Abstract
Introduction
Dextran sodium sulfate (DSS) is commonly used to induce a murine fulminant colitis model. Hepatocyte growth factor (HGF) has been shown to decrease the symptoms of inflammatory bowel disease (IBD) but the effect of its activator, HGFA, is not well characterized. Arginine reduces effects of oxidative stress but its effect on IBD is not well known. The primary aim is to determine whether HGF and HGFA, or arginine will decrease IBD symptoms such as pain and diarrhea in a DSS-induced fulminant colitis murine model.
Methods
A severe colitis was induced in young, male Fischer 344 rats with 4% (w/v) DSS oral solution for seven days; rats were sacrificed on day 10. Rats were divided into five groups of 8 animals: control, HGF (700 mcg/kg/dose), HGF and HGFA (10 mcg/dose), HGF and arginine, and high dose HGF (2800 mcg/kg/dose). Main clinical outcomes were pain, diarrhea and weight loss. Blinded pathologists scored the terminal ileum and distal colon.
Results
DSS reliably induced severe active colitis in 90% of animals (n = 36/40). There were no differences in injury scores between control and treatment animals. HGF led to 1.38 fewer days in pain (p = 0.036), while arginine led to 1.88 fewer days of diarrhea (P = 0.017) compared to controls. 88% of HGFA-treated rats started regaining weight (P < 0.001).
Discussion/Conclusion
Although treatment was unable to reverse fulminant disease, HGF and arginine were associated with decreased days of pain and diarrhea. These clinical interventions may reduce associated symptoms for severe IBD patients, even when urgent surgical intervention remains the only viable option.
Keywords: Inflammatory bowel disease, Fulminant colitis
Highlights
-
•
We developed a fulminant colitis model in adolescent rats.
-
•
The fulminant colitis model reproduces inflammatory bowel disease in humans.
-
•
The rats were treated with hepatocyte growth factor, its activator, and arginine.
-
•
The HGF treated rats had fewer days of pain.
-
•
The arginine treated rats had fewer days of diarrhea.
1. Introduction
The continuum of inflammatory bowel disease (IBD) includes Crohn's disease (CD), Ulcerative Colitis (UC) and indeterminate colitis. In adults and children alike, patients tend to present with abdominal pain, diarrhea, rectal bleeding, weight loss, and perforation [1], [2]. Children carry the additional burden of potential growth retardation in moderate to severe UC [3].
Goals of management are to control or minimize disease by suppressing inflammation and immune response modulation. Despite this, approximately 15–30% of patients undergo subtotal colectomy with end ileostomy due to disease progression, failure of medical therapy or personal preference. Another 20–30% of patients with fulminant colitis require urgent surgery [4], [5], [6], [7]. Although the focus of medical therapy for UC has been on suppression of inflammation, improved understanding is needed about the potential effects of growth factors geared at the mucosa in IBD.
Hepatocyte growth factor (HGF) is a heterodimer secreted by mesenchymal cells found in the liver, lung, central nervous system and the intestine and enhances epithelial cell proliferation in murine models [8]. HGF is known to decrease IBD symptomatology and lowers intestinal injury scores in IBD rat models [9], [10]. It has additional benefits of reducing inflammatory pathways and apoptosis in liver injury [11] and ischemia-reperfusion models [12]. Conversely, the effect of its activator, hepatocyte growth factor activator (HGFA), is not well delineated. Supplementing its activator should theoretically increase the effect of HGF and fulminant colitis recovery. Finally, arginine (ARG) is an amino acid that has protective effects against oxidative stress in healthy newborn rats, hypoxia-reoxygenation, exercise and diabetes [13], [14], [15]. It likely exerts effects via inducible nitric oxide synthase, which may increase epithelial wound repair [16]. Consequently, it may protect intestinal mucosa by ameliorating the inflammatory response seen so commonly amongst IBD patients.
We hypothesized clinical and pathologic improvement in colonic and terminal ileum mucosa in an experimental rat model of fulminant colitis versus control animals, by supplementing HGF treatment with HGFA and arginine. We also expected that HGF plus HGFA would synergistically promote earlier recovery from severe acute colitis in the rodent model. Primary endpoints included symptoms such as pain and diarrhea.
2. Materials and methods
2.1. Animal model
Following Institutional Animal Care and Use Committee approval (local IACUC protocol 210084), an active, severe colitis was induced in 40 young, male Fischer 344 rats (8–12 weeks of age) with a 4% (w/v) DSS oral solution for seven days via standard water bottles. The DSS solution was the water source for the animals during the period of injury. 40 rats were divided equally into 5 groups by treatment regimen. Group 1 (control, n = 8) was untreated and was a historical control; group 2 (HGF, n = 8) received 700 mcg/kg/dose of HGF (Genentech, South San Francisco, CA) subcutaneously on days 5, 7 and 9; group 3 (HGF+ARG) was given 700 mcg/kg/dose of HGF and administered arginine as a 1% (w/v) oral solution on days 5–10; group 4 (HGF+HGFA) was given 700 mcg/kg/dose of HGF and also given 10 mcg/dose of HGFA (human recombinant HGF activator, R&D Systems, Minneapolis, MN) subcutaneously on days 5 and 7; group 5 (HGFx4, high dose HGF) was given 2800 mcg/kg/dose of HGF. 700 mcg/kg/dose of HGF was chosen to mirror the total HGF dose of a prior model [10]. HGFA dosing was selected based on commercially available quantities, since there is no published data for HGFA dosing. Treatment started on day 5 based on our prior work that demonstrated the rats would have an ongoing active colitis. All rats were sacrificed on day 10 to allow for potential recovery after DSS injury ended on day 7. This would allow for five total days of treatment and corresponds to earlier surgical intervention, which is associated with improved postoperative outcomes [17]. This timing also corresponds to the point at which patients with steroid refractory UC should be considered for colectomy (5–7 days) [18]. One animal in the arginine group was sacrificed on day 9 due to significant pain, weight loss, lethargy and overall appearance.
2.2. Clinical monitoring
Rats were housed in standard polycarbonate individual caging systems. They were allowed access to standard rat chow and water ad libitum. On days 1–7, DSS was added to the water source in a 4% concentration (w/v) [16]. Similarly for HGF+ARG group, the water source also included arginine (1% w/v) on days 5–10 [16].
A veterinarian and veterinarian technologists closely monitored the rats. They were observed for overall health and their appearance (hunched, not groomed), presence of diarrhea (Type 6 or 7 on Bristol Stool Scale) [19] or gross blood, and need for pain medication was recorded. Rats were determined to be in pain if they maintained a hunched position or a ruffled, unkempt coat [20] and had binary documentation. If rats were deemed to be in pain on at least one of the twice-daily checks they were marked as being in pain for the day. Similarly, rats were marked as having diarrhea for the day if a single stool was diarrhea. Buprenorphine (0.01 mg/dose) was administered subcutaneously as necessary for pain control.
2.3. Histopathology
Rats were euthanized with carbon dioxide at time of necropsy. A midline laparotomy was used to harvest the similar segments of distal colon and distal ileum. The specimens were stored in 10% formalin, and delivered to two blinded pathologists. The small bowel was graded on a scale from 0 to 8 (0 = normal mucosa; 1 = subepithelial space at villus tip; 2 = extended subepithelial space; 3 = epithelial lifting along villus sides; 4 = denuded villi; 5 = loss of villus tissue; 6 = crypt layer infarction; 7 = transmucosal infarction; 8 = transmural infarction) [21]. The colon samples (Fig. 1) were graded on a scale from 0 to 3 (0 = no inflammation; 1 = mild inflammation with cryptitis; 2 = moderate inflammation with crypt abscesses; 3 = severe inflammation with crypt abscesses and surface erosion or ulceration) [10]. Injury scores were averaged between the two pathologists.
Fig. 1.
Hemotoxylin and eosin stained sections (40×) of colon from dextran sodium sulfate (DSS)-induced colitis in rats.
2.4. Statistical analysis
Analysis of variance (ANOVA) with Bonferroni correction was used to analyze the rats' appearance, pain, character of stool and percent weight loss. Binary logistic regression was used to determine if rats started to regain weight. ANOVA with Bonferroni correction was also used to analyze the rats' colon and small bowel injury scores. Statistical significance was set at P < 0.05.
2.5. Ethical considerations
Research on animals in this study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Research Council, 2010. This study was approved by the local Institutional Review Board and the Institutional Animal Care and Use Committee (IACUC) at the Madigan Army Medical Center. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. This work has been reported in line with the ARRIVE statement [22].
3. Results
Overall, 38 of 40 (95%) animals reliably developed an acute colitis; 36 (90%) animals developed a severe active colitis. Treatment with HGF, HGFA or arginine was unable to reverse this fulminant disease. Colon injury scores (out of 3) for the various groups were as follows: DSS = 3.00; HGF = 2.31; HGF + HGFA = 2.75; HGF + ARG = 3.00; HGFx4 = 2.69. Small bowel injury scores (out of 8) were: DSS = 2.63; HGF = 2.00; HGF + HGFA = 3.00; HGF + ARG = 2.25; HGFx4 = 2.50. There was no difference in colon or small bowel injury scores seen amongst treatment groups and the DSS-injured control animals (Table 1; colon P = 0.373; small bowel P = 0.545). All intervention groups had a significantly higher colon injury score than historical negative controls (Table 2). There was no difference between the small bowel injury scores of the DSS-injured animals and the treatment groups (Table 3).
Table 1.
Average ileum and colon injury scores by treatment vs. injured control animals (DSS alone).
| Treatment | Mean | Std. error | P value |
|---|---|---|---|
| Ileum (out of 8) | |||
| DSS alone | 2.63 | 0.35 | N/A |
| HGF | 2.00 | 0.35 | >0.999 |
| HGF+HGFA | 3.00 | 0.35 | >0.999 |
| HGF+ARG | 2.25 | 0.35 | >0.999 |
| HGFx4 | 2.50 | 0.35 | >0.999 |
| Colon (out of 3) | |||
| DSS alone | 3.00 | 0.27 | N/A |
| HGF | 2.31 | 0.27 | 0.81 |
| HGF+HGFA | 2.75 | 0.27 | >0.999 |
| HGF+ARG | 3.00 | 0.27 | >0.999 |
| HGFx4 | 2.69 | 0.27 | >0.999 |
Abbreviations: DSS = dextran sodium sulfate; HGF = hepatocyte growth factor; HFGA = hepatocyte growth factor activator; ARG = arginine; HGFx4 = high dose hepatocyte growth factor.
Table 2.
Difference between average colon injury scores: DSS vs. treatment vs. normal.
| (I) Treatment | (J) Treatment | Mean difference (I−J) | Std. error | P value | 95% Confidence interval |
|
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| DSS | HGF | 0.6875 | 0.36195 | 0.974 | −0.4443 | 1.8193 |
| HGF + ARG | 0.0000 | 0.36195 | >0.999 | −1.1318 | 1.1318 | |
| HGF + HGFA | 0.2500 | 0.36195 | >0.999 | −0.8818 | 1.3818 | |
| HGFx4 | 0.3125 | 0.36195 | >0.999 | −0.8193 | 1.4443 | |
| Normal | 3.0000∗ | 0.41269 | <0.001 | 1.7096 | 4.2904 | |
| HGF | DSS | −0.6875 | 0.36195 | 0.974 | −1.8193 | 0.4443 |
| HGF + ARG | −0.6875 | 0.36195 | 0.974 | −1.8193 | 0.4443 | |
| HGF + HGFA | −0.4375 | 0.36195 | >0.999 | −1.5693 | 0.6943 | |
| HGFx4 | −0.3750 | 0.36195 | >0.999 | −1.5068 | 0.7568 | |
| Normal | 2.3125∗ | 0.41269 | <0.001 | 1.0221 | 3.6029 | |
| HGF + ARG | DSS | 0.0000 | 0.36195 | >0.999 | −1.1318 | 1.1318 |
| HGF | 0.6875 | 0.36195 | 0.974 | −0.4443 | 1.8193 | |
| HGF + HGFA | 0.2500 | 0.36195 | >0.999 | −0.8818 | 1.3818 | |
| HGFx4 | 0.3125 | 0.36195 | >0.999 | −0.8193 | 1.4443 | |
| Normal | 3.0000∗ | 0.41269 | <0.001 | 1.7096 | 4.2904 | |
| HGF + HGFA | DSS | −0.2500 | 0.36195 | >0.999 | −1.3818 | 0.8818 |
| HGF | 0.4375 | 0.36195 | >0.999 | −0.6943 | 1.5693 | |
| HGF + ARG | −0.2500 | 0.36195 | >0.999 | −1.3818 | 0.8818 | |
| HGFx4 | 0.0625 | 0.36195 | >0.999 | −1.0693 | 1.1943 | |
| Normal | 2.7500∗ | 0.41269 | <0.001 | 1.4596 | 4.0404 | |
| HGFx4 | DSS | −0.3125 | 0.36195 | >0.999 | −1.4443 | 0.8193 |
| HGF | 0.3750 | 0.36195 | >0.999 | −0.7568 | 1.5068 | |
| HGF + ARG | −0.3125 | 0.36195 | >0.999 | −1.4443 | 0.8193 | |
| HGF + HGFA | −0.0625 | 0.36195 | >0.999 | −1.1943 | 1.0693 | |
| Normal | 2.6875∗ | 0.41269 | <0.001 | 1.3971 | 3.9779 | |
| Normal | DSS | −3.0000∗ | 0.41269 | <0.001 | −4.2904 | −1.7096 |
| HGF | −2.3125∗ | 0.41269 | <0.001 | −3.6029 | −1.0221 | |
| HGF + ARG | −3.0000∗ | 0.41269 | <0.001 | −4.2904 | −1.7096 | |
| HGF + HGFA | −2.7500∗ | 0.41269 | <0.001 | −4.0404 | −1.4596 | |
| HGFx4 | −2.6875∗ | 0.41269 | <0.001 | −3.9779 | −1.3971 | |
DSS = dextran sodium sulfate; HGF = hepatocyte growth factor; HFGA = hepatocyte growth factor activator; ARG = arginine; HGFx4 = high dose hepatocyte growth factor.
Table 3.
Difference between average small bowel injury scores: DSS vs. treatment vs. normal.
| (I) Treatment | (J) Treatment | Mean difference (I−J) | Std. error | P value | 95% Confidence interval |
|
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| DSS | HGF | 0.313 | 0.377 | >0.999 | −0.865 | 1.490 |
| HGF+ARG | 0.188 | 0.377 | >0.999 | −0.990 | 1.365 | |
| HGF+HGFA | −0.188 | 0.377 | >0.999 | −1.365 | 0.990 | |
| HGFx4 | 0.437 | 0.377 | >0.999 | −0.740 | 1.615 | |
| Normal | 2.750 | 0.429 | <0.001 | 1.407 | 4.093 | |
| HGF | DSS | −0.313 | 0.377 | >0.999 | −1.490 | 0.865 |
| HGF+ARG | −0.125 | 0.377 | >0.999 | −1.303 | 1.053 | |
| HGF+HGFA | −0.500 | 0.377 | >0.999 | −1.678 | 0.678 | |
| HGFx4 | 0.125 | 0.377 | >0.999 | −1.053 | 1.303 | |
| Normal | 2.437 | 0.429 | <0.001 | 1.095 | 3.780 | |
| HGF+ARG | DSS | −0.188 | 0.377 | >0.999 | −1.365 | 0.990 |
| HGF | 0.125 | 0.377 | >0.999 | −1.053 | 1.303 | |
| HGF+HGFA | −0.375 | 0.377 | >0.999 | −1.553 | 0.803 | |
| HGFx4 | 0.250 | 0.377 | >0.999 | −0.928 | 1.428 | |
| Normal | 2.562 | 0.429 | <0.001 | 1.220 | 3.905 | |
| HGF+HGFA | DSS | 0.188 | 0.377 | >0.999 | −0.990 | 1.365 |
| HGF | 0.500 | 0.377 | >0.999 | −0.678 | 1.678 | |
| HGF+ARG | 0.375 | 0.377 | >0.999 | −0.803 | 1.553 | |
| HGFx4 | 0.625 | 0.377 | >0.999 | −0.553 | 1.803 | |
| Normal | 2.937 | 0.429 | <0.001 | 1.595 | 4.280 | |
| HGFx4 | DSS | −0.437 | 0.377 | >0.999 | −1.615 | 0.740 |
| HGF | −0.125 | 0.377 | >0.999 | −1.303 | 1.053 | |
| HGF+ARG | −0.250 | 0.377 | >0.999 | −1.428 | 0.928 | |
| HGF+HGFA | −0.625 | 0.377 | >0.999 | −1.803 | 0.553 | |
| Normal | 2.312 | 0.429 | <0.001 | 0.970 | 3.655 | |
| Normal | DSS | −2.750 | 0.429 | <0.001 | −4.093 | −1.407 |
| HGF | −2.437 | 0.429 | <0.001 | −3.780 | −1.095 | |
| HGF+ARG | −2.562 | 0.429 | <0.001 | −3.905 | −1.220 | |
| HGF+HGFA | −2.937 | 0.429 | <0.001 | −4.280 | −1.595 | |
| HGFx4 | −2.312 | 0.429 | <0.001 | −3.655 | −0.970 | |
DSS = dextran sodium sulfate; HGF = hepatocyte growth factor; HFGA = hepatocyte growth factor activator; ARG = arginine; HGFx4 = high dose hepatocyte growth factor.
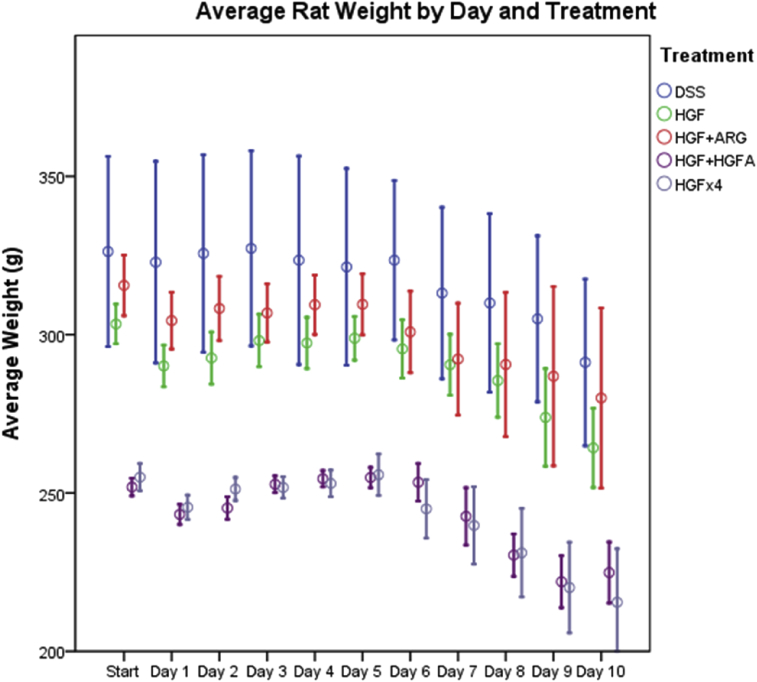
Treatment groups spent 0.88–1.38 fewer days in pain as compared to untreated control animals; however, only HGF-alone animals reached statistical significance with 1.38 less days of pain (P = 0.036, Table 4). Animals treated with HGF, high dose HGF and arginine also experienced 0.38–1.88 fewer days of diarrhea and hematochezia. Arginine supplementation was the only treatment with a significant difference with 1.88 fewer days of diarrhea (P = 0.017, Table 4). If it assumed that the one animal that was sacrificed on day 9 would also have had diarrhea on day 10, arginine supplementation still led to 1.75 fewer days of diarrhea (P = 0.028). There was no difference between groups with the amount of pain medication administered. Mean percent weight loss varied from 10.35% for untreated animals to 15.44% for high dose HGF treated animals (Table 4). Additionally, there was no significant difference in the overall percent weight loss amongst groups. However, 88% (7 out of 8) of HGFA-treated rats started to regain weight by day 10 (P < 0.001, Fig. 2).
Table 4.
Days with diarrhea, days in pain and percent weight loss. Treatment groups versus control.
| Treatment | Days with diarrhea | Days in Pain | % Weight Loss | |
|---|---|---|---|---|
| DSS alone | Mean | 4.13 | 1.87 | 10.35% |
| P value | n/a | n/a | n/a | |
| HGF | Mean | 3.38 | 0.50 | 12.94% |
| P value | 0.321 | *0.036 | 0.45 | |
| HGF+ARG | Mean | 2.25 | 1.00 | 11.26% |
| P value | *0.017 | 0.174 | 0.79 | |
| HGF+HGFA | Mean | 4.62 | 0.88 | 10.72% |
| P value | 0.506 | 0.122 | 0.914 | |
| HGFx4 | Mean | 3.75 | 0.63 | 15.44% |
| P value | 0.618 | 0.055 | 0.142 | |
DSS = dextran sodium sulfate; HGF = hepatocyte growth factor; HFGA = hepatocyte growth factor activator; ARG = arginine; HGFx4 = high dose hepatocyte growth factor.
Fig. 2.
Average rat weight by day and treatment.
4. Discussion
Toxic or fulminant colitis in humans with IBD remain challenging from a medical management perspective. Accurate modeling is essential for novel techniques. Our current rat model reproduced severe and fulminant disease in 90% of rats. This reliable model was used to evaluate the impact of HGF, HGFA and arginine.
Our results demonstrate low dose HGF is associated 1.38 fewer days of pain and oral arginine supplementation is associated with a significant decrease in the number of days of diarrhea and hematochezia. . Conversely, HGFA and high dose HGF were associated with a non-significant trend toward fewer days of pain with no differences between high and low dose HGF. This reinforces the concept of an HGF plateau point, after which increasing doses of HGF no longer have an effect [10].
There were no significant differences seen between treatment groups in percentage of weight loss. This may be limited by the duration of the study, since animals were only kept alive for 10 days post-DSS injury. Nearly all HGFA-treated animals (7 of 8, 88%) had started to regain weight by time of sacrifice (Fig. 2). This suggests that HGFA can potentially reverse cachexia and malnutrition seen in IBD, though a longer study period would is needed to fully evaluate this.
Unfortunately, the treatments showed no pathologic differences in colonic or small bowel samples. The majority of animals developed fulminant, unrecoverable disease over the course of the 5 treatment days. Although other groups have shown success with HGF treatment in both HLA-B27 transgene and trinitrobenzene sulfonic acid (TNBS) models, their animal models did not include toxic disease [8], [10]. This suggests that once IBD reaches toxic levels, disease is irreversible with currently available medical management modalities.
We acknowledge certain limitations to the present study. First, optimal dosing of HGFA is unknown in the literature and HGFA was administered in its zymogen form. HGFA is activated in vivo in response to tissue injury, likely by thrombin [23], [24], [25]; the DSS-induced colitis would have created the necessary tissue injury to activate HGFA. However, as a therapeutic agent, it may be more effective to activate HGFA in vitro prior to infusion. Finally, HGFA and arginine need to be compared as individual agents, instead of as combined supplements to HGF therapy. Additionally, arginine and DSS were administered as additives in drinking water. This relies on each rat to self-administer the colitis-inducing agent and the treatment, and does not ensure equal dosing. Lastly, intermittent subcutaneous injections of HGF may have led to variable concentrations, rather than continuous, therapeutic levels. Despite these limitations, we have demonstrated that a fulminant colitis model can be reliably created. We also reinforced that severe UC and fulminant colitis represent significant therapeutic challenges. The goal is to initiate therapy quickly, but not persist with futile treatment when surgery represents definitive care.
4.1. Conclusions
DSS can reliably create a significant and severe colitis in rats, replicating a human IBD model. Symptom improvement was demonstrated in the HGF and arginine groups however these treatments are unable to reverse fulminant disease. Therefore, HGA and arginine may improve clinical symptoms in IBD patients until an urgent/emergent operation is undertaken.
Ethical approval
Research on animals in this study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Research Council, 2010. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Sources of funding
Genentech provided hepatocyte growth factor.
NIH Surgical Oncology T32 CA163177.
Specific author contributions
Development of the protocol: Salgar, Zwintscher.
Performance of experiment: Salgar, Steele, Newton, Zwintscher.
Statistical Analysis: Steele, Zwintscher.
Writing of the Manuscript: Steele, Salgar, Zwintscher.
Critical Revision: Shah, Newton, Maykel, Samy, Jabir, Steele.
Conflicts of interest
The authors have no conflicts of interest.
Guarantor
Scott R. Steele.
Disclaimers
The clinical data in this manuscript was presented as a poster at the 2nd Annual European Paediatric Surgeons' Association – British Association of Paediatric Surgeons (EUPSA-BAPS) Joint Congress in Rome, Italy, June 13–16, 2012 and the pathology data was presented at the 8th Annual Academic Surgical Congress in New Orleans, LA, February 5–7, 2013. The views expressed are those of the author(s) and do not reflect the official policy of the Department of the Army, the Department of Defense or the U.S. Government. Research on animals in this study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Research Council, 2010. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Disclosure
All authors contributed significantly to the creation and revision of this manuscript. No authors have any significant disclosures related to this manuscript or its publication.
Acknowledgments
National Institutes of Health grant Surgical Oncology T32 CA163177 supported this work.
Contributor Information
Ahmed Samy, Email: Ahmedsamy.md@gmail.com.
Murad Jabir, Email: muradjab2010@gmail.com.
Scott R. Steele, Email: harkersteele@mac.com.
References
- 1.Turner D., Levine A., Escher J.C. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J. Pediatr. Gastroenterol. Nutr. 2012;55(3):340–361. doi: 10.1097/MPG.0b013e3182662233. [DOI] [PubMed] [Google Scholar]
- 2.Kugathasan S., Dubinsky M.C., Keljo D. Severe colitis in children. J. Pediatr. Gastroenterol. Nutr. 2005;41(4):375–385. doi: 10.1097/01.mpg.0000186272.65559.ce. http://www.ncbi.nlm.nih.gov/pubmed/16205502 (accessed 12.10.15) [DOI] [PubMed] [Google Scholar]
- 3.Cima R.R. Timing and indications for colectomy in chronic ulcerative colitis: surgical consideration. Dig. Dis. 2010;28(3):501–507. doi: 10.1159/000320409. [DOI] [PubMed] [Google Scholar]
- 4.Kirsner J.B. Historical origins of medical and surgical therapy of inflammatory bowel disease. Lancet (Lond. Engl.) 1998;352(9136):1303–1305. doi: 10.1016/S0140-6736(98)11132-7. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J.L., Strong S.A., Hyman N.H. Practice parameters for the surgical treatment of ulcerative colitis. Dis. Colon Rectum. 2005;48(11):1997–2009. doi: 10.1007/s10350-005-0180-z. [DOI] [PubMed] [Google Scholar]
- 6.Turner D., Travis S.P.L., Griffiths A.M. Consensus for managing acute severe ulcerative colitis in children: a systematic review and joint statement from ECCO, ESPGHAN, and the Porto IBD working group of ESPGHAN. Am. J. Gastroenterol. 2011;106(4):574–588. doi: 10.1038/ajg.2010.481. [DOI] [PubMed] [Google Scholar]
- 7.Hyman N.H., Cataldo P., Osler T. Urgent subtotal colectomy for severe inflammatory bowel disease. Dis. Colon Rectum. 2005;48(1):70–73. doi: 10.1007/s10350-004-0750-5. http://www.ncbi.nlm.nih.gov/pubmed/15690660 (accessed 12.10.15) [DOI] [PubMed] [Google Scholar]
- 8.Oh K., Iimuro Y., Takeuchi M. Ameliorating effect of hepatocyte growth factor on inflammatory bowel disease in a murine model. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288(4):G729–G735. doi: 10.1152/ajpgi.00438.2004. [DOI] [PubMed] [Google Scholar]
- 9.Ohda Y., Hori K., Tomita T. Effects of hepatocyte growth factor on rat inflammatory bowel disease models. Dig. Dis. Sci. 2005;50(5):914–921. doi: 10.1007/s10620-005-2664-z. http://www.ncbi.nlm.nih.gov/pubmed/15906768 (accessed 12.10.15) [DOI] [PubMed] [Google Scholar]
- 10.Arthur L.G., Schwartz M.Z., Kuenzler K.A., Birbe R. Hepatocyte growth factor treatment ameliorates diarrhea and bowel inflammation in a rat model of inflammatory bowel disease. J. Pediatr. Surg. 2004;39(2):139–143. doi: 10.1016/j.jpedsurg.2003.10.001. http://www.ncbi.nlm.nih.gov/pubmed/14966727 discussion 139-143. (accessed 12.10.15) [DOI] [PubMed] [Google Scholar]
- 11.Thatch K.A., Schwartz M.Z., Yoo E.Y., Mendelson K.G., Duke D.S. Modulation of the inflammatory response and apoptosis using epidermal growth factor and hepatocyte growth factor in a liver injury model: a potential approach to the management and treatment of cholestatic liver disease. J. Pediatr. Surg. 2008;43(12):2169–2173. doi: 10.1016/j.jpedsurg.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 12.Kuenzler K.A., Pearson P.Y., Schwartz M.Z. Hepatocyte growth factor pretreatment reduces apoptosis and mucosal damage after intestinal ischemia-reperfusion. J. Pediatr. Surg. 2002;37(7):1093–1097. doi: 10.1053/jpsu.2002.33884. http://www.ncbi.nlm.nih.gov/pubmed/12077779 discussion 1093-1097. (accessed 12.10.15) [DOI] [PubMed] [Google Scholar]
- 13.Kul M., Vurucu S., Demirkaya E. Enteral glutamine and/or arginine supplementation have favorable effects on oxidative stress parameters in neonatal rat intestine. J. Pediatr. Gastroenterol. Nutr. 2009;49(1):85–89. doi: 10.1097/MPG.0b013e318198cd36. [DOI] [PubMed] [Google Scholar]
- 14.Kochar N.I., Umathe S.N. Beneficial effects of L-arginine against diabetes-induced oxidative stress in gastrointestinal tissues in rats. Pharmacol. Rep. 2009;61(4):665–672. doi: 10.1016/s1734-1140(09)70118-5. http://www.ncbi.nlm.nih.gov/pubmed/19815948 (accessed 12.10.15) [DOI] [PubMed] [Google Scholar]
- 15.Huang C.-C., Lin T.-J., Lu Y.-F., Chen C.-C., Huang C.-Y., Lin W.-T. Protective effects of L-arginine supplementation against exhaustive exercise-induced oxidative stress in young rat tissues. Chin. J. Physiol. 2009;52(5):306–315. doi: 10.4077/cjp.2009.amh068. http://www.ncbi.nlm.nih.gov/pubmed/20034235 (accessed 12.10.15) [DOI] [PubMed] [Google Scholar]
- 16.Coburn L.A., Gong X., Singh K. L-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS One. 2012;7(3):e33546. doi: 10.1371/journal.pone.0033546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coakley B.A., Telem D., Nguyen S., Dallas K., Divino C.M. Prolonged preoperative hospitalization correlates with worse outcomes after colectomy for acute fulminant ulcerative colitis. Surgery. 2013;153(2):242–248. doi: 10.1016/j.surg.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Van Assche G., Vermeire S., Rutgeerts P. Treatment of severe steroid refractory ulcerative colitis. World J. Gastroenterol. 2008;14(36):5508–5511. doi: 10.3748/wjg.14.5508. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2746336&tool=pmcentrez&rendertype=abstract (accessed 12.10.15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis S.J., Heaton K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997;32(9):920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 20.Roughan J.V., Flecknell P.A. Evaluation of a short duration behaviour-based post-operative pain scoring system in rats. Eur. J. Pain. 2003;7(5):397–406. doi: 10.1016/S1090-3801(02)00140-4. [DOI] [PubMed] [Google Scholar]
- 21.Park P.O., Haglund U., Bulkley G.B., Fält K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery. 1990;107(5):574–580. http://www.ncbi.nlm.nih.gov/pubmed/2159192 (accessed 12.10.15) [PubMed] [Google Scholar]
- 22.Carol Kilkenny, William J Browne, Innes C Cuthill ME and DGA. ARRIVE guidelines | NC3Rs. National Centre for the Replacement Refinement and Reduction of Animals in Research. https://www.nc3rs.org.uk/arrive-guidelines. (accessed 12.10.15).
- 23.Shimomura T., Kondo J., Ochiai M. Activation of the zymogen of hepatocyte growth factor activator by thrombin. J. Biol. Chem. 1993;268(30):22927–22932. http://www.ncbi.nlm.nih.gov/pubmed/8226803 (accessed 12.10.15) [PubMed] [Google Scholar]
- 24.Kataoka H., Kawaguchi M. Hepatocyte growth factor activator (HGFA): pathophysiological functions in vivo. FEBS J. 2010;277(10):2230–2237. doi: 10.1111/j.1742-4658.2010.07640.x. [DOI] [PubMed] [Google Scholar]
- 25.Miyazawa K. Hepatocyte growth factor activator (HGFA): a serine protease that links tissue injury to activation of hepatocyte growth factor. FEBS J. 2010;277(10):2208–2214. doi: 10.1111/j.1742-4658.2010.07637.x. [DOI] [PubMed] [Google Scholar]