Abstract
Background.
There is little evidence on the long-term association between physical activity (PA) and depressive symptoms in old age. We examined the association of midlife PA and depressive symptoms in late life.
Methods.
A large community-based population residing in Reykjavik, Iceland, participated in a longitudinal study with an average of 25 years of follow up. Midlife PA was categorized as active and inactive groups (n = 4,140, Active = 1,292, Inactive = 2,848, mean age 52±7 years). The main outcome had six or higher depressive symptoms assessed by the 15-item Geriatric Depression scale. Participants who had a history of depression (n = 226), and were diagnosed with dementia (n = 393), and had incomplete cognitive data (n = 595) and incomplete analytical data (n = 422) were excluded. Level of weekly PA was ascertained by a questionnaire at midlife. Depressive symptoms were assessed on average 25 (±4) years later.
Results.
After controlling for demographic and health-related risk factors, those who were active at midlife were less likely to have high level of depressive symptomatology (6 or higher Geriatric Depression scale scores, odds ratio = 0.58, 95% confidence interval: 0.41–0.83, p < .005) compared with those who were inactive in midlife. After full adjustment of three domains of late-life cognitive function the results remained significant (odds ratio = 0.61, 95% confidence interval: 0.43–0.86, p = .005).
Conclusion.
Our study shows that midlife PA is associated with lower depressive symptoms 25 years later. Participating in regular PA in midlife may improve mental health in late life.
Key Words: Mid-life physical activity, Aging, Depressive symptomatology, Cognitive function.
Depression among older adults is associated with adverse health outcomes (1), such as decreased physical activity (PA) (2,3) and increased risk of losing physical function (4). Behavioral modification has a positive effect on depression among old people (5). In particular, participation in PA is associated with reduced depressive symptoms among older people with mild to moderate depression (6). However, evidence for a long-term association between PA and depressive symptoms in old age is lacking (7). Current evidence for the long-term association between PA and depressive symptoms is mostly based on short-term intervention studies (8,9) or studies where longitudinal follow-up time is less than 15 years (10,11). Previous longitudinal studies suggested that midlife PA levels are associated with cognitive (12) and physical function (13) measured 25 years later. Midlife PA level may also have an association with depressive symptoms among old people (14) with longitudinal follow-up time more than 25 years.
Depressive symptoms are also associated with cognitive impairment and development of dementia in older adults (15), but the relationship is complex (16,17). Previous studies mostly used a single global cognitive test as a confounder which may not capture domain specific or cognitive impairment. Whether the association between midlife PA and depressive symptoms in late life is independent of cognitive function needs to be assessed with appropriate data that include expanded cognitive battery.
The objective of the current study was to investigate the long-term association between midlife PA and late-life depressive symptoms, on average 25 years later, in a population free of clinical history of depression and diagnosis of dementia. In a secondary analysis, we further investigated whether the association was independent of cognitive function.
Methods
Study Population
Age Gene/Environment Susceptibility (AGES)—Reykjavik Study
The Icelandic Heart Association initiated the Reykjavik Study (RS) in 1967 to study cardiovascular disease and risk factors. The cohort was comprised of men and women living in Reykjavik who were born in 1907–1935 (n = 18,842). In 2002, surviving members (n = 11,308) of the RS cohort were re-invited to participate in the AGES-RS (Supplementary Table 1). Reexamination included cognitive testing, brain magnetic resonance imaging, and physical performance tests. Details of the study design and the baseline AGES-RS assessments have been described elsewhere (18). AGES-RS was approved by the Icelandic National Bioethics Committee (VSN 00-063) and by the Institutional Review Board of the U.S. National Institute on Aging, National Institutes of Health. Informed consent was signed by all participants.
Measurement in Midlife
Assessment of Physical Activity
At the midlife RS interview, participants were asked two questions about PA. The first question was about whether they regularly participated in sports or exercise. Participants who answered “yes” to the first question were then asked how many hours per week they were involved in sports or exercise separately in the winter and summer (three answers, [1] none, [2] 5 hours or less, and [3] more than 5 hours). The average hours of midlife PA per week were calculated from the total reported PA hours in summer and winter periods. Participants who reported neither sport nor exercise in both winter and summer were grouped as “Inactive.” Participants who reported any activity hours during summer or winter were defined as an “Active” group.
Measurement in Late Life
Assessment of Clinical Depression and Depressive Symptoms
In the AGES-RS examination, major depressive disorder was assessed with the Mini-International Neuropsychiatric Interview (19), and the diagnosis was processed according to international guidelines, Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition (DSM- IV) (20). Details of the major depressive disorder assessments have been described elsewhere (21). Depressive symptoms were measured using the 15-item Geriatric Depression scale (GDS) (22). The GDS scores range from 0 to 15, with higher scores indicating more symptoms of depression (see Supplementary Table 1). Individuals with 6 or higher GDS score (23) were defined as having a high level of depressive symptomatology (HDEPS).
Assessment of Cognition
Global cognitive function was examined using Mini-Mental State Examination (MMSE) (24). All participants were administered a battery of cognitive tests that included multiple tests of three cognitive domains (25). Composite scores were constructed for speed of processing (SP), memory (MEM), and executive function (EF) based on a theoretical grouping of tests, similar to other population-based studies (26). Details have been described in a previous study (22). A consensus diagnosis of dementia was made according to international guidelines, DSM- IV (17), by a geriatrician, neurologist, neuropsychologist, and a neuroradiologist.
Assessment of Covariates
There are several demographic and health factors that may influence the association between midlife PA and depressive symptoms in late life which we controlled for in the analyses.
Midlife Covariates
Measures based on the midlife RS included blood pressure (BP, mmHg), height, weight, serum cholesterol (mmol/L), and smoking status (never/previous/current). BP was measured in a semirecumbent position using a mercury sphygmomanometer and a cuff of appropriate size on the right arm after participants had rested for several minutes. Body mass index was calculated from measured height and weight. Serum cholesterol level was measured from a blood sample drawn at the in-person examination. Education was categorized into four levels (elementary school, high school, undergraduate, more than undergraduate education).
Late-life Covariates
Variables measured in the AGES-RS were also included. Diabetes was defined as self-reported doctor’s diagnosis, current medication use, or fasting glucose level ≥7.0 mmol/L. Coronary events included those identified in an ongoing surveillance system, self-reported history of myocardial infarction, coronary bypass surgery, heart bypass surgery, angioplasty, or other coronary artery diseases. Self-reported doctor’s diagnosis of stroke was assessed via questionnaire. Antidepressant medication use was registered at the time of examination of study participants in the AGES-RS.
Analytical Sample
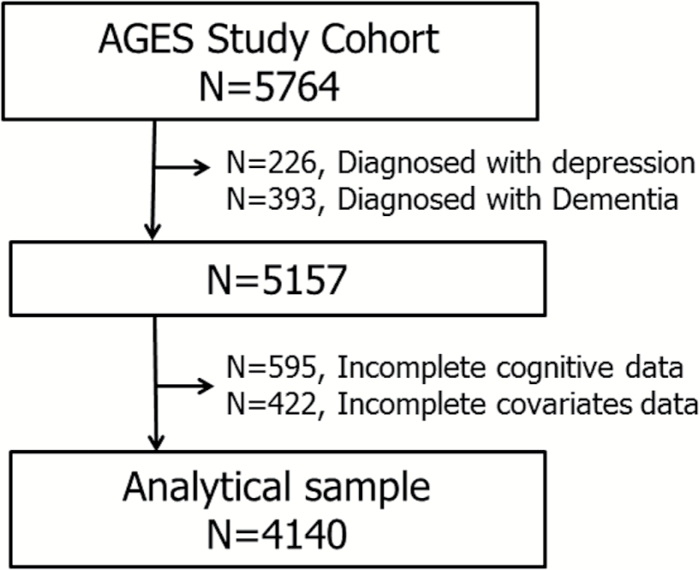
Out of a total population of 5,764, we excluded people who had clinical depression diagnosed by a doctor in the past or present (n = 226), and had been diagnosed with dementia by the AGES consensus panel (n = 393), as they are likely to have depressive symptoms in current life (13,14). Among 5,157 participants (2,185 men and 2,972 women), we further excluded those with incomplete data on cognitive function (n = 595) and other covariables (n = 422). Finally, 4,140 participants (1,781 men and 2,359 women; Active = 1,292, Inactive = 2,848) were available for the analysis (Figure 1).
Figure 1.
Flow-chart of the analytical sample.
Statistical Analysis
Chi-square test for categorical variables and general linear regression for continuous variables were used to compare characteristics of participants according to the midlife PA level. Logistic regression analysis was used to examine the hypothesis that people who were active at midlife are less likely to have HDEPS in late life compared with people who were inactive at midlife. The first model for logistic regression analysis (model 1) was adjusted for age, sex, and education. A second model (model 2) further included midlife covariates, including cholesterol level, systolic BP, smoking, and body mass index, and late-life covariates, including diabetes, history of coronary event, and self-reported physician diagnosis of stroke. The third model (model 3) was additionally adjusted for use of antidepressants. Then, model 3 was used as the standard model for adjusting different cognitive measurement. The model 3 was further included MMSE, SP, MEM, and EF separately. The final adjustment of model 3 included all three domains of SP, MEM, and EF together. Secondary analysis was performed to examine the association between midlife PA and late-life depressive symptoms by gender (Supplementary Table 2). Statistical analyses were performed using STATA software, version 10.
Results
Compared with those who were inactive in midlife (n = 2,848), those who were physically active (n = 1,292) in midlife had shorter number of follow-up years, had completed more years of schooling, had higher MMSE scores (all p < .001), and were generally healthier in late life (Table 1). Among 15 questions in the GDS, answers to nine questions were significantly different (p < .05) by midlife PA level, and four of these nine questions were highly significant (p < .001). The four questions were (i) Do you feel that your life is empty? (ii) Do you prefer to stay at home rather than go out and do new things? (iii) Do you feel pretty worthless the way you are now? and (iv) Do you feel full of energy? In a fully adjusted logistic regression analysis, including a single global cognitive score (Table 2), those who were active at midlife were 42% less likely to have HDEPS (GDS score of 6 or higher) in late life (odds ratio [OR] = 0.58, 95% confidence interval [CI] 0.41–0.83) compared with those who were inactive in midlife.
Table 1.
Characteristics of Participants by Midlife PA Level (excluding those with dementia or history of clinical depression)
| Total (n = 4,140) | ||||||
|---|---|---|---|---|---|---|
| Inactive (n = 2,848) | Active (n = 1,292) | p Value | p Value* | |||
| Follow-up time, mean (SD), y | 26.1 | (3.7) | 25.1 | (4.3) | <.0001 | <.0001 |
| Female, n (%) | 1,643 | (58.7) | 716 | (55.4) | .171 | .164 |
| Elementary education, n (%) | 725 | (25.5) | 193 | (14.9) | <.0001 | <.0001 |
| ApoE e4, n (%) | 756/2,790 | (27.1) | 317/1,264 | (25.1) | .388 | .159 |
| Midlife examination | ||||||
| Body mass index, mean (SD) | 3.7 | (25.3) | 25.2 | (3.2) | .473 | .516 |
| Cholesterol, mean (SD), mmol/L | 1.1 | (6,3) | 6.3 | (1.1) | .469 | .678 |
| Systolic blood pressure, mean (SD), mmHg | 132.6 | (17.0) | 131.6 | (16.4) | .078 | .141 |
| Midlife never smoked, n (%) | 1,106 | (38.8) | 415 | (32.1) | <.0001 | <.0001 |
| Current examination | ||||||
| Age, mean (SD), y | 76.3 | (5.4) | 76.1 | (5.3) | .113 | |
| Body mass index, mean (SD) | 27.2 | (4.5) | 27 | (4.3) | .288 | .200 |
| Systolic blood pressure, mean (SD), mmHg | 143.0 | (20.5) | 141.8 | (19.7) | .091 | .136 |
| Cholesterol, mean (SD), mmol/L | 5.6 | (1.2) | 5.58 | (1.2) | .238 | .232 |
| MMSE, mean (SD) | 26.9 | (2.4) | 27.3 | (2.0) | <.0001 | <.0001 |
| Diabetes, n (%) | 348 | (12.2) | 140 | (10.8) | .201 | .195 |
| Hypertension, n (%) | 2,340 | (82.2) | 1,014 | (78.5) | .014 | .035 |
| Coronary event, n (%) | 436 | (15.3) | 194 | (15.0) | .808 | .834 |
| Self-reported stroke, n (%) | 163 | (5.7) | 77 | (6.0) | .908 | .898 |
| Never smoked, n (%) | 1,180 | (41.4) | 596 | (46.1) | <.0001 | <.0001 |
| Cognitive function | ||||||
| Speed of processing, mean (SD) | 0.039 | (0.73) | 0.285 | (0.71) | <.0001 | <.0001 |
| Memory, mean (SD) | 0.057 | (0.85) | 0.230 | (0.90) | <.0001 | <.0001 |
| Executive function, mean (SD) | 0.032 | (0.64) | 0.173 | (0.65) | <.0001 | <.0001 |
Note: *Age adjusted.
Table 2.
Likelihood of Having High Depressive Symptom Burden in Late Life According to Midlife PA Levels
| Midlife PA Group (n = 4,140) |
|||
|---|---|---|---|
| Inactive | Active | ||
| (n = 2,848) 174 with HDEPS |
(n = 1,292) 42 with HDEPS |
||
| Odds Ratio (95% CI: low, high) | |||
| Six or higher late-life depressive symptoms | Model 1 | Reference | 0.57 (0.41, 0.81) |
| Model 2 | Reference | 0.58 (0.41, 0.83) | |
| Model 3 | Reference | 0.57 (0.40, 0.82) | |
| Model 3 + MMSE | Reference | 0.58 (0.41, 0.83) | |
| Model 3 + SP | Reference | 0.61 (0.42, 0.86) | |
| Model 3 + MEM | Reference | 0.59 (0.41, 0.84) | |
| Model 3 + EF | Reference | 0.59 (0.41, 0.84) | |
| Model 3 + SP, MEM, and EF | Reference | 0.61 (0.43, 0.86) | |
Notes: CI = confidence interval, HDEPS = high depressive symptoms; PA = physical activity.
Model 1 = adjusted for age, sex, and education. Model 2 = Model 1 + Midlife risk factors: body mass index (BMI), systolic blood pressure, current smoking, cholesterol, coronary event, never smoked, and self-reported stroke. Model 3 = Model 2 + antidepressant medication use. Model 3 + MMSE = Model 3 adjusted for MMSE. Model 3 + SP = Model 3 adjusted for speed of processing (SP). Model 3 + MEM = Model 3 adjusted for memory (MEM). Model 3 + EF = Model 3 adjusted for executive function (EF). Model 3 + SP, MEM & EF = Model 3 adjusted for all cognitive function (SP, MEM, and EF).
The analysis assessed the likelihood of having HDEPS in later life after adjusting for each cognitive domain. After additional adjustment with SP only, those who were active at midlife were 39% less likely to have HDEPS after 25 years (OR = 0.61, 95% CI: 0.42–0.86). The protective effect of being active at midlife was 41% when adjusted for MEM only (OR = 0.59, 95% CI: 0.41–0.84) and EF only (OR = 0.59, 95% CI: 0.41–0.84). After adjusting for all three cognitive domains in the same model, those who were active at midlife were 39% less likely to have HDEPS in late life compared with those who were inactive at midlife (OR = 0.61, 95% CI: 0.43–0.86) (Table 2). The protective effect of being physically active at midlife was reduced by 3% in a fully adjusted model including all three cognitive domains, compared with the model adjusted with MMSE. The secondary analysis by gender was performed and the trend was similar in both genders although it was only significant among women (Supplementary Table 2).
Discussion
In our longitudinal study over a period of 25 years, we found a strong association between midlife PA and depressive symptoms in late life among community dwelling old people who did not have a history of depression. Compared with those who were inactive at midlife, those who were active at midlife had significantly less depressive symptoms 25 years later even after controlling for demographics, physiologic markers, and various aspects of cognitive function.
The effects of PA on depressive symptoms among older people are still inconsistent (27,28) and the follow-up times in most longitudinal studies have a wide range (from 1 to 15 years) (29). The different age range of the cohorts in each study could have been the reason for inconsistent results because an older cohort is more likely to have depressive symptoms and low PA level than a younger cohort (30). There is no clear evidence for the long-term association of an earlier PA level and depressive symptoms in later years among older people. Our results confirmed positive findings from previous studies that being physically active at midlife has a long-term association with less depressive symptoms among older adults even after 25 years.
Cognitive impairment is significantly associated with depression in old people (13,14,31). However, only few studies have assessed the effect of PA on depression among old people with respect to various aspects of cognitive function (32). In the current study, we were able to examine the long-term association between midlife PA and depressive symptoms in late life after adjustment with MMSE as well as with various domains of cognitive function including SP, MEM, and EF. The results from the adjusted model with the cognitive domains were slightly attenuated compared with the original analysis adjusted for MMSE. We found that among all three cognitive domains, SP attenuated results the most (3%) compared with the original analysis with MMSE. In a previous study of the same cohort, SP had the strongest association with midlife PA (9). Our findings confirmed that the association between midlife PA and depressive symptoms is independent of various aspects of cognitive function in late life.
Although the mechanism by which PA may improve depressive symptoms remains uncertain, several hypotheses have been suggested. According to an endorphin hypothesis (33), PA increases endorphin levels in the brain, thereby reducing pain, anxiety, and depressive symptoms, and thus increasing the feeling of well-being (34). The vascular depression hypothesis is another possible explanation for the association. Depression is significantly associated with cardiovascular disease and regular exercise or PA which is well known for its protective effect against cardiovascular disease (35). Another possible explanation for the long-term association is that regular PA promotes positive feelings and higher self-esteem (36). Increased self-efficacy or self-esteem among those who were active in midlife may promote a better feeling of well-being and less depressive symptoms in late life. Another hypothesis connects the higher level of well-being with the effects of social activity or interaction because PA is often closely linked with social interaction which in turn provides mental support (37).
Our study has several strengths. First, our large, community-based cohort is well characterized by a variety of risk factors assessed at both midlife and late life. It allowed us to control for various health risk factors in both mid- and late life for the long-term association. Second, we assessed the long-term association between midlife PA measurement and late-life depressive symptoms over an average of 25 years. Depression and inactivity have a bidirectional relationship (30) thus longitudinal data are needed in order to establish temporality of the associations. Long interval time in the current study combined with exclusion of participants with a history of depression makes it less likely that the level of PA at midlife was influenced by depression or depressive symptoms in our population. Third, our study population was free of dementia. People who were clinically diagnosed with dementia were excluded because dementia is significantly associated with depression in older population (38). Fourth, we were able to examine the association between midlife PA and depressive symptoms in late life independent of cognitive function. Our study evaluated various aspects of cognitive function using multiple cognitive tests in late life.
However, our study has some limitations. Mortality rates by 2002 were lower and participation rates were higher among those who were active in midlife compared with those who were inactive in midlife during long follow-up years and this should be noted when interpreting our findings. At midlife examination, the standard set of PA questions at the time asked leisure related PA hours per week in summer and winter, respectively, but information was not collected with regards to details of intensity and frequency of PA at midlife. Information about depression and depressive symptoms was not available at the midlife examination. The results should be interpreted with caution as the association between midlife PA and depressive symptoms in late life did not include work-related PA and social activity in midlife. Information on work associated PA and social activity in midlife was not available in the current study.
Previous findings suggested that regular PA may prevent depression or depressive symptoms among older people with 1–15 years of follow-up time. Our results using population free of depression and dementia suggest that regular PA in midlife is significantly associated with reduced depressive symptoms 25 years later, even after adjusting multiple domains of cognitive function. Current findings lend support to the importance of promoting regular PA among middle aged people to maintain or improve physical function as well as to improve psychological well-being in later life. The physical and psychological benefits of regular PA in midlife demonstrate the importance of developing PA programs that help sedentary people adapting to regular PA early in life.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This study was funded by the National Institutes of Health (contract N01-AG-12100), the National Institute on Aging Intramural Research Program, the Icelandic Heart Association, Landspitali University Hospital, and the Icelandic Parliament.
Disclosures
All authors contributed significantly to the manuscript and declared that there is no financial interest/conflict of interest.
Supplementary Material
Acknowledgments
The study was approved by the National Bioethics Committee in Iceland (approval VSN-00-063) Data Protection Authority, and by the National Institute on Aging Intramural Institutional Review Board. Written informed consent was obtained from all participants. The study was previously presented in The Gerontological Society of America Annual Meeting in November, 2008, National Harbor, MD, 2008, USA.
References
- 1. Onder G, Penninx BW, Cesari M, et al. Anemia is associated with depression in older adults: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2005;60:1168–1172. PMID:16183958 [DOI] [PubMed] [Google Scholar]
- 2. Strawbridge WJ, Deleger S, Roberts RE, et al. Physical activity reduces the risk of subsequent depression for older adults. Am J Epidemiol. 2002;156(4):328–334. PMID:12181102 [DOI] [PubMed] [Google Scholar]
- 3. Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Prev Med. 2008;46:397–411. Epub January 26, 2008. doi:10.1016/j.ypmed.2008.01.009 [DOI] [PubMed] [Google Scholar]
- 4. Braam AW, Prince MJ, Beekman AT, et al. Physical health and depressive symptoms in older Europeans. Results from EURODEP. Br J Psychiatry. 2005;187:35–42. PMID:15994569 [DOI] [PubMed] [Google Scholar]
- 5. Isaac V, Stewart R, Artero S, et al. Social activity and improvement in depressive symptoms in older people: a prospective community cohort study. Am J Geriatr Psychiatry. 2009;17(8):688–696. doi:10.1097/JGP.0b013e3181a88441 [DOI] [PubMed] [Google Scholar]
- 6. Lindwall M, Rennemark M, Halling A, et al. Depression and exercise in elderly men and women: findings from the Swedish national study on aging and care. J Aging Phys Act. 2007;15(1):41–55. PMID:17387228 [DOI] [PubMed] [Google Scholar]
- 7. Sjösten N, Kivelä SL. The effects of physical exercise on depressive symptoms among the aged: a systematic review. Int J Geriatr Psychiatry. 2006;21:410–418. PMID:16676285 [DOI] [PubMed] [Google Scholar]
- 8. Lavretsky H, Alstein LL, Olmstead RE, et al. Complementary use of Tai Chi Chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. Am J Geriatr Psychiatry. 2011;19:839–850. doi:10.1097/JGP.0b013e31820ee9ef [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Penninx BW, Rejeski WJ, Pandya J, et al. Exercise and depressive symptoms: a comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. J Gerontol A Biol Sci Med Sci. 2002;57,124–132. PMID:11867660 [DOI] [PubMed] [Google Scholar]
- 10. Ku PW, Fox KR, Chen LJ, Chou P. Physical activity and depressive symptoms in older adults: 11-year follow-up. Am J Prev Med. 2012;42:355–362. doi:10.1016/j.amepre.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 11. Smith TL, Masaki KH, Fong K, et al. Effect of walking distance on 8-year incident depressive symptoms in elderly men with and without chronic disease: the Honolulu-Asia Aging Study. J Am Geriatr Soc. 2010;58:1447–1452. Epub July 28, 2010. doi:10.1111/j.1532-5415.2010.02981.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang M, Jonsson PV, Snaedal J, et al. The effect of midlife physical activity on cognitive function among older adults: AGES–Reykjavik Study. J Gerontol A Biol Sci Med Sci. 2010;65:1369–1374. Epub August 30, 2010. doi:10.1093/gerona/glq152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang M, Saczynski JS, Snaedal J, et al. Midlife physical activity preserves lower extremity function in older adults: age gene/environment susceptibility-Reykjavik study. J Am Geriatr Soc. 2013;61:237–242. Epub January 15, 2013. doi:10.1111/jgs.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Motl RW, McAuley E. Physical activity, disability, and quality of life in older adults. Phys Med Rehabil Clin N Am. 2010;21:299–308. doi:10.1016/j.pmr.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 15. Irie F, Masaki KH, Petrovitch H, et al. Apolipoprotein E epsilon4 allele genotype and the effect of depressive symptoms on the risk of dementia in men: the Honolulu-Asia Aging Study. Arch Gen Psychiatry. 2008;65:906–912. doi:10.1001/archpsyc.65.8.906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beevers CG. Cognitive vulnerability to depression: a dual process model. Clin Psychol Rev. 2005;25:975–1002. PMID:15905008 [DOI] [PubMed] [Google Scholar]
- 17. Beevers CG, Miller IW. Unlinking negative cognition and symptoms of depression: evidence of a specific treatment effect for cognitive therapy. J Consult Clin Psychol. 2005;73:68–77. PMID:15709833 [DOI] [PubMed] [Google Scholar]
- 18. Harris AH, Cronkite R, Moos R. Physical activity, exercise coping, and depression in a 10-year cohort study of depressed patients. J Affect Disord. 2006;93:79–85. PMID:16545873 [DOI] [PubMed] [Google Scholar]
- 19. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33;quiz 34. PMID:9881538 [PubMed] [Google Scholar]
- 20. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 21. Geerlings MI, Sigurdsson S, Eiriksdottir G, et al. Associations of current and remitted major depressive disorder with brain atrophy: the AGES-Reykjavik Study. Psychol Med. 2013;43:317–328. Epub May 30, 2012. doi:10.1017/S0033291712001110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. PMID:7183759 [DOI] [PubMed] [Google Scholar]
- 23. Sheikh J, Yesavage J. Geriatric depression scale (GDS); recent evidence and development of a shorter version. In: Brink T, ed. Clinical Gerontology: A Guide to Assessment. NewYork, NY: Hawthorne Press; 1986:165–173. [Google Scholar]
- 24. Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. PMID:1202204 [DOI] [PubMed] [Google Scholar]
- 25. Saczynski JS, Jonsdottir MK, Sigurdsson S, et al. White matter lesions and cognitive performance: the role of cognitively complex leisure activity. J Gerontol A Biol Sci Med Sci. 2008;63:848–854. PMID:18772473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. PMID:11851541 [DOI] [PubMed] [Google Scholar]
- 27. Singh NA, Clements KM, Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2001;56:M497–M504. PMID:11487602 [DOI] [PubMed] [Google Scholar]
- 28. Cooper-Patrick L, Ford DE, Mead LA, et al. Exercise and depression in midlife: a prospective study. Am J Public Health. 1997;87(4):670–673. PMID:9146452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blake H, Mo P, Malik S, Thomas S. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. 2009;23:873–887. PMID:9146452 [DOI] [PubMed] [Google Scholar]
- 30. Khalaila R, Litwin H. Changes in health behaviors and their associations with depressive symptoms among Israelis aged 50+. J Aging Health. 2014;26:401–421. doi:10.1177/0898264313516997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han L, McCusker J, Cole M, et al. 12-month cognitive outcomes of major and minor depression in older medical patients. Am J Geriatr Psychiatry. 2008;16(9):742–751. doi:10.1097/JGP.0b013e31817c6ad7 [DOI] [PubMed] [Google Scholar]
- 32. Castaneda AE, Tuulio-Henriksson A, Marttunen M, et al. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106(1–2):1–27. PMID:17707915 [DOI] [PubMed] [Google Scholar]
- 33. Fichna J, Janecka A, Costentin J, et al. The endomorphin system and its evolving neurophysiological role. Pharmacol Rev. 2007;59(1):88–123. PMID:17329549 [DOI] [PubMed] [Google Scholar]
- 34. Fichna J, Janecka A, Piestrzeniewicz M, et al. Antidepressant-like effect of endomorphin-1 and endomorphin-2 in mice. Neuropsychopharmacology. 2007;32(4):813–821. PMID:16823383 [DOI] [PubMed] [Google Scholar]
- 35. Frasure-Smith N, Lesperance F, Habra M, et al. Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation. 2009;120(2):134–140. doi:10.1161/CIRCULATIONAHA.109.851675 [DOI] [PubMed] [Google Scholar]
- 36. McAuley E, Mailey EL, Mullen SP, et al. Growth trajectories of exercise self-efficacy in older adults: influence of measures and initial status. Health Psychol. 2011;30:75–83. doi:10.1037/a0021567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ransford CP. A role for amines in the antidepressant effect of exercise: a review. Med Sci Sports Exerc. 1982;14:1–10. PMID:6280014 [DOI] [PubMed] [Google Scholar]
- 38. Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63 (5):530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.