been used as bathing water, but nowadays it is no longer in use as recreational lake because of yearly recurrent problems with harmful cyanobacterial blooms. The lake has a maximum depth of ~1 m, and surface area of ~0.1 km2. The shallow lake is well mixed by wind throughout the year.
During a dense summer bloom in July 2013, we monitored diel variation in gene expression and environmental conditions. The blooming area spanned the entire lake and the phytoplankton biomass peaked two times that year, from end of June till the 3rd week of July and in the 1st week of September. A small boat was used to sample the north side of the lake (Figure 1) at 13:00, 16:00, 19:00, and 22:45 of July 17, and at 4:45, 7:00, 10:00, and 13:00 of July 18. At each time point, the incident light intensity PAR just above the water surface, and vertical profiles of light intensity, water temperature, pH, dissolved oxygen and chlorophyll fluorescence were measured in triplicate at depth intervals of 0.1 m using a Hydrolab Surveyor with a Datasonde 4a (OTT Hydromet, Loveland, CO, USA). Furthermore, three independent water samples of 5 L each were collected at 0.2 m depth using plastic 10 L tanks. The samples were processed immediately for further analyses.
Cell Counts
Lugol’s iodine was added to 40 mL aliquots of fresh lake samples (1:100 v/v of a 5% solution) to preserve phytoplankton cells for microscopy. The samples were stored at 4°C until identification. Phytoplankton was counted according to the Utermöhl-method adjusted to the European standard protocol NEN-EN 15204 using a Lyca DM IRB inverted light microscope (Lyca Microsystems BV, Rijswijk, The Netherlands). Phytoplankton was identified to the genus level, and if possible to the species level. Biovolume was estimated from cellular dimensions and geometry (Hillebrand et al., 1999). Individual Microcystis cells were counted after disintegrating the colonies with KOH (Kardinaal et al., 2007).
Dissolved Inorganic Carbon and Sodium
For analysis of the DIC and sodium concentration, lake samples were filtered on site using 1.2 μm pore size 47 mm GF/C filters (Whatman GmbH, Dassel, Germany) followed by 0.45 μm pore size 47 mm polyethersulfone membrane filters (Sartorius AG, Goettingen, Germany). The filtrate was transferred to sterile plastic urine analysis tubes (VF-109SURI; Terumo Europe N.V., Leuven, Belgium) which were filled completely (with an inserted needle to release all air), and stored at 4°C until further analysis. A TOC-VCPH TOC analyzer (Shimadzu, Kyoto, Japan) was used to determine the DIC concentration, with 3–5 technical replicates per sample. Concentrations of dissolved CO2 (CO2(aq)), bicarbonate and carbonate were calculated from DIC and the pH and temperature of the lake (Stumm and Morgan, 1996). Sodium ion concentrations were measured using an Optima 8000 ICP-OES Spectrometer (Perkin Elmer, Waltham, MA, USA).
RNA Extraction
For RNA extraction, lake samples were filtered on-site with large 90 mm GF/C filters (Whatman GmbH, Dassel, Germany; 1.2 μm pore size) to concentrate phytoplankton biomass. Next, the 90 mm filters were dissected into several pieces using sterile surgical blades, which were transferred to 2 mL tubes that were filled with 1 mL TRIzol (Thermo Fisher Scientific, Waltham, MA, USA). The tubes were shaken vigorously, transported to the lab in a CX 100 Dry Shipper (Taylor Wharton, Theodore, AL, USA), and stored at -80°C until further analysis. RNA was extracted using 0.5 mm bashing beads (Zymo Research, Orange, CA, USA) to facilitate cell disruption, and purified according to Sandrini et al. (2015a) with the Direct-ZolTM RNA MiniPrep kit (Zymo Research, Orange, CA, USA) including in-column DNase I digestion. RNA concentrations were quantified using a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). All RNA samples had A260/A280 and A260/A230 values above 1.8.
Primer Development
Primers were developed to study expression of the Microcystis genes bicA, sbtA, cmpA, chpX, chpY, ccmR, ccmR2, rbcX, ccmM, ccaA, mcyB, gvpC, isiA, and flv4, using 16S rRNA as ‘reference gene.’ The complete list of primers is shown in Supplementary Table S1. The primers were designed to match the sequences of Microcystis strains NIES-843, PCC 7005, PCC 7941, PCC 7806, PCC 9432, PCC 9443, PCC 9701, PCC 9717, PCC 9806, PCC 9807, PCC 9808, PCC 9809, and T1-4. The bicA, sbtA and ccmR2 primers were also based on sequences of strains CCAP 1450/10, CCAP 1450/11, HUB 5-2-4, HUB 5-3, NICA-CYA 140, V145, and V163 (Sandrini et al., 2014). The primer design made use of IDT SciTools (Owczarzy et al., 2008) to develop primers with a similar melting temperature and GC content, such that all primers could be used under the same PCR conditions. We also used IDT SciTools to avoid undesirable secondary structures such as hairpins, self-dimers, or heterodimers. Furthermore, we performed primer-BLAST searches to assess the specificity of the primers (Ye et al., 2012). In this way, we ensured that the primers matched the gene sequences of Microcystis, but always included several mismatches with gene sequences of other cyanobacteria. The primers were tested on gDNA (obtained as described below) of 11 different Microcystis strains to ensure one target PCR product was formed. These PCR reactions were done with the GoTaq®Hot Start Polymerase kit (Promega Corporation, Madison, WI, USA) according to the supplier’s instructions. After an initial denaturation of 2 min at 95°C, 35 cycles were used that consisted of a denaturation step at 95°C for 45 s, an annealing temperature step at 60°C for 30 s and an extension step at 72°C for 3 min. Subsequently, a final extension step at 72°C was used for 5 min. The reactions contained 0.3 μmol L-1 primers and 10 ng gDNA in a total reaction volume of 25 μL. Other reaction components were added as instructed by the supplier. Gel electrophoresis showed that only the targeted gDNA sequences were amplified (no by-products were detected). As a negative control, the primers were tested on gDNA of Anabaena circinalis CCAP 1403/18, Aphanizomenon flos-aqua CCAP 1401/7, and Planktothrix agardhii CCAP 1460/1, which did not result in PCR amplification.
RT-qPCR Gene Expression Analysis
To quantify gene expression, cDNA was synthesized by reverse transcription of the RNA samples with Superscript III (Thermo Fisher Scientific, Waltham, MA, USA) according to Sandrini et al. (2015a). Subsequently, the qPCR Maxima®SYBR Green Master Mix (2x; Thermo Fisher Scientific, Waltham, MA, USA) was applied on the cDNA samples according to Sandrini et al. (2015a), in an ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The two-step cycling protocol was used, with a denaturation temperature of 95°C (15 s) and a combined annealing/extension temperature of 60°C (60 s) during 40 cycles. The reactions contained 0.3 μmol L-1 primers and 1 μL of 10 times diluted cDNA from the RT reaction in a total reaction volume of 25 μL. Other reaction components were added as instructed by the supplier. ROX solution was used to correct for any well-to-well variation. Melting curve analysis was performed on all measured samples to rule out non-specific PCR amplification. The melting curves confirmed that only one PCR product was amplified in each run.
Amplification efficiencies of individual runs (E) were calculated with LinRegPCR (version 2012.3; Ramakers et al., 2003; Ruijter et al., 2009) and were between 1.8 and 2.0 (Supplementary Table S1). Application of the primer sets to gDNA from six different Microcystis strains showed that the primer sets amplify the target genes from the different genotypes with similar efficiencies (see Supplementary Table S1 in Sandrini et al., 2015b). Time point 0 (13:00 of the 1st day) was used as ‘reference sample,’ and 16S rRNA was used as ‘reference gene.’ Each RT-qPCR plate contained a reference sample and samples with primers targeting 16S rRNA to correct for plate effects (differences in absolute fluorescence signal between individual plates). The data were baseline corrected using LinRegPCR, and the same software was used to calculate quantification cycle (Cq) values. LinRegPCR did not detect samples without amplification, without a plateau, with a baseline error or noise error, or with deviating amplification efficiencies. Negative control samples did not show significant amplification. Gene expression was quantified as the log2 ratio of the expression at a given time point relative to the mean expression over the 24-h period using the comparative CT method (Livak and Schmittgen, 2001). To determine if gene expression varied significantly between time points, one-way analysis of variance (ANOVA) was used with post hoc comparison of the means based on Tukey’s HSD test (α = 0.05) using SPSS version 20.0.
Hierarchical clustering was applied to compare the expression patterns of the studied genes. For each gene, time series of the expression values were normalized by the transformation (x - μ)/σ, where x is the original data point, μ is the mean of the time series, and σ is its standard deviation. Thus, all genes obtained normalized expression patterns with mean 0 and standard deviation 1. Hierarchical clustering and heatmap representation of the normalized gene expression data was done using the hclust and heatmap.2 functions of the gplots package in R version 3.0.2. We used the complete linkage clustering method for hierarchical clustering (Everitt et al., 2011).
Quantification of Microcystis Genotypes
To quantify the relative abundances of different Microcystis genotypes, we applied qPCR on purified gDNA (Supplementary Table S1). Lake samples were filtered on-site over 1.2 μm pore size 25 mm GF/C filters (Whatman GmbH, Dassel, Germany), and loaded filters were stored at -20°C. Subsequently, gDNA was extracted using the ZR Fungal/Bacterial DNA MiniPrepTM kit (Zymo Research, Orange, CA, USA) and further purified using the DNA Clean and ConcentratorTM-25 kit (Zymo Research) according to the supplier’s instructions. The gDNA samples were analyzed using a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), which resulted in A260/A280 values above 1.8 for all samples.
The Maxima®SYBR Green Master Mix (2x) kit (Thermo Fisher Scientific, Waltham, MA, USA) was applied to the purified gDNA according to the supplier’s instructions in an ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The two-step cycling protocol was used, and the reaction settings and melting curve analysis were the same as in the RT-qPCR section (see above). The reactions contained 0.3 μmol L-1 primers and 10 ng gDNA from lake samples in a total reaction volume of 25 μL. For data analysis, the LinRegPCR software tool (version 2012.3; Ramakers et al., 2003; Ruijter et al., 2009) and the comparative CT method (Livak and Schmittgen, 2001) were used.
To determine the relative abundances of the different Ci uptake genotypes, we used the bicA gene (primers bicA-F1 and bicA-R1; Supplementary Table S1) and the sbtA gene (primers sbtA-F1 and sbtA-R1; Supplementary Table S1) as ‘target genes.’ The bicA + sbtA gene of the bicA + sbtA strains (primers bicA-F2 and sbtA-R2; Supplementary Table S1) served as ‘reference gene.’ Purified gDNA of the axenic laboratory strains PCC 7005 and PCC 7941 (both bicA + sbtA strains; Sandrini et al., 2014) served as ‘reference samples,’ to calculate the relative ratios of (1) the bicA gene versus the bicA + sbtA gene, and (2) the sbtA gene versus the bicA + sbtA gene. We note that the bicA gene is present in both bicA strains and bicA + sbtA strains, and similarly the sbtA gene is present in both sbtA strains and bicA + sbtA strains. Hence, the relative abundances of the different Ci uptake genotypes can be calculated from the above two ratios based on the assumption that the sum of the bicA strains, sbtA strains and bicA + sbtA strains equals 100%.
To determine the relative abundance of potentially toxic genotypes, we used mcyB (primers mcyB-F and mcyB-R; Supplementary Table S1) as ‘target gene’ and the RuBisCO chaperone gene rbcX (primers rbcX-F and rbcX-R) present in all Microcystis strains as ‘reference gene.’ Purified gDNA of the axenic toxic strains PCC 7806 and PCC 7941 was used as ‘reference samples.’
Author Contributions
GS, HM, and JH designed the study. GS, RT, and HM performed the fieldwork, assisted by JMS and SB. GS analyzed most field samples. GS, RT, HM, and JH analyzed the data. GS, HM, and JH wrote the manuscript, and all authors commented on the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Veerle M. Luimstra for assistance with the cell counts. This research was supported by the Division of Earth and Life Sciences (ALW) of the Netherlands Organization for Scientific Research (NWO). JMS acknowledges support by the BE-BASIC Foundation.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00551
References
- Allahverdiyeva Y., Ermakova M., Eisenhut M., Zhang P., Richaud P., Hagemann M., et al. (2011). Interplay between flavodiiron proteins and photorespiration in Synechocystis sp. PCC 6803. J. Biol. Chem. 286 24007–24014. 10.1074/jbc.M111.223289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Price G. D., Long B. M., Woodger F. J. (2006). The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. J. Exp. Bot. 57 249–265. 10.1093/jxb/eri286 [DOI] [PubMed] [Google Scholar]
- Balmer M. B., Downing J. A. (2011). Carbon dioxide concentrations in eutrophic lakes: undersaturation implies atmospheric uptake. Inland Waters 1 125–132. 10.5268/IW-4.1.614 [DOI] [Google Scholar]
- Bersanini L., Battchikova N., Jokel M., Rehman A., Vass I., Allahverdiyeva Y.et al. (2014). Flavodiiron protein Flv2/Flv4-related photoprotective mechanism dissipates excitation pressure of PSII in cooperation with phycobilisomes in cyanobacteria. Plant Physiol. 164 805–818. 10.1104/pp.113.231969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer V. S., Stomp M., Rosso C., Van Beusekom S. A. M., Emmerich B., Stal L. J., et al. (2013). Low temperature delays timing and enhances the cost of nitrogen fixation in the unicellular cyanobacterium Cyanothece. ISME J. 7 2105–2115. 10.1038/ismej.2013.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnap R. L., Hagemann M., Kaplan A. (2015). Regulation of CO2 concentrating mechanism in cyanobacteria. Life (Basel) 5 348–371. 10.3390/life5010348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnap R. L., Nambudiri R., Holland S. (2013). Regulation of the carbon-concentrating mechanism in the cyanobacterium Synechocystis sp. PCC6803 in response to changing light intensity and inorganic carbon availability. Photosynth. Res. 118 115–124. 10.1007/s11120-013-9912-4 [DOI] [PubMed] [Google Scholar]
- Carmichael W. W. (2001). Health effects of toxin-producing cyanobacteria: “The CyanoHABs.” Hum. Ecol. Risk Assess. 7 1393–1407. 10.1080/20018091095087 [DOI] [Google Scholar]
- Chorus I., Bartram J. (1999). Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management. London: E & FN Spon. [Google Scholar]
- Codd G. A., Morrison L. F., Metcalf J. S. (2005). Cyanobacterial toxins: risk management for health protection. Toxicol. Appl. Pharmacol. 203 264–272. 10.1016/j.taap.2004.02.016 [DOI] [PubMed] [Google Scholar]
- Cole J. J., Bade D. L., Bastviken D., Pace M. L., Van de Bogert M. (2010). Multiple approaches to estimating air-water gas exchange in small lakes. Limnol. Oceanogr. Methods 8 285–293. 10.4319/lom.2010.8.285 [DOI] [Google Scholar]
- Cole J. J., Caraco N. F., Kling G. W., Kratz T. K. (1994). Carbon dioxide supersaturation in the surface waters of lakes. Science 265 1568–1570. 10.1126/science.265.5178.1568 [DOI] [PubMed] [Google Scholar]
- Crusius J., Wanninkhof R. (2003). Gas transfer velocities measured at low wind speed over a lake. Limnol. Oceanogr. 48 1010–1017. 10.4319/lo.2003.48.3.1010 [DOI] [Google Scholar]
- Dittmann E., Fewer D. P., Neilan B. A. (2013). Cyanobacterial toxins: biosynthetic routes and evolutionary roots. FEMS Microbiol. Rev. 37 23–43. 10.1111/j.1574-6976.2012.12000.x [DOI] [PubMed] [Google Scholar]
- Du J., Förster B., Rourke L., Howitt S. M., Price G. D. (2014). Characterisation of cyanobacterial bicarbonate transporters in E. coli shows that SbtA homologs are functional in this heterologous expression system. PLoS ONE 9:e115905 10.1371/journal.pone.0115905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut M., Aguirre von Wobeser E., Jonas L., Schubert H., Ibelings B. W., Bauwe H., et al. (2007). Long-term response toward inorganic carbon limitation in wild type and glycolate turnover mutants of the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiol. 144 1946–1959. 10.1104/pp.107.103341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B. S., Landau S., Leese M., Stahl D. (2011). Cluster Analysis, Hierarchical Clustering, 5 Edn Chichester: John Wiley & Sons, 71–110. [Google Scholar]
- Frangeul L., Quillardet P., Castets A. M., Humbert J. F., Matthijs H. C. P., Cortez D., et al. (2008). Highly plastic genome of Microcystis aeruginosa PCC 7806, a ubiquitous toxic freshwater cyanobacterium. BMC Genomics 9:274 10.1186/1471-2164-9-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B., Schelske C. L., Coveney M. F. (2011). Low carbon dioxide partial pressure in a productive subtropical lake. Aquat. Sci. 73 317–330. 10.1007/s00027-010-0179-y [DOI] [Google Scholar]
- Havaux M., Guedeney G., Hagemann M., Yeremenko N., Matthijs H. C. P., Jeanjean R. (2005). The chlorophyll-binding protein IsiA is inducible by high light and protects the cyanobacterium Synechocystis PCC6803 from photooxidative stress. FEBS Lett. 579 2289–2293. 10.1016/j.febslet.2005.03.021 [DOI] [PubMed] [Google Scholar]
- Hein M. (1997). Inorganic carbon limitation of photosynthesis in lake phytoplankton. Freshw. Biol. 37 545–552. 10.1046/j.1365-2427.1997.00180.x [DOI] [Google Scholar]
- Hihara Y., Kamei A., Kanehisa M., Kaplan A., Ikeuchi M. (2001). DNA microarray analysis of cyanobacterial gene expression during acclimation to high light. Plant Cell 13 793–806. 10.1105/tpc.13.4.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand H., Dürselen C. D., Kirschtel D., Pollingher U., Zohary T. (1999). Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 35 403–424. 10.1046/j.1529-8817.1999.3520403.x [DOI] [Google Scholar]
- Huisman J., Matthijs H. C. P., Visser P. M. (2005). Harmful Cyanobacteria. Berlin: Springer. [Google Scholar]
- Humbert J. F., Barbe V., Latifi A., Gugger M., Calteau A., Coursin T., et al. (2013). A tribute to disorder in the genome of the bloom-forming freshwater cyanobacterium Microcystis aeruginosa. PLoS ONE 8:e70747 10.1371/journal.pone.0070747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibelings B. W., Maberly S. C. (1998). Photoinhibition and the availability of inorganic carbon restrict photosynthesis by surface blooms of cyanobacteria. Limnol. Oceanogr. 43 408–419. 10.4319/lo.1998.43.3.0408 [DOI] [Google Scholar]
- Ishiura M., Kutsuna S., Aoki S., Iwasaki H., Andersson C. R., Tanabe A., et al. (1998). Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science 281 1519–1523. 10.1126/science.281.5382.1519 [DOI] [PubMed] [Google Scholar]
- Ito H., Mutsuda M., Murayama Y., Tomita J., Hosokawa N., Terauchi K., et al. (2009). Cyanobacterial daily life with Kai-based circadian and diurnal genome-wide transcriptional control in Synechococcus elongatus. Proc. Natl. Acad. Sci. U.S.A. 106 14168–14173. 10.1073/pnas.0902587106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H., Nishiwaki T., Kitayama Y., Nakajima M., Kondo T. (2002). KaiA-stimulated KaiC phosphorylation in circadian timing loops in cyanobacteria. Proc. Natl. Acad. Sci. U.S.A. 99 15788–15793. 10.1073/pnas.222467299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. I., Steunou A.-S., Bhaya D., Kühl M., Grossman A. R. (2011). In situ dynamics of O2, pH and cyanobacterial transcripts associated with CCM, photosynthesis and detoxification of ROS. ISME J. 5 317–328. 10.1038/ismej.2010.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöhnk K. D., Huisman J., Sharples J., Sommeijer B., Visser P. M., Stroom J. M. (2008). Summer heatwaves promote blooms of harmful cyanobacteria. Global Change Biol. 14 495–512. 10.1111/j.1365-2486.2007.01510.x [DOI] [Google Scholar]
- Kaneko T., Nakajima N., Okamoto S., Suzuki I., Tanabe Y., Tamaoki M., et al. (2007). Complete genomic structure of the bloom-forming toxic cyanobacterium Microcystis aeruginosa NIES-843. DNA Res. 14 247–256. 10.1093/dnares/dsm026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Reinhold L. (1999). CO2 concentrating mechanisms in photosynthetic microorganisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50 539–570. 10.1146/annurev.arplant.50.1.539 [DOI] [PubMed] [Google Scholar]
- Kardinaal W. E. A., Janse I., Kamst-Van Agterveld M., Meima M., Snoek J., Mur L. R., et al. (2007). Microcystis genotype succession in relation to microcystin concentrations in freshwater lakes. Aquat. Microb. Ecol. 48 1–12. 10.3354/ame048001 [DOI] [Google Scholar]
- Komárek J., Anagnostidis K. (1999). Cyanoprokaryota. 1. Teil: Chroococcales. Jena: Gustav Fischer Verlag, 225–236. [Google Scholar]
- Kosten S., Huszar V. L., Bécares E., Costa L. S., Donk E., Hansson L. A., et al. (2012). Warmer climates boost cyanobacterial dominance in shallow lakes. Global Change Biol. 18 118–126. 10.1111/j.1365-2486.2011.02488.x [DOI] [Google Scholar]
- Kucho K. I., Okamoto K., Tsuchiya Y., Nomura S., Nango M., Kanehisa M., et al. (2005). Global analysis of circadian expression in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 187 2190–2199. 10.1128/JB.187.6.2190-2199.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarino J. K., Bachmann R. W., Hoyer M. V., Canfield D. E., Jr. (2009). Carbon dioxide supersaturation in Florida lakes. Hydrobiologia 627 169–180. [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔC(T) method. Methods 25 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Maberly S. C. (1996). Diel, episodic and seasonal changes in pH and concentrations of inorganic carbon in a productive lake. Freshw. Biol. 35 579–598. 10.1111/j.1365-2427.1996.tb01770.x [DOI] [Google Scholar]
- Maeda S., Badger M. R., Price G. D. (2002). Novel gene products associated with NdhD3/D4-containing NDH-1 complexes are involved in photosynthetic CO2 hydration in the cyanobacterium, Synechococcus sp. PCC7942. Mol. Microbiol. 43 425–435. 10.1046/j.1365-2958.2002.02753.x [DOI] [PubMed] [Google Scholar]
- McGinn P. J., Price G. D., Badger M. R. (2004). High light enhances the expression of low-CO2-inducible transcripts involved in the CO2-concentrating mechanism in Synechocystis sp. PCC6803. Plant Cell Environ. 27 615–626. 10.1111/j.1365-3040.2004.01175.x [DOI] [Google Scholar]
- McGinn P. J., Price G. D., Maleszka R., Badger M. R. (2003). Inorganic carbon limitation and light control the expression of transcripts related to the CO2-concentrating mechanism in the cyanobacterium Synechocystis sp. strain PCC6803. Plant Physiol. 132 218–229. 10.1104/pp.019349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak A. M., Anderson E. J., Beletsky D., Boland S., Bosch N. S., Bridgeman T. B., et al. (2013). Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. U.S.A. 110 6448–6452. 10.1073/pnas.1216006110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T., Gohta S., Takahashi Y., Harano Y., Maeda S. (2001). Involvement of a CbbR homolog in low CO2-induced activation of the bicarbonate transporter operon in cyanobacteria. J. Bacteriol. 183 1891–1898. 10.1128/JB.183.6.1891-1898.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T., Price G. D., Badger M. R., Okamura M., Gohta S., Ogawa T. (1999). Identification of an ATP-binding cassette transporter involved in bicarbonate uptake in the cyanobacterium Synechococcus sp. strain PCC 7942. Proc. Natl. Acad. Sci. U.S.A. 96 13571–13576. 10.1073/pnas.96.23.13571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil J. M., Davis T. W., Burford M. A., Gobler C. J. (2012). The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae 14 313–334. 10.1016/j.hal.2011.10.027 [DOI] [Google Scholar]
- Otsuka S., Suda S., Shibata S., Oyaizu H., Matsumoto S., Watanabe M. M. (2001). A proposal for the unification of five species of the cyanobacterial genus Microcystis Kützing ex Lemmermann 1907 under the rules of the Bacteriological Code. Int. J. Syst. Evol. Microbiol. 51 873–879. 10.1099/00207713-51-3-873 [DOI] [PubMed] [Google Scholar]
- Owczarzy R., Tataurov A. V., Wu Y., Manthey J. A., McQuisten K. A., Almabrazi H. G., et al. (2008). IDT SciTools: a suite for analysis and design of nucleic acid oligomers. Nucleic Acids Res. 36 W163–W169. 10.1093/nar/gkn198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl H. W., Huisman J. (2008). Blooms like it hot. Science 320 57–58. 10.1126/science.1155398 [DOI] [PubMed] [Google Scholar]
- Penn K., Wang J., Fernando S., Thompson J. (2014). Secondary metabolite gene expression and interplay of bacterial functions in a tropical freshwater cyanobacterial bloom. ISME J. 8 1866–1878. 10.1038/ismej.2014.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G. D. (2011). Inorganic carbon transporters of the cyanobacterial CO2 concentrating mechanism. Photosynth. Res. 109 47–57. 10.1007/s11120-010-9608-y [DOI] [PubMed] [Google Scholar]
- Price G. D., Badger M. R., Woodger F. J., Long B. M. (2008). Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 59 1441–1461. 10.1093/jxb/erm112 [DOI] [PubMed] [Google Scholar]
- Price G. D., Maeda S., Omata T., Badger M. R. (2002). Modes of active inorganic carbon uptake in the cyanobacterium, Synechococcus sp. PCC7942. Funct. Plant Biol. 29 131–149. 10.1071/PP01229 [DOI] [PubMed] [Google Scholar]
- Price G. D., Pengelly J. J., Forster B., Du J., Whitney S. M., von Caemmerer S., et al. (2013). The cyanobacterial CCM as a source of genes for improving photosynthetic CO2 fixation in crop species. J. Exp. Bot. 64 753–768. 10.1093/jxb/ers257 [DOI] [PubMed] [Google Scholar]
- Price G. D., Woodger F. J., Badger M. R., Howitt S. M., Tucker L. (2004). Identification of a SulP-type bicarbonate transporter in marine cyanobacteria. Proc. Natl. Acad. Sci. U.S.A. 101 18228–18233. 10.1073/pnas.0405211101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin B., Zhu G., Gao G., Zhang Y., Li W., Paerl H. W., et al. (2010). A drinking water crisis in Lake Taihu, China: linkage to climatic variability and lake management. Environ. Manag. 45 105–112. 10.1007/s00267-009-9393-6 [DOI] [PubMed] [Google Scholar]
- Rae B. D., Forster B., Badger M. R., Price G. D. (2011). The CO2-concentrating mechanism of Synechococcus WH5701 is composed of native and horizontally-acquired components. Photosynth. Res. 109 59–72. 10.1007/s11120-011-9641-5 [DOI] [PubMed] [Google Scholar]
- Ramakers C., Ruijter J. M., Deprez R. H., Moorman A. F. (2003). Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339 62–66. 10.1016/S0304-3940(02)01423-4 [DOI] [PubMed] [Google Scholar]
- Raven J. A., Giordano M., Beardall J., Maberly S. C. (2012). Algal evolution in relation to atmospheric CO2: carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles. Phil. Trans. R. Soc. B 367 493–507. 10.1098/rstb.2011.0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijter J. M., Ramakers C., Hoogaars W. M., Karlen Y., Bakker O., van den Hoff M. J., et al. (2009). Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 37 e45. 10.1093/nar/gkp045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saker M. L., Fastner J., Dittmann E., Christiansen G., Vasconcelos V. M. (2005). Variation between strains of the cyanobacterium Microcystis aeruginosa isolated from a Portuguese river. J. Appl. Microbiol. 99 749–757. 10.1111/j.1365-2672.2005.02687.x [DOI] [PubMed] [Google Scholar]
- Sandrini G. (2016). Effects of Rising CO2 on the Harmful Cyanobacterium Microcystis. Ph.D. thesis, University of Amsterdam, Amsterdam. [Google Scholar]
- Sandrini G., Cunsolo S., Schuurmans J. M., Matthijs H. C. P., Huisman J. (2015a). Changes in gene expression, cell physiology and toxicity of the harmful cyanobacterium Microcystis aeruginosa at elevated CO2. Front. Microbiol. 6:401 10.3389/fmicb.2015.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini G., Jakupovic D., Matthijs H. C. P., Huisman J. (2015b). Strains of the harmful cyanobacterium Microcystis aeruginosa differ in gene expression and activity of inorganic carbon uptake systems at elevated CO2 levels. Appl. Environ. Microbiol. 81 7730–7739. 10.1128/AEM.02295-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini G., Matthijs H. C. P., Verspagen J. M. H., Muyzer G., Huisman J. (2014). Genetic diversity of inorganic carbon uptake systems causes variation in CO2 response of the cyanobacterium Microcystis. ISME J. 8 589–600. 10.1038/ismej.2013.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers P., Lürling M., Scheffer M. (2004). Increase of atmospheric CO2 promotes phytoplankton productivity. Ecol. Lett. 7 446–451. 10.1111/j.1461-0248.2004.00597.x [DOI] [Google Scholar]
- Schwarz D., Nodop A., Hüge J., Purfürst S., Forchhammer K., Michel K. P., et al. (2011). Metabolic and transcriptomic phenotyping of inorganic carbon acclimation in the cyanobacterium Synechococcus elongatus PCC 7942. Plant Physiol. 155 1640–1655. 10.1104/pp.110.170225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. (1997). The role of carbon dioxide in the initiation and maintenance of blue-green dominance in lakes. Freshw. Biol. 37 307–323. 10.1046/j.1365-2427.1997.00164.x [DOI] [Google Scholar]
- Siegenthaler U., Sarmiento J. L. (1993). Atmospheric carbon dioxide and the ocean. Nature 365 119–125. 10.1038/365119a0 [DOI] [Google Scholar]
- Sobek S., Tranvik L. J., Cole J. J. (2005). Temperature independence of carbon dioxide supersaturation in global lakes. Global Biogeochem. Cycles 19:GB2003 10.1029/2004GB002264 [DOI] [Google Scholar]
- Steffen M. M., Belisle B. S., Watson S. B., Boyer G. L., Bourbonniere R. A., Wilhelm S. W. (2015). Metatranscriptomic evidence for co-occurring top-down and bottom-up controls on toxic cyanobacterial communities. Appl. Environ. Microbiol. 81 3268–3276. 10.1128/AEM.04101-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen M. M., Belisle B. S., Watson S. B., Boyer G. L., Wilhelm S. W. (2014). Status, causes and controls of cyanobacterial blooms in Lake Erie. J. Great Lakes Res. 40 215–225. 10.1016/j.jglr.2013.12.012 [DOI] [Google Scholar]
- Straub C., Quillardet P., Vergalli J., De Marsac N. T., Humbert J. F. (2011). A day in the life of Microcystis aeruginosa strain PCC 7806 as revealed by a transcriptomic analysis. PLoS ONE 6:e16208 10.1371/journal.pone.0016208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm W., Morgan J. J. (1996). Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. New York, NY: Wiley-Interscience. [Google Scholar]
- Talling J. F. (1976). The depletion of carbon dioxide from lake water by phytoplankton. J. Ecol. 64 79–121. 10.2307/2258685 [DOI] [Google Scholar]
- Van de Waal D. B., Verspagen J. M. H., Finke J. F., Vournazou V., Immers A. K., Kardinaal W. E. A., et al. (2011). Reversal in competitive dominance of a toxic versus non-toxic cyanobacterium in response to rising CO2. ISME J. 5 1438–1450. 10.1038/ismej.2011.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verspagen J. M. H., Passarge J., Jöhnk K. D., Visser P. M., Peperzak L., Boers P., et al. (2006). Water management strategies against toxic Microcystis blooms in the Dutch delta. Ecol. Appl. 16 313–327. 10.1890/04-1953 [DOI] [PubMed] [Google Scholar]
- Verspagen J. M. H., Van de Waal D. B., Finke J. F., Visser P. M., Van Donk E., Huisman J. (2014). Rising CO2 levels will intensify phytoplankton blooms in eutrophic and hypertrophic lakes. PLoS ONE 9:e104325 10.1371/journal.pone.0104325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser P. M., Verspagen J. M. H., Sandrini G., Stal L. J., Matthijs H. C. P., Davis T. W., et al. (2016). How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae (in press). [DOI] [PubMed] [Google Scholar]
- Wang H. L., Postier B. L., Burnap R. L. (2004). Alterations in global patterns of gene expression in Synechocystis sp. PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator. J. Biol. Chem. 279 5739–5751. 10.1074/jbc.M311336200 [DOI] [PubMed] [Google Scholar]
- Weiss R. (1974). Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Mar. Chem. 2 203–215. 10.1016/0304-4203(74)90015-2 [DOI] [Google Scholar]
- Woodger F. J., Badger M. R., Price G. D. (2003). Inorganic carbon limitation induces transcripts encoding components of the CO2-concentrating mechanism in Synechococcus sp. PCC7942 through a redox-independent pathway. Plant Physiol. 133 2069–2080. 10.1104/pp.103.029728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Kong F., Zhang M. (2011). Photoinhibition of colonial and unicellular Microcystis cells in a summer bloom in Lake Taihu. Limnology 12 55–61. 10.1007/s10201-010-0321-5 [DOI] [Google Scholar]
- Yamamoto Y., Tsukada H. (2009). Measurement of in situ specific growth rates of Microcystis (cyanobacteria) from the frequency of dividing cells. J. Phycol. 45 1003–1009. 10.1111/j.1529-8817.2009.00723.x [DOI] [PubMed] [Google Scholar]
- Ye J., Coulouris G., Zaretskaya I., Cutcutache I., Rozen S., Madden T. L. (2012). Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13:134 10.1186/1471-2105-13-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeremenko N., Kouril R., Ihalainen J. A., D’Haene S., Van Oosterwijk N., Andrizhiyevskaya E. G., et al. (2004). Supramolecular organization and dual function of the IsiA chlorophyll-binding protein in cyanobacteria. Biochemistry 43 10308–10313. 10.1021/bi048772l [DOI] [PubMed] [Google Scholar]
- Zhang P., Allahverdiyeva Y., Eisenhut M., Aro E. M. (2009). Flavodiiron proteins in oxygenic photosynthetic organisms: photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS ONE 4:e5331 10.1371/journal.pone.0005331 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.