Abstract
Magnesium (Mg)-based biomaterials have shown great potential in clinical applications. However, the cytotoxic effects of excessive Mg2+ and the corrosion products from Mg-based biomaterials, particularly their effects on neurons, have been little studied. Although viability tests are most commonly used, a functional evaluation is critically needed. Here, both methyl thiazolyl tetrazolium (MTT) and lactate dehydrogenase (LDH) assays were used to test the effect of Mg2+ and Mg-extract solution on neuronal viability. Microelectrode arrays (MEAs), which provide long-term, real-time recording of extracellular electrophysiological signals of in vitro neuronal networks, were used to test for toxic effects. The minimum effective concentrations (ECmin) of Mg2+ from the MTT and LDH assays were 3 mmol/L and 100 mmol/L, respectively, while the ECmin obtained from the MEA assay was 0.1 mmol/L. MEA data revealed significant loss of neuronal network activity when the culture was exposed to 25% Mg-extract solution, a concentration that did not affect neuronal viability. For evaluating the biocompatibility of Mg-based biomaterials with neurons, MEA electrophysiological testing is a more precise method than basic cell-viability testing.
Keywords: Magnesium, Microelectrode array, Neuroelectrophysiology, Neuron viability, Neuronal network
1. Introduction
In recent years, magnesium (Mg) and its alloys have been investigated for applications as implantable biomedical materials because of their excellent mechanical properties and complete biodegradability[1]. Clinical application of Mg-based biomaterials has shown great potential in orthopedic implants and cardiovascular stents[2,3], and suitability for applications in nerve regeneration is currently being studied. Vennemeyer[4] created a pure Mg microfilament to serve as a temporary scaffold within a biodegradable poly-caprolactone nerve conduit. Although the range of clinical applications continues to increase, the cytotoxic effects of excessive magnesium ions (Mg2+, a more highly concentrated than normal physiological level) and corrosion products from Mg-based biomaterials have been less studied. Recent reports on Mg-related cytotoxicity have focused mainly on bone and vessel cells and fibroblasts[5]. Few studies have focused on neuronal cytotoxicity.
Cytocompatiblity of Mg-based biomaterials is typically studied using cell-viability tests, such as the methyl thiazolyl tetrazolium (MTT) and lactate dehydrogenase (LDH) assays. However, such tests do not provide information on nonlethal damage to cells. Mg2+, the main corrosion product of Mg-based biomaterial, plays an important role in neuroelectrophysiological function: A voltage-dependent blocker of glutamate N-methyl-D-aspartate (NMDA) receptors, Mg2+ is associated with the entry of calcium ions into neurons and the initiation of action potentials[6]. Although increases in extracellular Mg2+ concentration caused by biomaterial implantation may not affect cell viability, they can elevate the risk of suppression of neuronal excitability[7]. Therefore, a more precise functional method is required to evaluate the biocompatibility of Mg-based implantable biomaterials.
The microelectrode array (MEA) has been recently used in in vitro electrophysiological testing to supplement patch clamp testing, the traditional method of intracellular electrophysiological recording. MEA assessment provides noninvasive, long-term, real-time extracellular-signal recording with high-throughput and multiparameter outputs. In vitro neuronal networks on MEAs, having been developed as biosensors, are widely used in neuropharmacological tests and are now viewed as the physiologically based neurotoxicity-testing platform of the 21st century[8]. However, few studies report electrophysiological testing of biomaterials on the MEA.
Here we studied the effect of the corrosion products of pure Mg and defined concentrations of Mg2+ ([Mg2+]) on neurons. First, we studied the effects on neuronal viability by conducting MTT and LDH assays using our chick forebrain neuron (CFBN) culture model. Then, we used our recently established CFBN network–microelectrode array (CFBNN–MEA) biosensor to study neuronal electrophysiology effects. Our CFBNN has been proven to have a comparative development process and firing pattern with rodent cortical neurons that is widely used in MEA study, but with the advantages of abundant resources, cost-effective cultures, and an easy dissection process[9].
2. Materials and Methods
2.1. Material preparation
2.1.1. Preparation of defined concentrations of standard Mg2+solutions
2.1.1.1. Viability test
Magnesium chloride hexahydrate (M2670, Sigma) was dissolved at 100 mmol/L in serum-free neurobasal (NB)culture media (Neurobasal Medium; 21103-049, Gibco, Invitrogen)supplemented with 2% B27 (17504-044, Gibco, Invitrogen), 1% GluMax (35050-061, Gibco, Invitrogen), and 0.5% penicillin/streptomycin (P/S, 10378-016, Gibco, Invitrogen). The medium was then diluted to 30, 10, 3, 1, 0.3, and 0.1 mmol/L [Mg2+] for use in the viability test as standard Mg2+ solutions. Because cell-culture medium contains Mg2+ at approximately physiological level, [Mg2+] specified in this paper is given as a relative concentration; that is, the amount is our addition to the original [Mg2+] in the media.
2.1.1.2. Electrophysiological test
Magnesium chloride hexahydrate was dissolved in phosphate buffered saline (PBS) at 2 mol/L and was serially diluted to 200, 20, and 2 mmol/L [Mg2+] for use in electrophysiological testing as stock solutions.
2.1.2. Preparation of Mg-extract solutions
Pure Mg (99.95% in purity, Hunan Rare-Earth Metal Research Institute of China) was cut into 1.5 cm square specimens (1.0 mm height). They were polished successively with 400#, 1000#, and 2000# grit paper, ultrasonically cleaned in pure ethanol for 20 min, sterilized in ultraviolet light for 30 min, and dried in a laminar hood. The Mg-extract solutions were prepared according to ISO 10993:12: The Mg specimens were immersed in serum-free NB culture media for 24 h in a standard cell-culture incubator (37 °C, 5% CO2). The volume of the medium was determined by the specimen's surface area-to-media-volume ratio of 3 cm2/mL. The medium was then collected, its pH value was measured, and its [Mg2+] was tested using inductively coupled plasma atomic emission spectrometry (ICP-AES, Optima5300DV, USA). Then the collected medium was filtered through a Ø0.22 μm membrane and diluted with untreated culture media to 50% and 25% concentrations. The original and diluted media were then stored as Mg-extract solutions at 4 °C.
2.2. Neuron viability test
2.2.1. Primary neuronal cultures for viability testing
Forebrain tissues were harvested from Day 7 White Leghorn chick embryos as described by Heidemann[10]. The tissues were dissociated enzymatically in 0.25% trypsin with EDTA (T4049, Sigma) for 5 min at 37 °C and were mechanically dissociated by trituration through a 1 mL pipette tip. After centrifugation at 1000 r/min for 5 min, the cells were suspended in M199 media (M4530, Sigma) supplemented with 2% B27 and 1% P/S. Prior to seeding, the 96-well culture plates were coated with 0.05% w/v polyethylenimine (P3143, Sigma) for 2 h and then washed 3 times in deionized water. Cells were plated onto coated culture plates at a density of 6.4 × 104 cells/well (2000 cells/mm2) and cultured for 24 h in a standard incubator (37 °C, 95% humidity, and 5% CO2). Then the medium was changed to NB culture media for maintenance. After 3 days, half of the media was replaced with fresh culture media with 2 μmol/L cytosine arabinoside (Ar-AC, C1768, Sigma) to prevent glial proliferation. After 5 days, the medium was completely replaced with 150 μL of standard solution with defined Mg2+ concentrations or Mg-extract solutions; fresh culture medium was used as the negative control. The cells were subsequently used for MTT and LDH tests at Day 1, Day 3, and Day 5. Five identical wells were prepared for statistical analysis.
2.2.2. MTT and LDH measurements
At Day 1, Day 3, and Day 5, 50 μL of supernatant was transferred from each well of the culture plates to the corresponding well of new 96-well culture plates for LDH tests. Then, 10 μL of 5 mg/mL MTT (M5655, Sigma) in PBS was added to each well, and the culture plates were incubated for 4 h. The supernatant in each well was carefully removed by aspiration, and 150 μL of dimethylsulfoxide (DMSO, D8418, Sigma) was added. The plates were shaken for 10 min to thoroughly resolve purple formazan and placed on a multidetection microplate reader (BioTek, Synergy™ 4) to measure optical density (OD) at 570 nm; the reference wavelength was 630 nm. The results were expressed as:
LDH leakage was measured using a commercial LDH Kit (88953, Pierson) according to the manufacturer's protocol. In brief, the 50 μL of medium that was removed before adding MTT was mixed with 50 μL of reaction buffer for 30 min at room temperature in the dark, followed by the addition of 50 μL of stop solution. Using a multidetection microplate reader (BioTek, Synergy™ 4) and a reference wavelength of 680 nm, the OD of the mixtures was measured at 490 nm. The results were expressed as:
2.3. Neuronal electrophysiology test
2.3.1. Primary neuronal cultures for electrophysiological testing
For the MEA test, forebrain tissues that contained a high proportion of glial cells were harvested from Day 9 chick embryos. The dissection and dissociation methods were the same as those described in Section 2.2.1. Cells were suspended in M199 media with 2% B27 at a density of approximately 3 × 106 cells/mL. A 20 μL of cell suspension was plated at the center of a standard MEA (60MEA200/30iR-Ti, Multi-Channel Systems, Germany, Fig. 1(a)) according to Hales's protocol[11]. Prior to cell plating, the MEA surface was activated by oxygen plasma treatment (PDC-32G, Harrick) for 3 min and coated with 0.05% w/v polyethylenimine (P3143, Sigma) for 30 min at 37 °C; it was then washed 3 times in deionized water. After plating, the MEA chip was covered with a Teflon® lid (MEA-MEM5, ALA Scientific) that was permeable to gases, but not water and bacteria, to maintain proper culture osmolality and avoid contamination[12] (Fig. 1(b)). Then, the chip was placed in a 100 mm Petri dish and cultured in a humidified incubator at 37 °C with 5% CO2. 1 mL of M199 media was added to the chip after cells were attached. After 24 h in the culture, the cells were maintained in the NB culture medium with 2% fetal bovine serum (10437-077, Gibco) for long-term culture. Because glia, particularly astrocytes, is fundamental for development of neuronal-network activity, an antimitotic drug was not added to prevent glial proliferation. One-half of the culture media was changed every 3 days. After 3–4 weeks in the culture, spontaneous activity became stable, and firing occurred in a stereotypical, periodical, and synchronized pattern (Fig. 1(c)). To make conditions consistent across all tests, the medium was changed to NB without FBS 3 days before testing; even without FBS, at this stage there was no significant fluctuation in signals, and glia and neurons formed a dense, confluent multilayer (Fig. 2(a)).
Fig. 1.
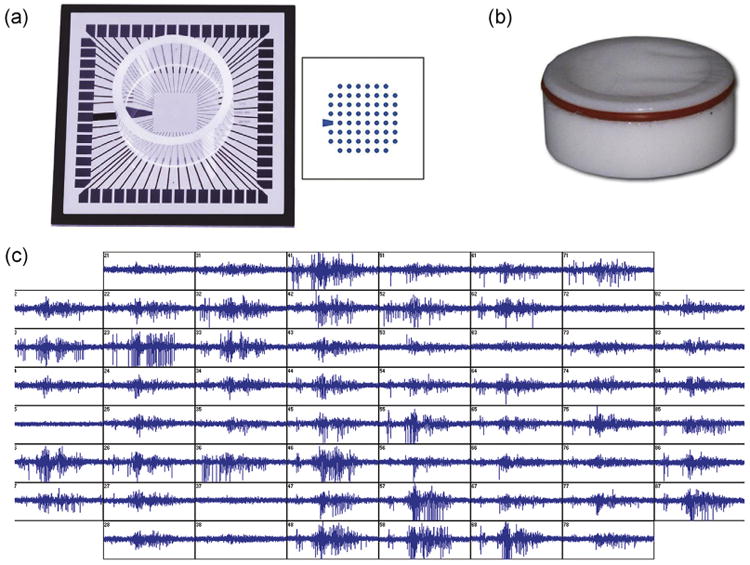
(a) Standard MEA chip used in this study with 60 electrodes, Multi-Channel System; (b) Teflon lid used to prevent water evaporation and bacterial contamination, ALA scientific; (c) Typical spontaneous and synchronized electrophysiological activities of a mature CFBNN.
Fig. 2.
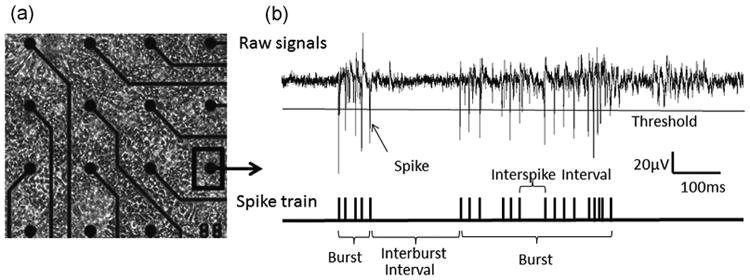
(a) Morphology of a neuronal and glial co-cultured CFBNN growing on an MEA chip (DIV 28); microelectrodes (30 mm diameter, 200 mm spacing). (b) Raw signals recorded from one electrode by MC_Rack, and the processed signals after spike train analysis by NeuroMEA; threshold set −5.5 SDs.
2.3.2. Multichannel recording and parameter detection
To record signals, the MEA chip was placed in a dry incubator (65% humidity) and maintained at 37 °C with 5% CO2. The signals were amplified by MCS 1060-INV amplifier (Multi-Channel Systems, Germany) and recorded by MC_Rack software (Multi-Channel Systems, Germany) at a 25 kHz sampling rate. Raw electrophysiological signals recorded by each electrode were composed of information-containing signals (spikes and bursts) and background noise (Fig. 2(b)). A “spike” is an action potential-related extracellular field potential; a “burst” is a dense sequence of spikes. In this study, spikes were detected by MC_Rack with a threshold of −5.5 standard deviations below background noise. A burst met the following criteria: The maximum spike interval within a burst was no more than 100 ms; the minimum burst duration was no less than 10 ms; the minimum number of spikes within a burst was no less than 3. Bursts were quantitatively identified via spike-train analysis using a lab-designed Matlab program, “NeuroMEA”. Multiparametric endpoints were extracted to describe network activity patterns. These included firing-rate-related parameters such as spike rate per minute (SR) and burst rate per minute (BR) and burst-structure-related parameters such as burst duration (BD) and spike frequency in burst (SFB). A channel with at least 3 bursts per 10 min was considered an active channel, and an MEA chip with over 10 active channels qualified for the study.
2.3.3. Experimental protocols of electrophysiological testing
2.3.3.1. Acute effects of Mg2+ (30-min exposure)
This experiment was designed to explore the effects of Mg2+ dose through dose-dependent administration. Before drug administration, spontaneous network activity was recorded for 30 min as reference activity. To minimize mechanical and osmotic disturbance, 100 μL of the medium was removed from the culture. This medium was mixed with the three stock solutions described in Section 2.1.1 and then carefully returned to the culture. Table 1 specifies the volume of the added stock solutions, which were designed to cumulatively achieve logarithmic concentration in the culture from 0.01 to 3 mmol/L in 30-min intervals. Electrical activity was recorded immediately after each administration of a drug; however, only the last 10 min of each recording (when activity had stabilized) was used for comparison with the reference activity.
Table 1. Steps of dose-dependent administration in the study of acute effects of Mg2+ on neuronal electrophysiology.
| Dose | Volume (μL) | Stock solution (mmol/L) | Final concentration (mmol/L) |
|---|---|---|---|
| 1 | 5 | 2 | 0.01 |
| 2 | 10 | 2 | 0.03 |
| 3 | 3.5 | 20 | 0.1 |
| 4 | 10 | 20 | 0.3 |
| 5 | 3.5 | 200 | 1 |
| 6 | 10 | 200 | 3 |
2.3.3.2. Chronic effects of Mg-extract solution (3-day exposure)
This experiment was designed to explore changes in electrophysiological activity during and after exposure to Mg-extract solution, which has a complex composition. Addition of the extract media intensely disturbs signals when compared with addition of standard Mg2+ solutions. Thus, 3-day-exposure experiments on the effects of the Mg-extract solution were designed to allow the disturbance to be averaged. Spontaneous network activity was traced 30 min daily for 3 days. On the 3rd day, one quarter of the medium was replaced with the Mg-extract solution at the original concentration. On the 6th day, all media were replaced with fresh media. Cultures with 3 mmol/L standard Mg2+ solution were used as the positive control, and cultures with fresh media were used as the negative control. For comparison, the 3-day mean of the signals obtained before addition of the test solutions was designated as reference, the 3-day mean of the signals obtained during exposure was designated as exposure, and the 3-day mean of the signals obtained after washing was designated as recovery.
2.4. Statistical analysis
There was a large variation in response of MEA electrodes, so all parameters for electrophysiological testing were normalized for each electrode according to the corresponding values of the reference activity. Statistical analysis was performed using SPSS V20 statistical software. Significant changes were tested by analysis of variance (ANOVA) followed by Dunnett's multiple comparison post hoc test with reference activity as the common control. All results were expressed as mean ± SEM; p < 0.05 was considered significant.
To determine the half-maximal effective concentration (EC50), the data points obtained from the experiments described in Sections 2.2.2 and 2.3.3.1 were fitted using the sigmoidal dose–response model of nonlinear analysis in Graphpad Prism 5.0 to form dose–response curves. The software requires selection of “top” and “bottom” parameters, respectively; they were “less than 100” and “greater than zero”.
3. Results
3.1. Characterization of Mg-extract solutions
The pH value of the Mg-extract solution was 7.86 ± 0.25, much higher than that of standard cell-culture media (7.2–7.4). ICP-AES testing showed that [Mg2+] of the Mg-extract solution was 10.60 ± 0.40 mmol/L in total. Considering that the [Mg2+] in a standard cell-culture media is 0.84 mmol/L, the excessive [Mg2+] extracted from pure Mg was 9.76 mmol/L. Accordingly, the excessive [Mg2+] of the 50% and 25% Mg-extract solutions were 4.88 and 2.44 mmol/L, respectively.
3.2. Neuronal viability test
3.2.1. Effects of Mg2+ concentrations on neuronal viability
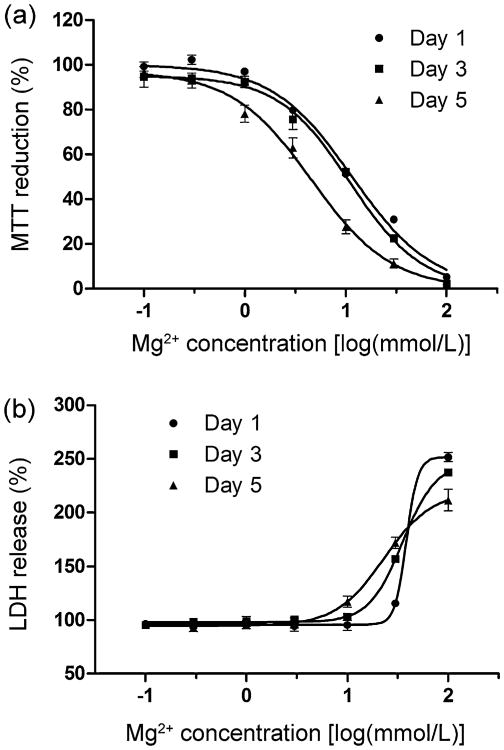
Standard Mg2+ solutions described in Section 2.1.1 with [Mg2+] levels from 0.1 to 100 mmol/L were used to investigate effects of Mg2+ concentrations on neuronal viability. Fig. 3 shows that MTT viability gradually decreases as [Mg2+] increases and that over 95% of viability is lost when [Mg2+] reaches 100 mmol/L. The dose-dependent curves for Day 1, Day 3, and Day 5 left-shift, indicating that viability decreases with exposure time. LDH assay demonstrates that LDH release does not increase until [Mg2+] is over 30 mmol/L. The slopes of the dose-dependent curves for Day 1, Day 3, and Day 5 gradually decrease. The estimated EC50 values fitted by a dose–response sigmoidal model and the minimum concentrations that caused significant changes (ECmin, p < 0.05) in both MTT and LDH assays are shown in Table 2. EC50 values decrease as exposure time increases. In addition, the EC50 and ECmin values from the MTT assay are much lower than those from the LDH assay.
Fig. 3.
Effects of different concentrations of Mg2+ on neuron viability evaluated by (a) MTT and (b) LDH assays ([Mg2+]: 0.1–100 mmol/L, n = 5).
Table 2. Fitted EC50 and ECmin values of [Mg2+] evaluated by MTT and LDH assays (mmol/L).
| EC50_MTT | ECmin_MTT | EC50_LDH | ECmin_LDH | |
|---|---|---|---|---|
| Day 1 | 11.6 ± 1.1 | 3 | 38.6 ± 2.0 | 100 |
| Day 3 | 11.0 ± 1.2 | 3 | 35.4 ± 1.1 | 30 |
| Day 5 | 4.6 ± 1.2 | 1 | 23.0 ± 1. 2 | 30 |
3.2.2. Effects of Mg-extract solutions on neuronal viability
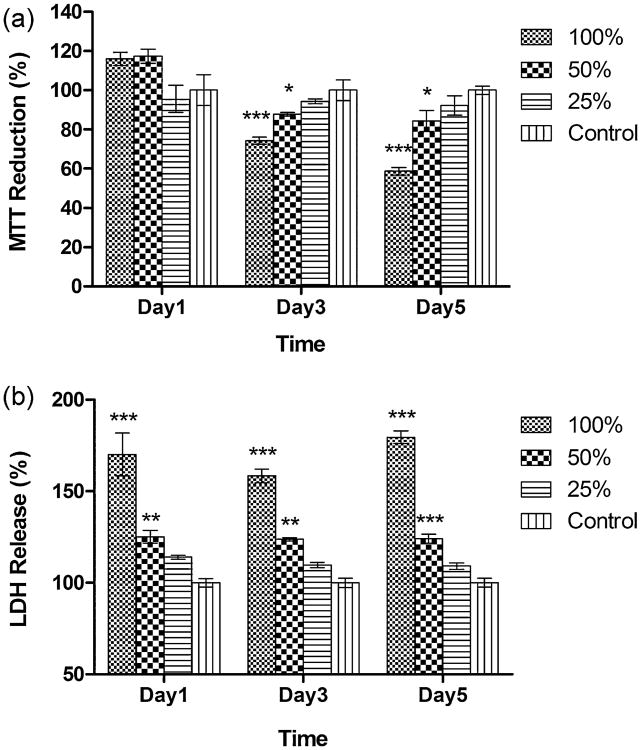
The effects of Mg-extract solutions on neuronal viability were tested with 3 concentrations: 100% (original concentration), 50% diluted, and 25% diluted (Fig. 4); fresh medium was used as control. The MTT assay shows that after 1 day of exposure, no significant MTT reduction is detected for the 3 concentrations; after 3 days of exposure, cell viability decreases significantly in the 100% (p < 0.001) and 50% concentrations (p < 0.05); and at no time is a significant MTT reduction detected for the 25% concentration. The LDH assay shows that after 1 day of exposure, significant increases in LDH release are detected at the 100% (p < 0.001) and 50% concentrations (p < 0.01); at no time is a significant LDH release detected for the 25% concentration. MTT viability decreases with exposure time, but LDH release does not change significantly with exposure time.
Fig. 4.
Effects of 100%, 50%, and 25% concentrations of Mg-extract solutions on neuronal viability evaluated by (a) MTT and (b) LDH assays (control = 100%, n = 5, Dunnett's multiple comparison post hoc test: *p < 0.05, **p < 0.01, ***p < 0.001).
3.3. Electrophysiological test
3.3.1. Acute effects of Mg2+ solutions on neuronal electrophysiology Neuronal electrophysiological changes were monitored using our
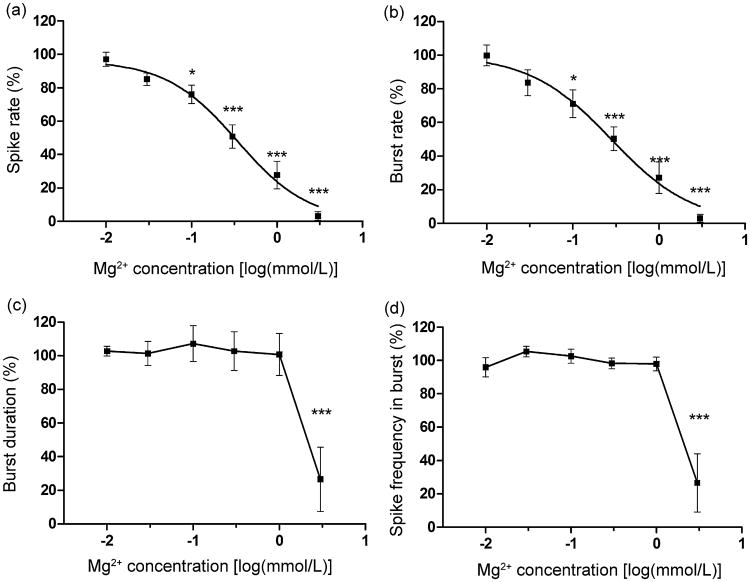
CFBNN–MEA biosensor. Acute effects in response to 0.01–3 mmol/L accumulations of Mg2+ were tested. The firing rate of the network shows an obvious dose-dependent reduction, and while over 95% the activity is inhibited when [Mg2+] reaches 3 mmol/L, synchronicity among channels and oscillatory features do not change with increasing [Mg2+] (Fig. 5).
Fig. 5.
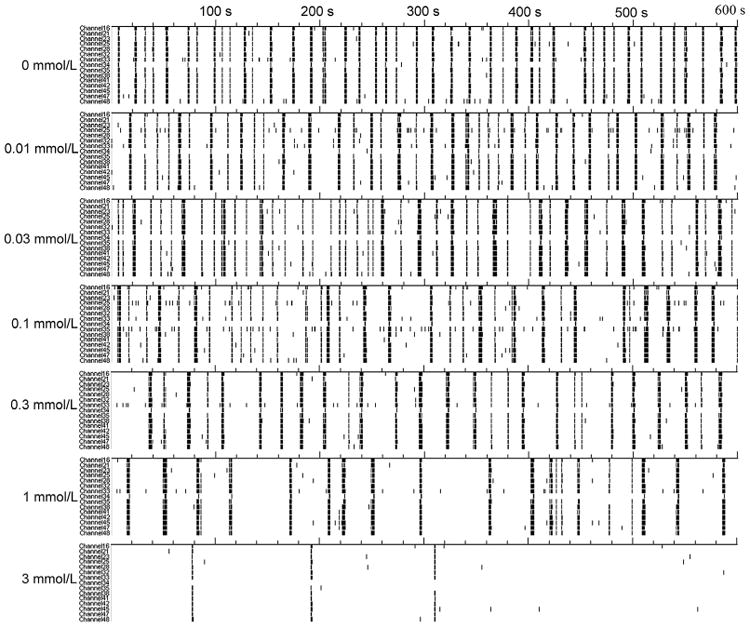
Typical activity change of neuronal network electrophysiology when application of Mg2+ increased from 0.01 to 3 mmol/L (10 min recording from 15 electrodes on one MEA).
Quantitative electrophysiological changes with [Mg2+] are obtained from 177 effective channels from six MEAs (Fig. 6). Firing-related parameters SR and BR (95% of spikes were in bursts) show a similar trend of dose-dependent change—the reduction is significant when [Mg2+] reaches 0.1 mmol/L. The EC50 for the spike rate obtained with the sigmoidal dose–response model-based curve-fitting is 0.34 mmol/L. Burst-structure-related parameters BD and SFB do not show a significant difference until [Mg2+] reaches 3 mmol/L, thus, they are not as sensitive as SR and BR.
Fig. 6.
Effects of increasing Mg2+ on neuronal-network electrophysiological activity: (a) spike rate, (b) burst rate, (c) burst duration, and (d) spike frequency in burst ([Mg2+]: 0.01-3 mmol/L, values are expressed as mean ± SEM based on normalized activity, reference = 100%, n = 6, Dunnett's multiple comparison post hoc test, *p < 0.5, ***p < 0.001).
3.3.2. Chronic effects of Mg-extract solution on neuronal electrophysiology
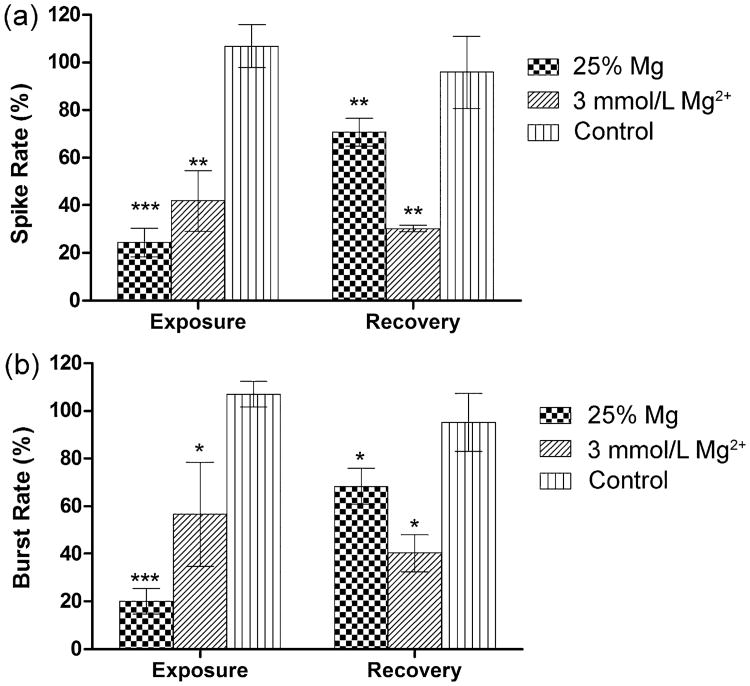
The effects of 25%-diluted Mg-extract solution (test group), 3 mmol/L Mg2+ solution (positive control), and fresh media (negative control) on neuronal network activity (Fig. 7) were tested on three MEAs per group. During exposure, firing rate-related parameters SR and BR are significantly inhibited (p < 0.001); the inhibitory effects on the test group are stronger than those of the 3 mmol/L Mg2+ group. After being washed, the inhibited SR and BR of the test group recover to around 70% of the reference level (p < 0.01), but the SR and BR of the 3 mmol/L Mg2+ group are further inhibited, to approximately 30%–40% of the reference level (p < 0.001).
Fig. 7.
Effects of 25% Mg-extract solution and 3 mmol/L Mg2+ on neuronal network electrophysiological activity. (a) Spike rate, (b) burst rate (values are expressed as mean ± SEM based on normalized activity, reference = 100%, n = 3, Dunnett's multiple comparison post hoc test, *p < 0.5, **p < 0.01, ***p < 0.001).
4. Discussion
4.1. Neuron viability test
The cytotoxicity of Mg-based biomaterials on bone-related cells, vessel-related cells, and fibroblasts has been extensively investigated, but their effect on neuronal cells has not been reported. In a report of Feyerabend[13], EC50 viability values of [Mg2+] determined by MTT assays for MG63 (human osteosarcoma-cell line), HUCPV (human umbilical cord perivascular cells), and RAW 264.7 (mouse macrophage) were 50, 73, and 53 mmol/L, respectively, after a 2-day exposure. The estimated EC50 of [Mg2+] obtained from an MTT assay was approximately 11–12 mmol/L during exposure periods lasting 1–3 days (Table 2).
The ECmin of Mg2+ from the MTT assay was 3 mmol/L, but that from LDH assay was 100 mmol/L; the LDH assay detected that the 50% Mg-extract solution lowered viability obviously after 1 day exposure, but the MTT assay could not detect a difference of 100% Mg-extract solution. Although both the MTT and LDH assays indicated viability, their mechanisms for doing this differed. MTT quantifies mitochondrial activity and correlates with viability when adenosine triphosphate (ATP) levels fall below a critical threshold. The LDH assay measures necrosis when the cell membrane is compromised[14]. Most cytotoxicity tests of Mg-based biomaterials were based on the MTT assay only, and the large difference between our MTT and LDH assays suggests that two or more assays are necessary for viability testing to thoroughly evaluate Mg-based biomaterials.
Due to the high pH (7.86) and complicated ingredients of Mg-extract solution, its results differed from the corresponding concentrations of Mg2+. MTT assay results indicated that the effect of Mg-extract solutions was less than that of the control solution with corresponding amounts of Mg2+. Fischer et al.[15] reported that the corrosion products of pure Mg can convert tetrazolium-salt-based assays to formazan. So, the detection sensitivity of MTT assay is lower for Mg extract than that for defined concentrations of Mg2+. LDH assay results showed that the cell viability of Mg-extract solutions ([Mg2+]: 9.76 mmol/L) was lower than that of the control solution with corresponding amounts of Mg2+ (ECmin = 30 mmol/L), which indicated that some ingredients other than Mg2+ increased LDH release. Simpson et al.[16] measured LDH values after murine insulinoma ßTC3 cells were exposed for 4 h to acidic (pH: 6.37), neutral (pH: 7.07), or alkaline (pH: 7.78) media. The LDH-release in the alkaline (51.1 units) media was significantly higher than in the neutral (−11.4 units) media and in the acidic (−5.9 units) media. Therefore, it is the high pH of the Mg-extract solutions that caused the LDH release.
4.2. Neuronal electrophysiology test
Although the inhibitory function of Mg2+ on the NMDA receptor is well known, a few studies have used MEA to quantitatively investigate the effect of Mg2+ on neural activity. In the acute study of electrophysiological disturbance by Mg2+, the network activity showed dose-dependent reduction when [Mg2+] increased. This is because the increasing Mg2+ blocks NMDA channels, causing Ca2+ influx reduction and preventing initiation of action potentials[17]. The ECmin and EC50 of [Mg2+] for SR calculated from the MEA recording of the CFBN network (Fig. 6) were 0.1 and 0.34 mmol/L, respectively, and the values calculated from the MTT assay (Table 2) were 3 and 11.6 mmol/L, respectively; the latter was around 30-fold higher than the former. Thus, the MEA electrophysiological assessment of cellular effects of Mg2+ was far more sensitive than the conventional viability assessment.
The electrophysiological disturbance takes effect instantaneously, but the viability effect is not immediate. When the Mg2+ solution reached a concentration higher than 3 mmol/L, neuronal viability significantly decreased until neuronal activity was totally shut down. Possibly, silencing of neuronal activity can be used to determine the concentration threshold for viability decrease. Gleichmann[18] has reported that the gene-expression changes in neurons, where spontaneous network activities are inhibited, overlap gene-expression changes in Alzheimer's disease, in which neuro-nal viability is dramatically decreased. Detecting the change of neuronal activity is a timely manner which is imperative for effective neurotoxic evaluation.
Our data demonstrated that burst-structure parameters (BD and SFB) were not as sensitive to [Mg2+] as firing-rate parameters (SR and BR) (Fig. 6), suggesting that firing-rate parameters were the more effective evaluation of the effect of Mg2+ on electrophysiology. The raster plot showed that the firing pattern did not change when [Mg2+] was lower than 3 mmol/L (Fig. 5), indicating that the effect of Mg2+ did not change the firing pattern of spontaneous action potentials. In addition, it is unlikely that inhibition of the activity was caused by Cl−; its maximum concentration, produced by addition of MgCl2 to create excessive Mg2+, was negligible when compared with the [Cl−] level (approximately 140 mmol/L) in the culture media.
In the tests of Mg-extract solution, a longer time-period (3 days) was chosen to test the relative activity changes during drug exposure and after drug removal. Our results showed that firing rate was significantly inhibited by the 25% Mg-extract solution (p < 0.001), and after 3 days of exposure, it could not be recovered to the reference level. However, the MTT and LDH assays using the same test solution showed no decrease in viability, suggesting again that electrophysiological activity was more sensitive than cell viability in evaluating the effects of Mg-based biomaterials on neurons. Further, our results showed that the corresponding concentration of the standard Mg2+ solution had a more severe impact on network activity than the Mg extract. It has been reported that high pH levels can increase neuronal activity[19], thus, increasing pH of Mg extract may offset part of the inhibition effect from Mg2+, but the quantitative relation needs to be further studied.
4.3. Practical significance and limitations
The large difference in EC50 values of Mg2+ between the electro-physiological test (0.34 mmol/L) and viability test (11.6 mmol/L) means neurons tolerated large amounts of Mg2+ even when network function was inhibited. Our compatibility study is limited to in vitro extract solution of Mg. Because there is high corrosion rate in vitro, the [Mg2+] in vitro might be far higher than that in vivo[20]. In in vitro study, it is also found that inhibition caused by Mg extract is slighter than that by corresponding concentrations of Mg2+. Our findings suggested that although Mg-based biomaterials might have a promising application on neuron system, more studies of the slight inhibitions associated with certain concentrations (0.03–0.1 mmol/L) in neural activity are needed in the future.
5. Conclusion
This study was the first to investigate the effects of Mg-based biomaterials on neurons in vitro. Although MTT and LDH assays were popularly used in biocompatibility studies, the two assays showed inconsistent outcomes during evaluation of the Mg2+ and the Mg extract solution. MEA electrophysiological tests revealed that the Mg2+ solution and the Mg extract inhibited neuronal electrical activity far before loss of viability, a more than 30-fold increase in sensitivity over cell-viability tests. Thus, MEA-based electrophysiological testing could be of more practical use than traditional viability test for evaluation of neurotoxicity of Mg-based biomaterials.
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program, No. 2012CB619102); the National Natural Science Foundation of China (Nos. 31070847 and 31370956); the National Science and Technology Support Program (No. 2012BAI18B01); the Strategic New Industry Development Special Foundation of Shenzhen, China (No. JCYJ20130402172114948); the Guangdong Provincial Department of Science and Technology, China (No. 2011B050400011); and NIH NIGMS COBRE (No. NIH P20GM103444).
Contributor Information
Tingfei Xi, Email: xitingfei@pku.edu.cn.
Bruce Z. Gao, Email: zgao@clemson.edu.
References
- 1.Li N, Zheng YF, Mater J. Sci Technol. 2013;29:489–502. [Google Scholar]
- 2.Staiger MP, Pietak AM, Huadmai J, Dias G. Biomaterials. 2006;27:1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Waksman R. EuroIntervention. 2009;5:F94–F97. doi: 10.4244/EIJV5IFA16. [DOI] [PubMed] [Google Scholar]
- 4.Vennemeyer JJ. PhD thesis. University of Cincinnati: 2013. Investigation of Magnesium-based Interventions for Central and Peripheral Nervous Tissue Regeneration. [Google Scholar]
- 5.Gu X, Zheng Y, Cheng Y, Zhong S, Xi T. Biomaterials. 2009;30:484–498. doi: 10.1016/j.biomaterials.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 7.Dribben WH, Eisenman LN, Mennerick S. Cell Death Dis. 2010;1:e63. doi: 10.1038/cddis.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnstone AFM, Gross GW, Weiss DG, Schroeder OHU, Gramowski A, Shafer TJ. Neurotoxicology. 2010;31:331–350. doi: 10.1016/j.neuro.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Kuang SY, Wang Z, Huang T, Wei L, Xi T, Kindy M, Gao BZ. Biotechnol Lett. 2015;37:499–509. doi: 10.1007/s10529-014-1704-1. [DOI] [PubMed] [Google Scholar]
- 10.Heidemann SR, Reynolds M, Ngo K, Lamoureux P. Methods Cell Biol. 2003;71:51–65. doi: 10.1016/s0091-679x(03)01004-5. [DOI] [PubMed] [Google Scholar]
- 11.Hales CM, Rolston JD, Potter SM. J Vis Exp. 2010;39:e2056. doi: 10.3791/2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potter SM, DeMarse TB. J Neurosci Meth. 2001;110:17–24. doi: 10.1016/s0165-0270(01)00412-5. [DOI] [PubMed] [Google Scholar]
- 13.Feyerabend F, Fischer J, Holtz J, Witte F, Willumeit R, Drucker H, Vogt C, Hort N. Acta Biomater. 2010;6:1834–1842. doi: 10.1016/j.actbio.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Lobner D. J Neurosci Meth. 2000;96:147–152. doi: 10.1016/s0165-0270(99)00193-4. [DOI] [PubMed] [Google Scholar]
- 15.Fischer J, Prosenc MH, Wolff M, Hort N, Willumeit R, Feyerabend F. Acta Biomater. 2010;6:1813–1823. doi: 10.1016/j.actbio.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Simpson NE, Bennett LK, Papas KK, Sambanis A, Constantinidis I. Biochem Biophys Res Commun. 2000;273:937–941. doi: 10.1006/bbrc.2000.3022. [DOI] [PubMed] [Google Scholar]
- 17.Pollet S, Pollet SE, Maudanz MJ, Baraliakos X, Kisters K. Trace Elem Electrolytes. 2013;30:59–65. [Google Scholar]
- 18.Gleichmann M, Zhang Y, Wood WH, III, Becker KG, Mughal MR, Pazin MJ, van Praag H, Kobilo T, Zonderman AB, Troncoso JC, Markesbery WR, Mattson MP. Neurobiol Aging. 2012;33:205.e1–205.e8. doi: 10.1016/j.neurobiolaging.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinning A, Hübner CA. FEBS Lett. 2013;587:1923–1928. doi: 10.1016/j.febslet.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 20.Chen YG, Yan J, Wang ZG, Yu S, Wang XH, Yuan ZM, Zhang XN, Zhao CL, Zheng Q. Mater Sci Eng C. 2014;42:116–123. doi: 10.1016/j.msec.2014.05.014. [DOI] [PubMed] [Google Scholar]