Abstract
PURPOSE: Increased murine double minute 2 (MDM2) expression, independent of p53 status, is associated with increased cancer-specific mortality for men with prostate cancer treated with radiotherapy. We assessed MI-219, a small molecule inhibitor of MDM2 with improved pharmacokinetics over nutlin-3, for sensitization of prostate cancer cells to radiotherapy and androgen deprivation therapy, a standard treatment option for men with high-risk prostate cancer. EXPERIMENTAL DESIGN: The effect of MDM2 inhibition by MI-219 was assessed in vitro and in vivo with mouse xenograft models across multiple prostate cancer cell lines containing varying p53 functional status. RESULTS: MDM2 inhibition by MI-219 resulted in dose- and time-dependent p53 activation and decreased clonogenic cell survival after radiation in a p53-dependent manner. Mechanistically, radiosensitization following inhibition of MDM2 was largely the result of p53-dependent increases in apoptosis and DNA damage as evidenced by Annexin V flow cytometry and γ-H2AX foci immunofluorescence. Similarly, treatment with MI-219 enhanced response to antiandrogen therapy via a p53-dependent increase in apoptotic cell death. Lastly, triple therapy with radiation, androgen deprivation therapy, and MI-219 decreased xenograft tumor growth compared with any single- or double-agent treatment. CONCLUSION: MDM2 inhibition with MI-219 results in p53-dependent sensitization of prostate cancer cells to radiation, antiandrogen therapy, and the combination. These findings support MDM2 small molecule inhibitor therapy as a therapy intensification strategy to improve clinical outcomes in high-risk localized prostate cancer. TRANSLATIONAL RELEVANCE: The combination of radiotherapy and androgen deprivation therapy is a standard treatment option for men with high-risk prostate cancer. Despite improvements in outcomes when androgen deprivation therapy is added to radiation, men with high-risk prostate cancer have significant risk for disease recurrence, progression, and even death within the first 10 years following treatment. We demonstrate that treatment with MI-219 (an inhibitor of MDM2) results in prostate cancer cell sensitization to radiation and androgen deprivation therapy in vitro and in vivo. Triple therapy with MI-219, radiation, and androgen deprivation therapy dramatically decreased tumor growth compared with any single- or double-agent therapy. These findings provide evidence that inhibition of MDM2 is a viable means by which to enhance the efficacy of both radiation and androgen deprivation therapy and thereby improve outcomes in the treatment of prostate cancer. As such, further investigation is warranted to translate these findings to the clinical setting.
Introduction
Prostate cancer is the second leading cause of cancer-specific death among men [1]. The majority of deaths caused by prostate cancer occur in patients initially diagnosed with high-risk disease as defined by the National Comprehensive Cancer Network. In fact, patients with high-risk disease have approximately a 14-fold increased risk of dying from prostate cancer as compared with their low-risk counterparts [2]. Radiotherapy plus concurrent androgen deprivation therapy (ADT) is a standard treatment modality for men diagnosed with high-risk prostate cancer [3]. Multiple clinical trials have demonstrated improved patient outcomes and improved patient survival when ADT is combined with radiotherapy (RT) [2], [4], [5], [6]. Despite the benefits obtained with the addition of ADT to radiation for men with high-risk prostate cancer, recurrence rates following treatment remain unacceptably high. In the first 10 years, approximately 50% of these men may experience a prostate-specific antigen (PSA) recurrence, 15% may develop metastatic disease, and up to 12% may eventually die from prostate cancer [4]. Given the high rates of recurrence, development of metastatic disease, and high burden of prostate cancer–specific deaths in men diagnosed with high-risk prostate cancer and treated with radiotherapy plus ADT, means by which to further intensify treatment are greatly needed. One approach to improve outcomes in this setting is through identification of molecular pathways whose targeting results in radiosensitization and/or improved efficacy of antiandrogen therapy.
In analysis of results from a multi-institutional randomized trial where men with prostate cancer were treated with RT + ADT, increased murine double minute 2 (MDM2) expression was demonstrated to increase the risk for recurrence, metastasis, and death from prostate cancer independently of patient p53 status [7]. Consistent findings were also noted in a previous analysis of a smaller cohort of men prospectively treated with RT + ADT [8]. The MDM2 human orthologue oncoprotein is a ubiquitin ligase which when activated targets p53, among other clients, for degradation and also represses p53 transcriptional targets, thereby acting as a negative regulator of p53 [9], [10], [11], [12], [13], [14]. Thus, overexpression of MDM2 results in inactivation of the p53 pathway and inhibits p53-mediated cell cycle arrest [15], [16]. Antisense MDM2 knockdown sensitized prostate cancer cell lines to radiotherapy, ADT, and the combination in a p53-dependent and -independent manner [17], [18], [19], [20], thus confirming MDM2 as a therapeutic target for intensification in prostate cancer.
Indeed, the prototypical MDM2 small molecule inhibitor, nutlin-3, induces radiosensitization to ADT in vitro and in vivo [21], [22]. However, nutlin-3 has poor pharmacokinetics, and similar studies with clinically relevant MDM2 inhibitors have not been performed. MI-219 is a small molecule inhibitor of MDM2 with improved pharmacokinetics that has single-agent activity in vivo [23]. Therefore, we assessed the potential for MI-219, a clinically relevant small molecule inhibitor of MDM2, to induce sensitization to radiotherapy, antiandrogen therapy, and the combination therapy in vitro and in vivo across multiple prostate cancer cell lines.
Materials and Methods
Cell Culture and Cell Lines
Androgen-dependent (22RV1 and LNCaP) cell lines were obtained from ATCC and maintained as per the guidelines. All cell lines were purchased between January 2012 and June 2013. Before the initiation of our analyses, all cell lines were authenticated, characterized, and genotyped by fragment analysis and ProfilerID at our DNA sequencing core facility. Sample fragments were compared against the cell line standards provided by ATCC. Cells were cultured at 37°C in a 5% CO2 humidified incubator. P53 knockdown cells were generated by transfecting the cells with lentivrus-based shRNAs (Addgene, Cambridge, MA, pLKO-p53-shRNA-427 [24]) and growing them in puromycin containing media for 5 to 6 days. PC3 and DU145 cells were maintained in DMEM supplemented with 10% FBS. The p53 status and androgen sensitivity of the cell lines used in the study are listed in Supplementary Table 1.
Immunoblot (Western Blot) Analysis
Western blot analysis was carried out using standard protocols. Cells were grown in culture dishes and treated with indicated treatments for designated time periods, and cell lysates were resolved on SDS-PAGE gels to transfer on to polyvinylidene difluoride membranes. Membranes were probed against specific primary antibodies against p53 (Cell Signaling #9282), MDM2 (Santa Cruz Biotech #SMP14), p21 (Cell Signaling #2947), PUMA (Cell Signaling #4976), and GAPDH (Cell Signaling #2118), followed by HRP-conjugated secondary antibodies. Membranes were visualized using the Enhanced Chemiluminescence Western Blotting System (GE Healthcare, Piscataway, NJ).
Clonogenic Survival Assays
Cells were trypsinized and plated in triplicate into six-well plates at different densities based on cell types and doses of radiation. 22RV1, LNCaP, DU145, and PC3 cells were plated at a density of 2000 to 10,000 cells/well, 100 to 600 cells/well, and 100 to 500 cells/well, respectively. Cells were treated with 5 μM MI-219 for 2 hours and then treated with radiation (2 to 6 Gy). Twenty-four hours after radiation treatment, the medium containing MI-219 was removed, and cells were maintained in normal culturing medium. Twelve days after the cells were plated, they were washed and stained with crystal violet, and the colonies containing > 50 cells were counted. Plating efficiency was calculated by dividing the average number of cell colonies per well by the amount of cells plated. Survival fractions were calculated by normalization to the plating efficiency of appropriate control groups.
Immunofluorescence
22RV1 and LNCaP cells (with or without p53 knockdown) were seeded onto coverslips in six-well plates and incubated overnight at 37°C. Cells were treated with either MI-219 or radiation or a combination of both. Following the indicated treatment for the described time points, cells were fixed with ice-cold methanol:acetone (50:50). Cells were washed three times with cold PBS and then permeabilized with 0.5% Triton X-100 in PBS for 5 minutes at room temperature. Cells were then blocked overnight at 4°C with blocking buffer (0.1% Triton X-100, 1% BSA in PBS). Blocked cells were incubated with anti–phospho-histone H2A.X (Ser139) monoclonal antibody (Cell Signaling #9718) for 1 hour at a 1:10,000 dilution in blocking buffer at room temperature. After washing three times with washing buffer (0.1% Triton X-100 in PBS), cells were incubated for 1 hour at room temperature with Alexa Fluor 488 goat anti-rabbit secondary antibody in blocking buffer. Cells were then washed with PBS, and images were acquired.
Immunohistochemistry
Tumors from mice treated with MI-219 (300 mg/kg), radiation, or a combination of both, and tumors from castrated mice were excised and fixed in formalin. The tumors were processed at the Tissue Core Facility of University of Michigan and stained with anti-CD31 antibody (1:100, Abcam #ab28364), and blood vessel density was determined by manual counting at least five high-power fields. A two-sided Student’s t test was performed to assess statistical significance.
Flow Cytometry
Cells were treated with MI-219 or radiation and collected after indicated time points. Cells were washed with PBS and fixed in 1 ml of cold 70% ethanol, added dropwise. Cells were then pelleted by centrifugation and washed twice with PBS at RT. Cells were then treated with 50 μl of ribonuclease A, after which propidium iodide was added to cells. Cells were incubated for 15 minutes and were acquired on FACSAria (BD biosciences) for the analysis of sub-G1 cells.
The Annexin V apoptosis assay was carried out using ApoScreen Annexin V Apoptosis Kit (Southern Biotech) per the manufacturer's protocol. Briefly, cells treated casodex (5 μM), MI-219 (10 μM) or both, or were grown in charcoal-stripped media and treated with MI-219. After the treatment, cells were washed with cold PBS, suspended in cold 1 × binding buffer, stained with Annexin V and propidium iodide, and subjected to flow cytometry by FACSAria Cell Sorter (BD Biosciences).
Reporter Assays
The MDM2 or p21 3′UTR subcloned into the pRL-TK vector. 22RV1, LNCaP (with or without p53-KD), and DU145 cells were plated onto 24-well plates and transfected with these 3′UTR luciferase constructs as well as pRL-TK vector as internal control for luciferase activity. After 48 hours of incubation, luciferase reporter assay were conducted with the dual luciferase assay kit (Promega, Madison, WI) as per the recommended protocol.
In Vivo Xenograft Studies
Xenograft studies were performed in accordance with National Institutes of Health guidelines, and animal protocols were approved by the University of Michigan. 22RV1 or 22RV1 p53-KD cells (3 × 106) were combined 1:1 with Matrigel (BD Biosciences, 354234) and injected subcutaneously into the flanks of 6-week-old, intact-male athymic nude/SCID mice. Once the tumors reached 100 mm3 in size, mice were subjected to treatment with radiation (delivered in four fractions of 2 Gy on days 1 to 4), MI-219 (delivered orally at 300 mg/kg twice daily on days 1 to 10), or both. Tumor sizes were measured for 18 days following a 2-week engraftment period. For VCaP xenografts studies, VCaP (3 × 106) cells were injected subcutaneously into the dorsal flanks of male athymic nude/SCID mice, and tumors were allowed to grow. Treatment was initiated when tumors were approximately 100 mm3 in size. Radiation was delivered on days 0, 2, and 4, with 2 Gy delivered on each of these days for a total dose of 6 Gy. Castration was performed on day 0. MI-219 was given orally at a dose of 100 mg/kg for 20 consecutive days beginning on day 0. Tumor growth was followed for 91 days posttreatment. Graphs were plotted using Graphpad prism.
Statistical Analysis
All data are expressed as means ± SD or SEM. All experimental assays were performed in duplicate or triplicate. Statistical analysis was performed by two-sided Student’s t test and one-way analysis of variance with a Student-Newman-Keuls follow-up test. Significance was indicated by “*” when P < .05.
Results
MI-219 Results in p53 Activation in a Dose- and Time-Dependent Manner
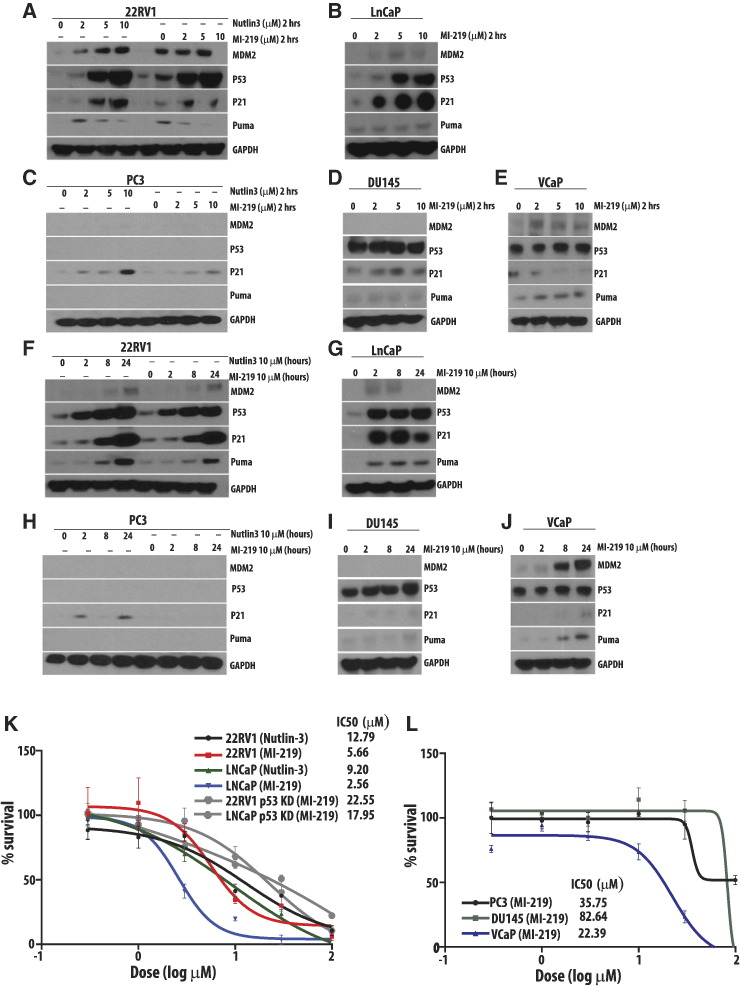
To assess the dose and time response to MI-219, we used two wt-p53 PCa cell lines as well as their corresponding p53-knockdown (p53-KD) cell lines. LNCaP and 22RV1 cell lines (wt-p53) treated with increasing doses of MI-219 (2 to 10 μM) demonstrated a dose-dependent increase in MDM2 and p53, as well as p53 targets p21, and the proapoptotic protein PUMA (p53 upregulated modulator of apoptosis), with a maximum effect noted between 2 and 5 μM (Figure 1, A and B). Knockdown of p53 using specific lentiviral-based shRNAs resulted in undetectable levels of p53 in both cell lines with corresponding downregulation of the p53-regulated proteins p21 and PUMA (Supplementary Figure S1, A and B).
Figure 1.
MI-219 results in p53 activation in a dose- and time-dependent manner. (A–E) Western blot analyses of prostate cancer cells treated with indicated doses of MI-219 or nutlin-3. (F–J) Cells were treated with 10 μM MI-219 or nutlin-3 for indicated time points, and Western blot analysis was carried out. GAPDH documented equal loading. (K) 22RV1and LNCaP cells, with or without p53 knockdown, were treated with increasing doses of MI-219 and nutlin-3 in a WST-1 cytotoxicity assay. (L) cells with altered p53 status (PC3, DU145, and VCaP) were treated with increasing doses of MI-219 in a similar fashion to determine IC50 values of MI-219. Bars represent ± SEM of three independent experiments.
Given the apparent p53-dependent dose response to MI-219 as demonstrated in the wt-p53 and p53-KD LNCaP and 22RV1 cell lines, we next assessed the dose response to MI-219 in three PCa cell lines with altered p53 status. PC3 (p53 null), DU145 (p53 homozygous mutant), and VCaP (p53 heterozygous mutant) cell lines were treated with increasing doses of MI-219 (Figure 1, C–E). As expected, p53 was undetectable in the p53 null PC3 line, and p53 levels were not significantly altered in the DU145 and VCaP cell lines. p21, a known p53-independent client of MDM2 [25], showed dose-dependent increase in levels across these cell lines. Interestingly, the VCaP cell line exhibited an apparent active p53 response with regard to dose-dependent increase in levels of MDM2 and PUMA expression, suggesting at least partial p53 functionality in this mutant. Promoter assay analysis confirmed this functional p53 response in the VCaP cell line (Supplementary Figure 1).
Having established a p53-dependent dose response, we next evaluated for a time-dependent response in the cell lines described above. Cells were assayed at 2, 8, and 24 hours following treatment with 10 μM MI-219 (Figure 1, F–J). In the wt-p53 22RV1 line, p53, p21, and PUMA levels increased over 24 hours, whereas these levels peaked around 2 hours posttreatment in the LNCaP line. This time-dependent response was greatly diminished in the 22RV1 and LNCaP p53-KD cell lines (Supplementary Figure 2, A–D); however, there was a modest increase of expression of p21 at 24 hours posttreatment in the p53-KD lines, again consistent with p21 being a target of MDM2. The p53 null cell line, PC3, also showed a modest increase in p21 levels, and the VCaP line showed an apparent p53-dependent time response with regard to MDM2 and PUMA expression.
We next assessed the effect of MI-219 on cellular proliferation. (Figure 1, K and L). The wt-p53 22RV1 and LNCaP cell lines had similar responses to treatment with MI-219, with IC50 values between 2 and 6 μM, which was similar to the IC50 values for nutlin-3 (ranging from 9 to 13 μM). The p53-KD correspondents showed a diminished response to MI-219 with IC50 values between 17 and 23 μM, as did the PC3 and DU145 cell lines. VCaP cells had a response more similar to the p53-KD cell lines with an IC50 value of 22.39 μM. In total, these findings suggest a definite p53-dependent dose and time response and a p53-dependent cell survival response to MDM2 inhibition with MI-219.
MI-219 Radiosensitizes Prostate Cancer Cells in a p53-Dependent Manner
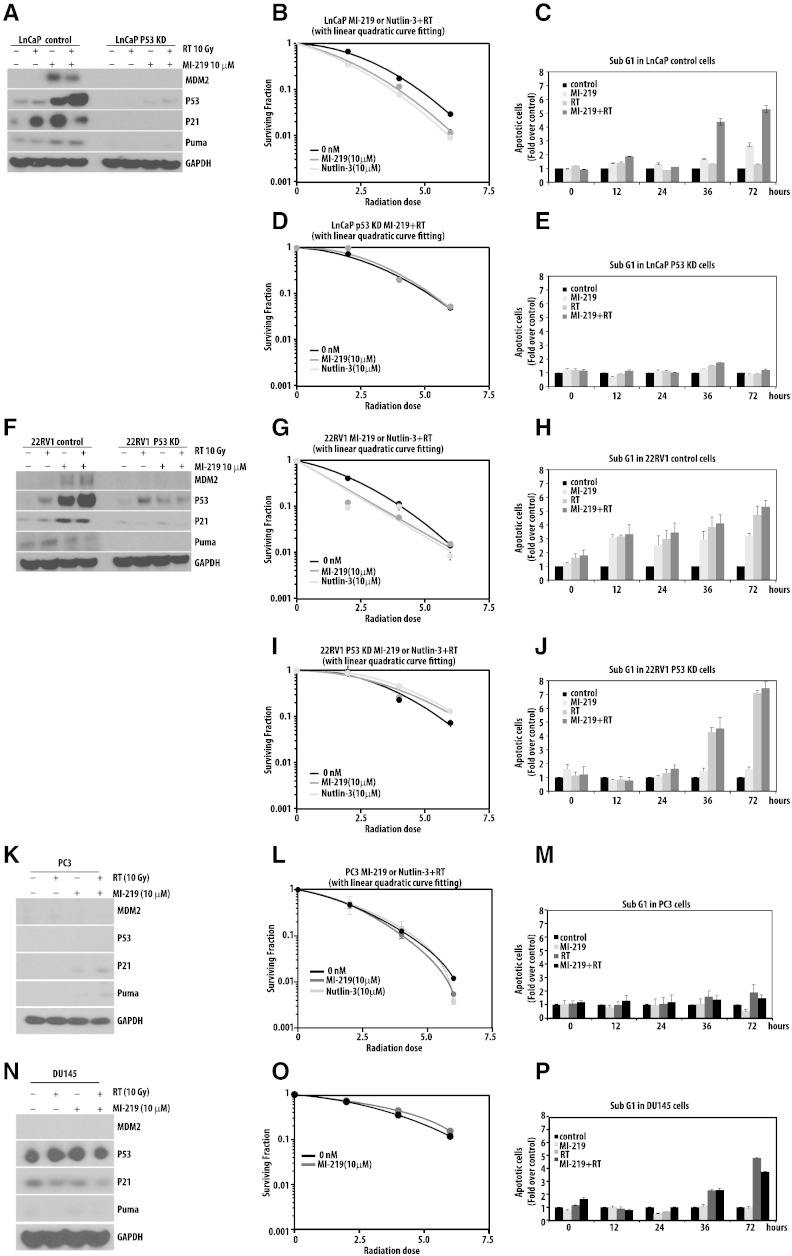
Radiation therapy forms a major treatment modality in the clinical management of prostate cancer. Thus, compounds that can sensitize prostate cancer cells to radiation doses are of high clinical importance. To determine if MI-219 sensitizes prostate cancer cells to ionizing radiation, we next sought to assess the impact of combining inhibition of MDM2 via MI-219 with radiation through clonogenic survival assays and analysis of sub-G1 DNA content as a marker of apoptosis. For clonogenic survival analysis, cells were treated with MI-219 or radiation as described in methods. The timing of administration of MI-219 was not found to be schedule dependent (Supplementary Figure 3). The combination of MI-219 and radiation resulted in upregulation of p53, p21, and PUMA (Figure 2, A and F) in the wt-p53 22RV1 and LNCaP cell lines. Treatment with MI-219 in addition to radiation resulted in significant radiosensitization, with radiation enhancement ratios of 1.30 (SD 0.07) for 22RV1 and 1.35 (SD 0.05) for LNCap (Figure 2, B and G). We next asked if treatment with MI-219 and/or radiation results in increased apoptosis of cells and if the apoptosis is p53 dependent. DNA content was determined via flow cytometry following treatment with 5 μM MI-219 with and without radiation (10 Gy). We found an increased sub-G1 DNA content at 72 hours for 22RV1 and LNCaP cell lines following treatment with RT alone, MI-219 alone, and RT + MI-219. The combinatorial treatment of MI-219 and radiation further resulted in a significant increase in sub-G1 DNA content compared with treatment with single therapy (Figure 2, C and H). The addition of MI-219 to radiation did not result in radiosensitization in the p53-KD 22RV1 and LNCaP cell lines, or in the PC3 or DU145 cell lines (Figure 2, E, J, M, and P). Similarly, upregulation of p53, p21, and PUMA was lost in PC3 or DU145 cell lines. p53-KD LNCaP and PC3 cell lines showed no increase in sub-G1 DNA content at 72 hours, whereas the p53-KD 22RV1 and DU145 cell lines had an increase in sub-G1 DNA content following treatment with radiation with minimal impact with the addition of MI-219 to radiation. Taken together, these results suggest that MDM2 inhibition via MI-219 sensitizes prostate cancer cells to ionizing radiations in a p53-dependent manner.
Figure 2.
MI-219 radiosensitizes prostate cancer cells in a p53-dependent manner. (A) Western blot analysis of LNCaP cells after treatment with 10 μM MI-219 and/or 10 Gy of radiation. (B) Clonogenic survival assays of LNCaP cells after treatment with either 10 μM MI-219 or 10 μM nutlin-3 and radiation (0, 2, 4, and 6 Gy). (C) Apoptosis (sub-G1 population) was analyzed in LNCaP cells after treatment with 10 μM MI-219, radiation (10 Gy), or both via FACS analysis. (D–E) Similar experiments were performed with LNCaP p53 knockdown cells. (F–P) Western blot analyses, clonogenic cell survival assays, and quantification of apoptotic cells were carried out in a similar fashion in 22RV1, PC3, and DU145 cells.
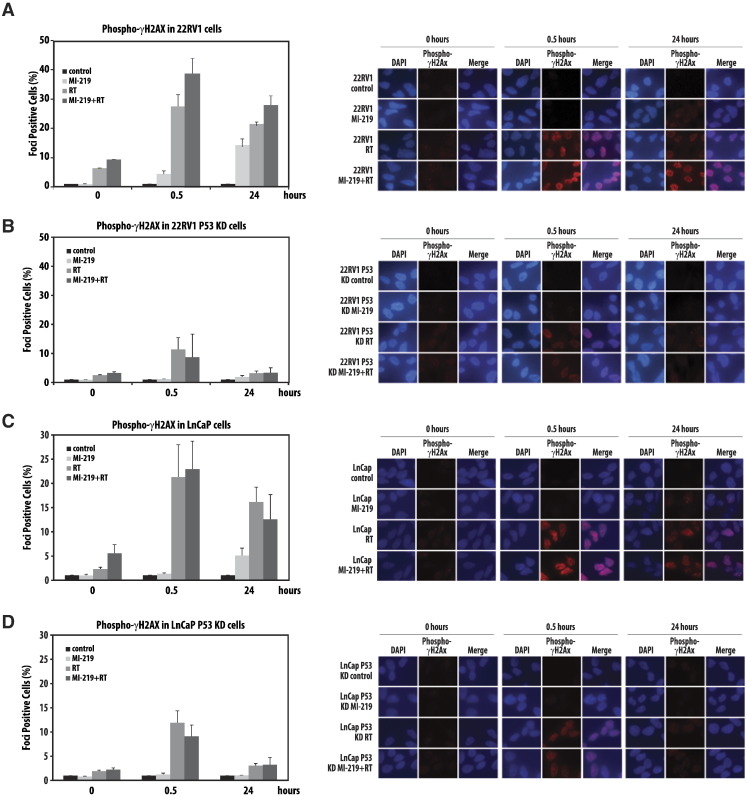
The Combination of MI-219 and Radiation Results in a p53-Dependent Increase in DNA Damage
Although the addition of MI-219 to radiation resulted in a significant increase in apoptosis, it is unlikely that this finding alone is responsible for the radiation enhancement observed with MDM2 inhibition. To further ascertain the mechanism of MDM2 inhibition–induced radiosensitization, we evaluated unrepaired DNA damage following treatment with RT, MI-219, and RT + MI-219 at 30 minutes and 24 hours posttreatment via γ-H2AX foci formation in wt-p53 and p53-KD 22RV1 and LNCaP cell lines. Treatment with MI-219 alone resulted in a moderate increase in the number of γ-H2AX foci at 24 hours posttreatment in both wt-p53 cell lines (Figure 3A and C). The addition of MI-219 to radiation caused a significant increase in the percentage of γ-H2AX foci–positive cells at 30 minutes and 24 hours posttreatment in the wt-p53 22RV1 cell line and at 30 minutes posttreatment in the wt-p53 LnCaP cell line compared with treatment with radiation alone. Knockdown of p53 abolished these findings (Figure 3, B and D).
Figure 3.
MI-219 increases DNA damage in a p53-dependent manner. Foci formation assays were conducted on (A–B) 22RV1 cells with or without p53 knockdown and (C–D) LNCaP cells with or without p53 knockdown. Cells were treated with 10 μM MI-219, radiation (10 Gy), or both and stained for H2Ax phosphorylation (ƴH2AX) at indicated time points. Graphs represent the quantification of cells with positive ƴH2AX foci. Bars represent ± SEM.
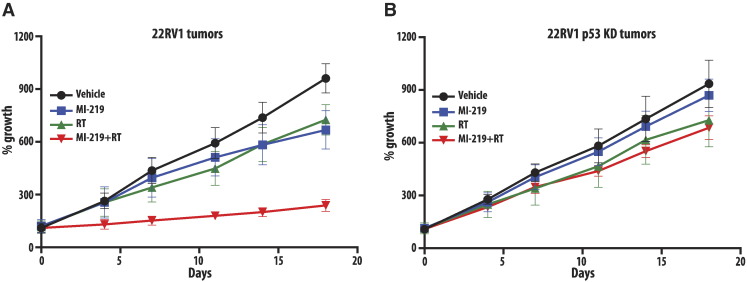
MI-219 Causes In Vivo Radiosensitization in a p53-Dependent Manner
Having defined the p53-dependent radiosensitization of MI-219 in vitro, we sought to verify these findings in a 22RV1 mouse xenograft model. Animal groups were treated with RT alone, MI-219 alone, or the combination of the two and compared with a control group, which received no treatment. The treatment with radiation or MI-219 was given as described in methods. Tumor sizes were measured two to three times a week following a 2-week engraftment period. Single-modality treatment with radiation or MI-219 resulted in equivalent tumor growth delay in the wt-p53 22RV1 model (Figure 4A). The combination of radiation and MI-219 resulted in a statistically significant decrease in tumor growth that was evident by day 4 and persisted through day 18. In the p53-KD 22RV1 xenografts, there were no response to treatment with MI-219 and no enhancement when MI-219 was combined with RT (Figure 4C). These results indicate the promise of MI-219 in sensitizing prostate cancer tumors with wt-p53 to ionizing radiations in vivo.
Figure 4.
MI-219 radiosensitizes prostate cancer xenografts in a p53-dependent manner. Xenografts arising from (A) 22RV1 cells or (B) 22RV1 (p53 knockdown) cells were treated with MI-219 (300 mg/kg body weight), radiation (four fractions of 2 Gy on days 1 to 4), or a combination of both, and tumor growth was monitored for 18 days. Bars represent ± SEM.
Androgen Receptor Antagonism Combined with MI-219 Treatment Results in Increased Apoptotic Cell Death in a p53-Dependent Manner
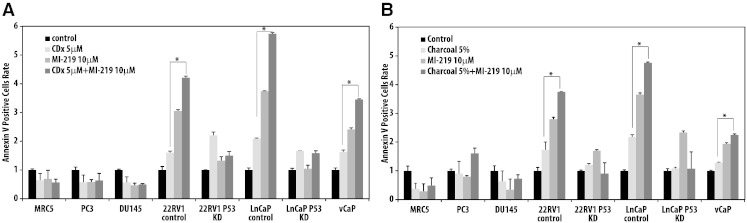
Androgen deprivation combined with radiation therapy is a standard treatment modality for high-risk prostate cancer. Given the demonstrated p53-dependent benefits of MI-219 when combined with radiation, we next evaluated the impact of combining MI-219 with androgen deprivation therapy in vitro. 22RV1 and LNCaP (both androgen responsive) wt-p53 and p53-KD cell lines were treated with 5 μM casodex (a clinically available antiandrogen with mixed agonist/antagonist effects) or 10 μM MI-219, or evaluated in a charcoal-stripped media to simulate androgen withdrawal. Cells were also treated with paired combinations of these three modalities. Antagonism of the androgen receptor with either charcoal-stripped media or casodex resulted in a p53-dependent activation of p53, p21, and PUMA that was enhanced with the addition of MI-219 (Supplementary Figure 4, A and B). Next, we assessed apoptosis via analysis of Annexin V following treatment with casodex/charcoal-stripped media, MI-219, or the combination of two in wt-p53 22RV1 and LNCaP cell lines, p53-KD 22RV1 and LNCaP cell lines, and VCaP, PC3, and DU145 cell lines (Figure 5, A and B). The combination of MI-219 with casodex or charcoal-stripped media resulted in a statistically significant increase in the number of Annexin V–positive cells in the wt-p53 22RV1, wt-p53 LNCaP, and VCaP cell lines compared with treatment with androgen deprivation or MI-219 alone, demonstrating a p53-dependent increase in apoptosis. This increase in apoptosis was not noted in the p53-KD cell lines or in the PC3 or DU145 cell lines.
Figure 5.
Androgen receptor antagonism combined with MI-219 treatment results in increased apoptotic cell death in a p53-dependent manner. Apoptosis measured by Annexin V in multiple cell lines after treatment with (A) MI-219, casodex, or both and (B) charcoal-stripped medium, MI-219 or both. *P < .05.
MI-219 Enhances the Effects of Combined Radiation and Androgen-Deprivation Therapy In Vivo
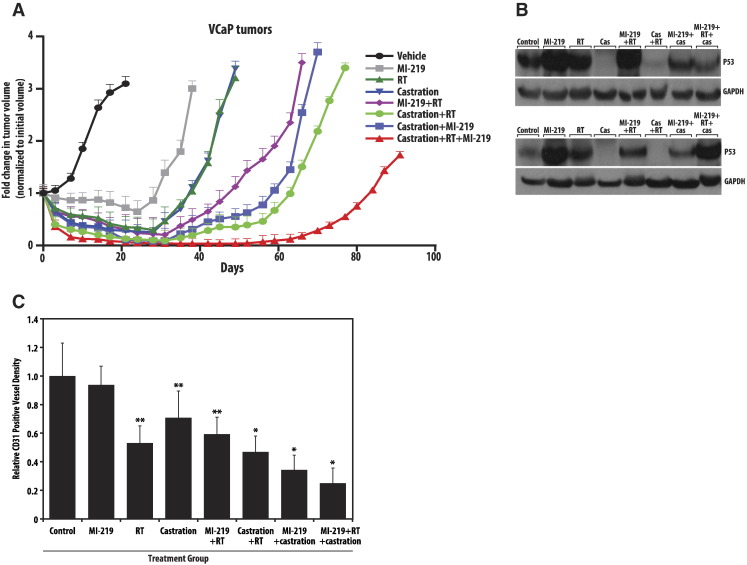
Androgen deprivation therapy and radiation are the standard of care for clinical management of prostate cancer. Given the findings that MI-219 can increase the effect of both of these treatment modalities in vitro, we sought to evaluate triple therapy with MI-219, radiation, and androgen deprivation therapy in vivo. A VCaP mouse xenograft model was established with six to eight animals in each treatment group. Animal groups were treated with RT alone, MI-219 alone, castration alone, RT + castration, RT + MI-219, castration + MI-219, and RT + castration + MI-219. Single therapy with either RT or castration was equivalent in delaying tumor growth, slightly outperforming therapy with MI-219 alone (Figure 6A). Combination therapy with RT + castration, RT + MI-219, and castration + MI-219 all delayed tumor growth similarity, outperforming any single-modality treatment. Triple therapy substantially delayed tumor growth compared with all other treatment combinations and appears to have a greater than additive effect on tumor growth delay. Triple therapy resulted in near-complete regression of the tumor at 50 days and only a 1.5-fold increase from initial tumor volume at 91 days posttreatment. As expected, MI-219, radiation, or a combination of two, as well as the triple therapy, resulted in an increase in p53 levels in xenografts (Figure 6B). As has been seen in other studies on sensitization to radiotherapy and antiandrogen therapy, we saw that treatment response correlated well with blood vessel densities as indicated by CD31 staining [26]. Whereas single therapies resulted in a slight decrease in the vessel density, a combination of MI-219 with radiation or castration was more effective in reducing the density of blood vessels. Interestingly, the triple therapy outperformed all the therapies and resulted in a drastic reduction of blood vessel density, indicating the effectiveness of the combination of MI-219 with radiation and ADT (Figure 6C).
Figure 6.
MI-219 enhances the effects of combined radiation and androgen-deprivation therapy in vivo. (A) VCaP xenografts were subjected to indicated treatments, and tumor growth was monitored. Graphs represent the fold change of tumor volumes, normalized to initial tumor volume. (B) Western blot analyses of p53 in the treated xenograft samples (two independent xenografts per treatment are shown). (C) Xenografts were formalin fixed, sectioned, and stained with CD31 antibodies to quantitate the blood vessel densities after the treatments. *P < .05 compared with triple therapy. ** P < .05 compared with double therapy.
Discussion
Although mutations of the TP53 gene, which encodes the tumor suppressor p53 protein, are thought to be the most abundant genetic alterations occurring in cancer, the relative prevalence of p53 mutations in clinically localized prostate cancer is low [27], [28], [29], [30]. Thus, although the presence of p53 mutations in localized prostate cancer has been associated with worse overall patient outcomes for men treated with radiation plus androgen deprivation, approximately 80% of men diagnosed with prostate cancer will not harbor such a mutation [28], [29], [30]. However, even cancers, which harbor wild-type p53, may have a reduced or inhibited function of p53. This inhibition is most commonly due to increased expression of MDM2, a ubiquitin ligase with both p53-dependent as well as -independent functions. MDM2 overexpression in prostate cancer has also been shown to be associated with worse clinical outcomes [7]. In analysis of 478 men treated with conventional dose radiation plus short- or long-term androgen deprivation on Radiation Therapy Oncology Group 92-02 [4], overexpression of MDM2 was prognostic for development of metastatic disease as well as overall mortality, with a trend toward cause-specific survival as well [7], [31]. Notably, approximately 24% of men in this analysis were found to overexpress MDM2. Combined together, the above observations suggest that inhibition of MDM2 in prostate cancer may have a dual suppressive effect by virtue of blocking MDM2 function and activating p53 functions. Thus, MDM2 inhibition represents an attractive and viable strategy for the treatment of cancers with infrequent p53 alterations, such as prostate cancer, especially in combination with current standard of care therapies.
Along with this enthusiasm, there are important questions about MDM2 inhibition therapy that remain to be answered. For example, it is extremely important to assess if MDM2 treatment will cause aberrations or mutations in the p53 pathway, as there is some evidence that leukemic cells can acquire p53 mutations during treatment with MDM2 inhibitors [32]; whether this is true for prostate cancer or other solid tumors is unknown. It is also very important to assess if there are other pathways that cancer cells may use after anti-MDM2 therapy to evade the treatment. Finally, although the MDM2 inhibition seems to be effective in tumors with intact p53 functions, it will be interesting to design combination therapies that can use MDM2 inhibition in cancers with mutant p53 functions.
In the present study, we demonstrate that inhibition of MDM2 results in in vitro and in vivo p53-dependent radiosensitization of prostate cancer cells. Cell lines harboring wt-p53 or mutated yet functional p53 signaling displayed a time- and dose-dependent upregulation of p53, p21, and PUMA following inhibition of MDM2, and inhibition of MDM2 likewise resulted in a p53-dependent decrease in clonogenic cell survival. Mechanistically, radiosensitization following inhibition of MDM2 was largely due to p53-dependent increases in DNA damage and apoptosis. Combining MDM2 inhibition with androgen deprivation therapy similarly resulted in a p53-dependent upregulation of p53, p21, and PUMA, as well as increased rates of apoptosis. Finally, triple therapy consisting of radiation therapy, androgen deprivation, and MDM2 inhibition substantially decreased xenograft tumor growth compared with any single- or double-agent treatment. In total, these findings underscore the potential of MDM2 inhibition as a means of inducing sensitization to both radiation and androgen deprivation therapy and thereby improving clinical outcomes in prostate cancers with functional p53 status.
The biggest strength of our study lies in the clinical relevance of our findings, which support MDM2 inhibition with MI-219 as a strategy for therapy intensification in prostate cancer. Although MI-219 has suboptimal dosing and delivery characteristics, SAR405838, an analogue of MI-219 with improved pharmacokinetic profile, has shown remarkable single-agent efficacy in multiple xenograft tumor models and is being evaluated in humans [33]. Thus, our study provides a strong rationale for investigating SAR405838 in clinical trials of ADT and/or radiotherapy combinations in prostate cancer. The combination of low prevalence of p53 mutations in localized prostate cancer, worse clinical outcomes with MDM2 overexpression, and the unacceptably high recurrence of localized high-risk prostate cancer, indicates that inhibition of MDM2 may serve as a means by which improved outcomes for high-risk localized prostate cancer can be achieved.
The following are the supplementary data related to this article.
P53 status and androgen sensitivity of cell lines used in the study
Supplementary Figure S1. MI-219 results in p53 activation in a dose- and time-dependent manner. (A–C) Western blot analyses of 22RV1 and LNCaP cells (p53 knockdown) after treatment with indicated doses of MI-219 or nutlin-3. (D) LNCaP cells were treated with 10 μM MI-219 for indicated time points, and Western blot analysis was performed. GAPDH accounts for the equal loading.
Supplementary Figure S2. P53 response is intact in 22RV1, LNCaP, and VCaP cells. Luciferase reporter assays on 22RV1 and LNCaP (wt-p53), DU145 (mt-p53), and VCaP (heterozygous mutant, functional p53) were performed to check the intact p53 response. Cells were transfected with luciferase constructs driven by MDM2 or P21 promoters and were treated with radiation (10 Gy), 10 μM MI-219, or both, and luciferase activity was measured after 48 hours. Renilla luciferase was cotransfected in each sample to normalize the transfection efficiency. Data represent luciferase activity compared with control.
Supplementary Figure S3. Response to MI-219 is independent of drug scheduling. (A) Western blot analysis of 22RV1 cells, with or without p53 knockdown, was performed after the indicated schedule of treatment with MI-219 and/or radiation. (B–C) Clonogenic cell survival assays were performed after treatment with MI-219 or nutlin-3.
Supplementary Figure S4. Androgen receptor antagonism with either casodex or stripped media results in activation of p53, P21, and PUMA in a p53-dependent manner. Western blot analyses of LNCaP and 22RV1 cells (with or without p53 knockdown) after treatment with casodex, MI-219, or charcoal- stripped media or a combination. GAPDH was used as a loading control.
Acknowledgements
We would like to thank Steven Kronenberg for his expertise in helping prepare the figures for this publication.
Grant Information: This work was supported in part by Young Investigator Awards to Daniel Hamstra and Felix Feng from the Prostate Cancer Foundation.
Footnotes
Funding: This work was supported by grants to Drs. Hamstra and Feng from the Prostate Cancer Foundation.
Conflicts: S. W. is a cofounder of Ascenta Therapeutics, which has licensed MI-219 and its analogues for clinical development. S. W. owns stock in Ascenta. The remaining authors declare no conflict of interests in these studies.
Contributor Information
Felix Y. Feng, Email: felix.feng@ucsf.edu.
Daniel A. Hamstra, Email: dhamm@umich.edu.
References
- 1.D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol. 2003;21:2163–2172. doi: 10.1200/JCO.2003.01.075. [DOI] [PubMed] [Google Scholar]
- 2.Bolla M, de Reijke TM, Van Tienhoven G, den Bergh AC Van, Oddens J, Poortmans PM, Gez E, Kil P, Akdas A, Soete G. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 3.Mehra R, Shi Y, Udager AM, Prensner JR, Sahu A, Iyer MK, Siddiqui J, Cao X, Wei J, Jiang H. A novel RNA in situ hybridization assay for the long noncoding RNA SChLAP1 predicts poor clinical outcome after radical prostatectomy in clinically localized prostate cancer. Neoplasia. 2014;16:1121–1127. doi: 10.1016/j.neo.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, Brereton HD, Venkatesan V, Lawton CA, Rosenthal SA, Sandler HM. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 5.Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, Hug EB, Asbell SO, Grignon D. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 6.Pinthus JH, Bryskin I, Trachtenberg J, Lu JP, Singh G, Fridman E, Wilson BC. Androgen induces adaptation to oxidative stress in prostate cancer: implications for treatment with radiation therapy. Neoplasia. 2007;9:68–80. doi: 10.1593/neo.06739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khor LY, Bae K, Paulus R, Al-Saleem T, Hammond ME, Grignon DJ, Che M, Venkatesan V, Byhardt RW, Rotman M. MDM2 and Ki-67 predict for distant metastasis and mortality in men treated with radiotherapy and androgen deprivation for prostate cancer: RTOG 92-02. J Clin Oncol. 2009;27:3177–3184. doi: 10.1200/JCO.2008.19.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khor LY, Desilvio M, Al-Saleem T, Hammond ME, Grignon DJ, Sause W, Pilepich M, Okunieff P, Sandler H, Pollack A. MDM2 as a predictor of prostate carcinoma outcome: an analysis of Radiation Therapy Oncology Group Protocol 8610. Cancer. 2005;104:962–967. doi: 10.1002/cncr.21261. [DOI] [PubMed] [Google Scholar]
- 9.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 10.Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 11.Xiao ZX, Chen J, Levine AJ, Modjtahedi N, Xing J, Sellers WR, Livingston DM. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature. 1995;375:694–698. doi: 10.1038/375694a0. [DOI] [PubMed] [Google Scholar]
- 12.Martin K, Trouche D, Hagemeier C, Sorensen TS, La Thangue NB, Kouzarides T. Stimulation of E2F1/DP1 transcriptional activity by MDM2 oncoprotein. Nature. 1995;375:691–694. doi: 10.1038/375691a0. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y. E3 ubiquitin ligases as cancer targets and biomarkers. Neoplasia. 2006;8:645–654. doi: 10.1593/neo.06376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Xu T, Jiang Y, Xu H, Yan Y, Fu D, Chen J. The challenges and the promise of molecular targeted therapy in malignant gliomas. Neoplasia. 2015;17:239–255. doi: 10.1016/j.neo.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CY, Oliner JD, Zhan Q, Fornace AJ, Jr., Vogelstein B, Kastan MB. Interactions between p53 and MDM2 in a mammalian cell cycle checkpoint pathway. Proc Natl Acad Sci U S A. 1994;91:2684–2688. doi: 10.1073/pnas.91.7.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ljungman M. Dial 9-1-1 for p53: mechanisms of p53 activation by cellular stress. Neoplasia. 2000;2:208–225. doi: 10.1038/sj.neo.7900073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoyanova R, Hachem P, Hensley H, Khor LY, Mu Z, Hammond ME, Agrawal S, Pollack A. Antisense-MDM2 sensitizes LNCaP prostate cancer cells to androgen deprivation, radiation, and the combination in vivo. Int J Radiat Oncol Biol Phys. 2007;68:1151–1160. doi: 10.1016/j.ijrobp.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mu Z, Hachem P, Agrawal S, Pollack A. Antisense MDM2 sensitizes prostate cancer cells to androgen deprivation, radiation, and the combination. Int J Radiat Oncol Biol Phys. 2004;58:336–343. doi: 10.1016/j.ijrobp.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 19.Mu Z, Hachem P, Agrawal S, Pollack A. Antisense MDM2 oligonucleotides restore the apoptotic response of prostate cancer cells to androgen deprivation. Prostate. 2004;60:187–196. doi: 10.1002/pros.20044. [DOI] [PubMed] [Google Scholar]
- 20.Mu Z, Hachem P, Hensley H, Stoyanova R, Kwon HW, Hanlon AL, Agrawal S, Pollack A. Antisense MDM2 enhances the response of androgen insensitive human prostate cancer cells to androgen deprivation in vitro and in vivo. Prostate. 2008;68:599–609. doi: 10.1002/pros.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tovar C, Higgins B, Kolinsky K, Xia M, Packman K, Heimbrook DC, Vassilev LT. MDM2 antagonists boost antitumor effect of androgen withdrawal: implications for therapy of prostate cancer. Mol Cancer. 2011;10:49. doi: 10.1186/1476-4598-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Supiot S, Hill RP, Bristow RG. Nutlin-3 radiosensitizes hypoxic prostate cancer cells independent of p53. Mol Cancer Ther. 2008;7:993–999. doi: 10.1158/1535-7163.MCT-07-0442. [DOI] [PubMed] [Google Scholar]
- 23.Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S, Nikolovska-Coleska Z, Ding K, Wang G, Chen J. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci U S A. 2008;105:3933–3938. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JS, Lee C, Bonifant CL, Ressom H, Waldman T. Activation of p53-dependent growth suppression in human cells by mutations in PTEN or PIK3CA. Mol Cell Biol. 2007;27:662–677. doi: 10.1128/MCB.00537-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Wang H, Li M, Agrawal S, Chen X, Zhang R. MDM2 is a negative regulator of p21WAF1/CIP1, independent of p53. J Biol Chem. 2004;279:16000–16006. doi: 10.1074/jbc.M312264200. [DOI] [PubMed] [Google Scholar]
- 26.Huamani J, Willey C, Thotala D, Niermann KJ, Reyzer M, Leavitt L, Jones C, Fleishcher A, Caprioli R, Hallahan DE. Differential efficacy of combined therapy with radiation and AEE788 in high and low EGFR-expressing androgen-independent prostate tumor models. Int J Radiat Oncol Biol Phys. 2008;71:237–246. doi: 10.1016/j.ijrobp.2007.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall MC, Navone NM, Troncoso P, Pollack A, Zagars GK, von Eschenbach AC, Conti CJ, Chung LW. Frequency and characterization of p53 mutations in clinically localized prostate cancer. Urology. 1995;45:470–475. doi: 10.1016/s0090-4295(99)80018-1. [DOI] [PubMed] [Google Scholar]
- 28.Che M, DeSilvio M, Pollack A, Grignon DJ, Venkatesan VM, Hanks GE, Sandler HM, Rtog Prognostic value of abnormal p53 expression in locally advanced prostate cancer treated with androgen deprivation and radiotherapy: a study based on RTOG 9202. Int J Radiat Oncol Biol Phys. 2007;69:1117–1123. doi: 10.1016/j.ijrobp.2007.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Amico AV, Halabi S, Vollmer R, Loffredo M, McMahon E, Sanford B, Archer L, Vogelzang NJ, Small EJ, Kantoff PW. p53 protein expression status and recurrence in men treated with radiation and androgen suppression therapy for higher-risk prostate cancer: a prospective phase II Cancer and Leukemia Group B Study (CALGB 9682) Urology. 2008;71:933–937. doi: 10.1016/j.urology.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Grignon DJ, Caplan R, Sarkar FH, Lawton CA, Hammond EH, Pilepich MV, Forman JD, Mesic J, Fu KK, Abrams RA. p53 status and prognosis of locally advanced prostatic adenocarcinoma: a study based on RTOG 8610. J Natl Cancer Inst. 1997;89:158–165. doi: 10.1093/jnci/89.2.158. [DOI] [PubMed] [Google Scholar]
- 31.Pollack A, Dignam JJ, Diaz DA, Wu Q, Stoyanova R, Bae K, Dicker AP, Sandler H, Hanks GE, Feng FY. A tissue biomarker-based model that identifies patients with a high risk of distant metastasis and differential survival by length of androgen deprivation therapy in RTOG protocol 92–02. Clin Cancer Res. 2014;20:6379–6388. doi: 10.1158/1078-0432.CCR-14-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Hoffman-Luca CG, Ziazadeh D, McEachern D, Zhao Y, Sun W, Debussche L. Elucidation of acquired resistance to Bcl-2 and MDM2 inhibitors in acute leukemia in vitro and in vivo. Clin Cancer Res. 2015;21(11):2558–2568. doi: 10.1158/1078-0432.CCR-14-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Sun W, Zhao Y, McEachern D, Meaux I, Barriere C, Stuckey JA, Meagher JL, Bai L, Liu L. SAR405838: an optimized inhibitor of MDM2-p53 interaction that induces complete and durable tumor regression. Cancer Res. 2014;74:5855–5865. doi: 10.1158/0008-5472.CAN-14-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
P53 status and androgen sensitivity of cell lines used in the study
Supplementary Figure S1. MI-219 results in p53 activation in a dose- and time-dependent manner. (A–C) Western blot analyses of 22RV1 and LNCaP cells (p53 knockdown) after treatment with indicated doses of MI-219 or nutlin-3. (D) LNCaP cells were treated with 10 μM MI-219 for indicated time points, and Western blot analysis was performed. GAPDH accounts for the equal loading.
Supplementary Figure S2. P53 response is intact in 22RV1, LNCaP, and VCaP cells. Luciferase reporter assays on 22RV1 and LNCaP (wt-p53), DU145 (mt-p53), and VCaP (heterozygous mutant, functional p53) were performed to check the intact p53 response. Cells were transfected with luciferase constructs driven by MDM2 or P21 promoters and were treated with radiation (10 Gy), 10 μM MI-219, or both, and luciferase activity was measured after 48 hours. Renilla luciferase was cotransfected in each sample to normalize the transfection efficiency. Data represent luciferase activity compared with control.
Supplementary Figure S3. Response to MI-219 is independent of drug scheduling. (A) Western blot analysis of 22RV1 cells, with or without p53 knockdown, was performed after the indicated schedule of treatment with MI-219 and/or radiation. (B–C) Clonogenic cell survival assays were performed after treatment with MI-219 or nutlin-3.
Supplementary Figure S4. Androgen receptor antagonism with either casodex or stripped media results in activation of p53, P21, and PUMA in a p53-dependent manner. Western blot analyses of LNCaP and 22RV1 cells (with or without p53 knockdown) after treatment with casodex, MI-219, or charcoal- stripped media or a combination. GAPDH was used as a loading control.