Abstract
Background
The vast majority of abdominal aortic aneurysms found in screening programs are small, and as no effective treatment exits, many will expand until surgery is indicated. Therefore, it remains intriguing to develop a safe and low cost treatment of these small aneurysms, that is able to prevent or delay their expansion.
In this study, we investigated whether intraluminal delivered pentagalloyl glucose (PGG) can impair the early AAA development in a porcine model.
Methods
The infrarenal aorta was exposed in thirty pigs. Twenty underwent an elastase based AAA inducing procedure and ten of these received an additional intraluminal PGG infusion. The final 10 were sham operated and served as controls.
Results
All pigs who only had an elastase infusion developed macroscopically expanding AAAs. In pigs treated with an additional PGG infusion the growth rate of the AP-diameter rapidly returned to physiological values as seen in the control group. In the elastase group, histology revealed more or less complete resolution of the elastic lamellae in the media while they were more abundant, coherent and structurally organized in the PGG group. The control group displayed normal physiological growth and histology.
Conclusion
In our model, intraluminal delivered PGG is able to penetrate the aortic wall from the inside and impair the early AAA development by stabilizing the elastic lamellae and preserving their integrity. The principle holds a high clinical potential if it can be translated to human conditions, since it, if so, potentially could represent a new drug for stabilizing small abdominal aneurysms.
Keywords: Abdominal aortic aneurysm, Animal model, Pentagalloyl glucose, AAA inhibition
Highlights
-
•
Pentagalloyl glucose impairs the early AAA development in a porcine model.
-
•
Pentagalloyl glucose stabilizes arterial elastic lamellae and preserves their integrity.
-
•
Pentagalloyl glucose can penetrate the arterial wall in large AAA prone arteries from the inside.
-
•
Pentagalloyl glucose, a potential new drug for stabilizing small abdominal aneurysms.
1. Introduction
It is estimated that the global number of aortic aneurysm related deaths has increased from around a 100.000 in 1990 to around 152.000 in 2013, corresponding to a 52% increase [1].
The risk of developing an abdominal aortic aneurysm (AAA) increases with age, and screening programs have revealed a prevalence of about 3.3–4.9% in European men aged 64–73 (highest in the Caucasian subgroup) [2], [3], [4], [5]. The MASS study found that the majority (71%) of these aneurysms were small 3–4.4 cm. A definitive surgical treatment (open or endovascular) is in general only recommended for aneurysms with an external diameter ≥ 5.5 cm, aneurysms that expand >1 cm/year or become symptomatic [6], [7]. However, since smaller aneurysms (3.5–4 cm) grow on average 2.5–3 mm/year with an exponentially increasing growth rate of about 1.62 mm/year per 1-cm increase in aneurysm diameter, many will eventually reach a size where surgery is indicated. It has been found, that 3.5 cm and 4.5 cm AAAs would take on average 6.2 years and 2.3 years respectively to reach 5.5 cm [7], [8].
Despite intensive research into the complex multifactorial etiopathogenesis of this degenerative disease, the exact mechanisms involved are not yet fully understood and no effective medical treatment exits.
Therefore, it remains intriguing to develop a safe and low cost treatment (medically/minimal invasive) of these small aneurysms, that is able to prevent or delay their expansion.
Increased proteolytic elastase and collagenase activity leading to progressive degradation of the aortic walls connective tissue resulting in decreased tensile strength, has been a key early finding [9], [10]. In vivo stabilization of infrarenal aortic elastin by means of periadventitially delivered pentagalloyl glucose (PGG) in adult male Sprague-Dawley rats, exposed to CaCl2-mediated aortic elastin injury, has shown to prevent early AAA formation by binding to elastin and thereby preserving the integrity of the elastic lamellae [11]. However, translation of experimental results in rodents to humans has shown difficulties. Consequently, we hypothesized, that intraluminal delivered PGG into the infrarenal aortic segment of our previously described elastase based porcine AAA model in the 30–38 kg weight range [12], also is able to penetrate the arterial wall in large arteries from the inside and stabilize the elastic lamellae, preserving their integrity and hereby impair the early development of AAAs in our model.
2. Materials and methods
Thirty female Danish Landrace pigs in the 30–38 kg weight range were divided into 3 groups of 10. Group A were subjected to balloon dilatation, elastase and a juxtrarenal stenosing cuff (n = 10, mean weight 34 kg (range 31–38 kg)). Group B were subjected to balloon dilatation, elastase, PGG and a juxtrarenal stenosing cuff (n = 10, mean weight 33 kg (range 31–35 Kg)), and control group C underwent a sham procedure (n = 10, mean weight 34 kg (range 30–38 kg)).
2.1. Anesthesia and surgical procedure
Anesthesia was induced by im. injection (mg/kg BW) of 1.25 mg tiletamine, 1.25 mg zolazepam, 0.25 mg butorphanol, 1.25 mg ketamine and 1.25 mg xylazine. After tracheal intubation, the animals were placed in the supine position and ventilated with oxygen 4 L/min and atm. air (1:1, v/v) and anesthesia was extended by continuous intravenous infusion of 10 mg propofol and 25 μg fentanyl per kg BW/h.
With the animals in the supine position a transabdominal ultrasound scan of the infrarenal aorta was performed in both the transverse and longitudinal plane to measure the systolic preoperative external anterior-posterior diameter (AP0).
Through a midline longitudinal laparotomy and a retrocolic prerenal transperitoneal approach the aorta was dissected from the lowest renal artery to the trifurcation, whereafter the lumbars, inferior mesenteric artery and the aorta itself were temporarily clamped. Group C had the clamps removed after 30 min. In group A and B a 2.5 mm arteriotomy in the aorta was made at the level of the inferior mesenteric artery through which a 10 mm × 4 cm high-pressure balloon catheter was placed and inflated for 5 min. Hereafter a curved beaded knop needle was introduced through which 10 ml of porcine pancreatic elastase (Sigma-Aldrich Denmark A/S, E1250-100MG, Type-I ≥4.0 units'/mg protein) was gradually manually infused into the aortic lumen over 30 min. Then a 12 mm × 4 cm high-pressure balloon catheter was introduced and inflated for 5 min. After the second balloon dilatation the first five pigs in group B had 25 MG and the last five pigs in group B had 50 MG of PGG (Penta-O-galloyl-β-d-glucose hydrate, Sigma-Aldrich Denmark A/S, G7548, ≥96% (HPLC)) dissolved in 10 ml of isotonic saline infused into the lumen and flushed for 30 min. Hereafter the arteriotomy was sutured and the aorta declamped. To establish turbulent flow in the infrarenal aortic segment of group A and B, a stenosing nylon cable-tie strap (120 mm × 5 mm) was placed as a cuff around the aorta just below the renal arteries and narrowed until a thrill indicating turbulent flow in the infrarenal aortic segment could be palpated and postoperatively be visualized by Doppler sonography. Finally, the retroperitoneum and laparotomy were closed.
2.2. Postoperative care
Postoperative the animals were monitored the subsequent 28 days. On the 3rd 7th 14th 21st and 28th postoperative days the pigs were anaesthetized and the AP-diameter of the infrarenal aorta was again measured using transabdominal ultrasound. Hereafter the animals were euthanized with a lethal overdose of intravenous phenobarbital and the infrarenal aortic segment was removed and fixed in 10% buffered formalin.
2.3. Histology
The specimens were sliced and stained with routine protocols for human tissue for x100 and x400 microscopy with hematoxylin and eosin stain, Verhoeff's stain for elastin and immunohistochemical staining for human smooth muscle actin (that was found to react satisfactory with tissue from pigs). Actin staining was done in a Ventana autostainer using Actin, Muscle Specific Antibody clone HHF35 from CELL MARQUE (6600 Sierra College Blvd. Rocklin, CA 95677 USA.) that labels smooth and striated muscle cells.
2.4. Statistics
Using mixed models for repeated measures a multivariate analysis of variance (MANOVA) was performed to compare the correlations of the aortic AP-diameters in the three groups over time. In order to determine whether the slopes of the increase in AP-diameter between the groups were comparable Wald chiˆ2 tests were carried out. One-way ANOVAs were used to compare mean weights between groups. Results were presented as means ± SD and with 95% confidence intervals P-values < 0.05 were considered significant.
3. Results
The three groups (A, B and C) were comparable in terms of mean baseline weight Kg0 (P = 0.45) and AP-diameter AP0 (P = 0.36). Throughout the experiment the mean weight did not differ significantly between the three groups (Table 1).
Table 1.
Schematic representation of the comparison between Group A, B and C.
| Group A/Group B/Group C | Day 0 | Day 3 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|---|
| Mean weight, Kg ± SD | ||||||
| Group A | 33.8 ± 2.44 | 34.3 ± 2.66 | 36.2 ± 1.99 | 40.7 ± 2.41 | 45.9 ± 2.69 | 50.4 ± 3.37 |
| Group B | 32.8 ± 1.62 | 34.2 ± 1.55 | 36.7 ± 1.42 | 40.3 ± 1.57 | 46.1 ± 1.52 | 51.8 ± 2.1 |
| Group C | 34.0 ± 2.58 | 34.4 ± 3.31 | 37.8 ± 2.10 | 42.2 ± 1.76 | 46.1 ± 3.05 | 51.2 ± 2.39 |
| P values | 0.45 | 0.99 | 0.16 | 0.09 | 0.98 | 0.51 |
| Mean AP diameter, mm ± SD | ||||||
| Group A | 10.36 ± 0.11 | 11.6 ± 0.18 | 12.85 ± 0.32 | 13.83 ± 0.44 | 14.74 ± 0.54 | 16.26 ± 0.93 |
| Group B | 10.28 ± 0.15 | 11.45 ± 0.15 | 11.57 ± 0.18 | 11.68 ± 0.12 | 11.83 ± 0.17 | 12.17 ± 0.13 |
| Group C | 10.37 ± 0.19 | 10.46 ± 0.13 | 10.64 ± 0.11 | 10.87 ± 0.19 | 11.06 ± 0.2 | 11.33 ± 0.13 |
| P values | 0.36 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Mean increase, %a ± SD | ||||||
| Group A | 11.98 ± 2.62 | 24.04 ± 3.67 | 33.52 ± 5.19 | 42.32 ± 6.31 | 57.03 ± 10.17 | |
| Group B | 11.39 ± 1.2 | 12.56 ± 1.55 | 13.64 ± 1.88 | 15.09 ± 1.92 | 18.41 ± 2.11 | |
| Group C | 0.89 ± 1.59 | 2.63 ± 1.91 | 4.85 ± 1.85 | 6.69 ± 2.14 | 9.26 ± 1.37 | |
| Identical expansion rates/slopes | Group A:B | Group B:C | Group A:B | Group B:C | ||
| P values | 0.45 | <0.001 | <0.001 | 0.06 | ||
Mean increase, % with respect to the preoperative/Day 0 aortic AP-diameter.
The mixed models MANOVA analysis revealed a significant difference in AP-development between the groups (P < 0.001) (Table 1).
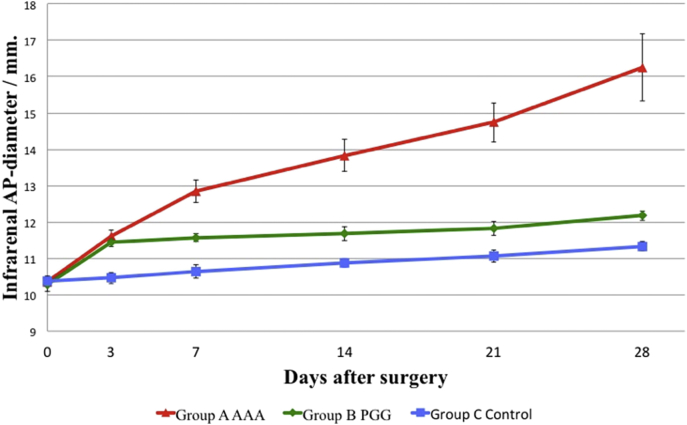
After 28 days all pigs in group A developed macroscopically AAAs with a mean increase in AP-diameter to 16.26 ± 0.93 mm equivalent to an increase of 57% ± 10.17 SD (Range 31%). In group B the mean increase was lower and equal to 12.17 ± 0.13 corresponding to 18.4% ± 2.11 SD (Range 6.5%) and whether the PGG dose given was 25 or 50 MG had no clear influence on the resulting expansion. In control group C there were no signs of developing aneurysms, as mean AP-diameter only increased to 11.33 ± 0.13 mm equivalent to 9.3% ± 1.37 SD (Range 3.5%) in accordance with normal physiological growth (Table 1 and Fig 1).
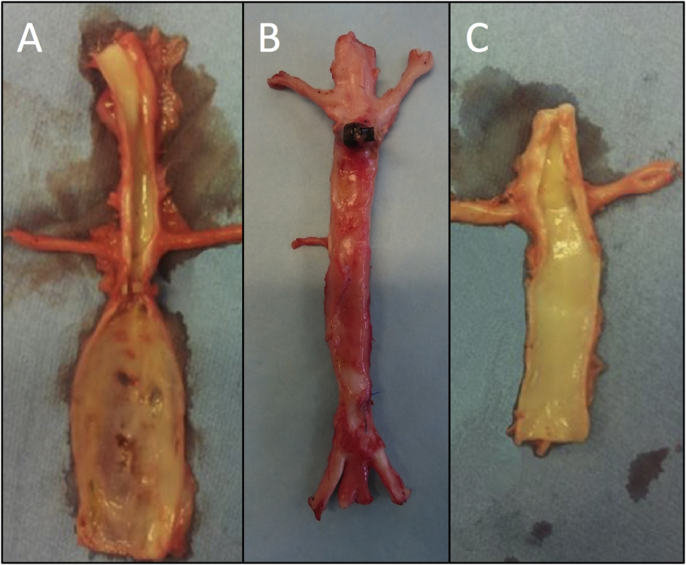
Fig. 1.
Notice the significantly lager infrarenal AP-diameter (AP28) in group A (left side) 28 days after surgery compared to the PGG treated group B aorta in the middle (not opened) and control group C (right side). Please note that the shown specimens representing group A and C are specimens from our previous study [12].
The Wald chiˆ2 analysis revealed that group A and B had identical expansion rates from day 0 to day 3 (P = 0.45) but a significant reduction in the expansion rate in group B compared to group A was seen from day 3 and onwards (P < 0.001) (Table 1 and Fig 2).
Fig. 2.
In group A a progressive aneurysmatic expansion of the infrarenal aortas were seen. After the first postoperative measurement the slope of the increase in AP-diameter in group B and C were a like, indicating that the expansion rate of the AP-diameter in Group B had returned to physiological values, as control group C only showed physiological expansion/growth.
The final mean AP-diameter (AP28) in group B was significantly larger than in group C (P < 0.001), but when adjusted for the immediate mechanical dilating effect of the balloon dilatation subjected to group B, by calculating the increase from the day 3 (AP3) measurement, we saw no significant difference in the expansion between group B and C (0.72 mm ± 0.07 SD vs. 0.87 mm ± 0.04 SD). After day 3 the Wald chiˆ2 test confirmed identical slopes in group B and C (P = 0.06), indicating that the growth rate of the AP-diameter in group B had returned to physiological values after day 3 (Table 1 and Fig. 2).
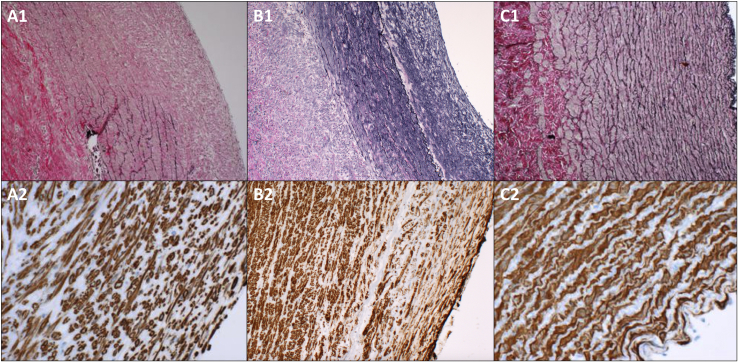
Microscopy of the removed aortic segments showed a preserved endothelium in all groups and as previously described more or less complete resolution of the elastic tissue in the tunica media in group A. In small areas with partially preserved elastic lamellae (all localized in the anti-luminal side of the tunica media) the fibers seemed disorganized, thinned and fragmented. In group B, the elastic fibers also seemed depleted in the luminal side of the media but more abundant, coherent and structurally organized towards the anti-luminal side, indicating possible preserved integrity with elastic properties (Fig. 3). In group A and B the Immunoperoxidase staining for smooth muscle actin revealed light to moderate irregular scattered focal muscle atrophy in the tunica media.
Fig. 3.
Histological findings in the three groups. First, second and third column representing group A, B and C respectively (the luminal side to the right). Top row: Verhoeff's stain for elastic tissue enlarged x100. While the elastic lamellae in group A are very dissolved, disorganized, thinned and fragmented, the fibers in group B seem more abundant and organized indicating preserved integrity, especially towards the anti-luminal side in the media. Bottom row: Immunoperoxidase staining for smooth muscle actin enlarged x400 (B2 x100) revealing light to moderate focal muscle atrophy towards the luminal side of the media in group A and B. Group C reveals normal aortic histology in both stains. Please note that the shown specimens representing group A and C are specimens from our previous study [12].
Widespread calcifications in the lamina elastica externa were seen in group A and B, but only invasion of a few inflammatory cells could be seen in the media and adventitia using a standard hematoxylin and eosin stain.
The tunica adventitia appeared largely unaffected. Group C displayed normal histology in all layers (Fig. 3).
4. Discussion
Subjecting the pigs for these three different types of operations resulted in a significant difference in AP-development. Group A developed AAAs with a mean increase in AP-diameter of 57%. In the PGG treated group B the growth rate rapidly returned to physiological values and the final increase was 18.4%. In group C the increase in AP-diameter was only 9.3% in accordance with normal physiological growth.
Histology revealed more or less complete resolution of the elastic tissue in the tunica media in group A, but in group B they were more abundant, coherent and structurally organized indicating possible preserved integrity with elastic properties. Only a few inflammatory cells could be seen in the media and adventitia.
Despite our open approach we have shown, that also endovascular delivered PGG is able to penetrate the arterial wall and stabilize the elastic lamellae from the inside in large AAA prone arteries, and we believe this route is more clinically feasible.
As mentioned, this study is partly based upon data derived from our previous described elastase based porcine AAA model [12], where we operated and obtained the data from the pigs in group A and C. This might be seen as a limitation, but due to animal ethics/economic reasons, we chose to reuse these data and combine these with the newly operated pigs in group B. However, we think the groups and their data are still comparable, as we used pigs that were comparable at baseline (same race and sex from the same breeder, the same mean weight, the same surgeon doing the same type of surgery and the same operative- and postoperative facilities etc.).
Previous studies of the protective properties of PGG on the elastic fibers in AAA prone arteries have been carried out in a rat model [11]. While the main results in that rat study and our study are fairly alike, we believe that this pig model is more transformable to the human setting, due to the physical size of the vasculature with similar arterial morphology.
In contrast to the rat study, we did not clarify as to how far PGG also is able to limit AAA progression in established expanding aneurysms. This study would have been obvious since it, if so, potentially could have a great clinical impact, as such treatment could hold the potential also to be beneficial in larger aneurysms not suitable to repair due to e.g. comorbidity or hostile anatomy. Since we believe, such a study ideally should be carried out by means of a totally endovascular approach, and since we did not have the requisite facilities to perform such interventions, we chose to concentrate on the impairment of early AAA formation.
The human AAA etiopathogenesis has in short been described as a chronic inflammatory condition with a proteolytic imbalance. Besides increased proteolytic elastase and collagenase activity, a chronic inflammatory condition in the aortic wall, dominated by macrophages, monocytes, lymphocytes, and plasma cells which excrete cytotoxic mediators, with further proteolytic activities and thus induces additional elastolytic breakdown and smooth muscle apoptosis, is also an important characteristic that further weakens the aortic wall [13], [14], [15]. Microscopy of a specimen removed 24 h after surgery (intended to be in group A but excluded due to lower limp paralysis), revealed a severe inflammatory response in the media with invasion of inflammatory cells.
In this study however, only very sparse inflammatory changes could be detected at the time of harvest on the 28th postoperative day in group A and B. Attenuating inflammation over time is in contrast to the nature of human AAAs, which could imply, that the porcine aorta has a partial healing potential. This theory, is supported by the findings of Hynecek et al. [16], who in a similar porcine model of AAAs reported a decrease in inflammation, reendothelialization of the damaged intimal surface, recovery of smooth muscle cells, but no sign of recovery or reorganization of the damaged elastic fibers after 6 weeks. Similar findings of such a self-healing phenomenon has been reported in elastase based rabbit aneurysm models [17], [18], [19] and has been described as the “response to injury” hypothesis. In these models, smooth muscle cell proliferation and elastin regeneration occurs after AAA induction, and even gradual aneurysm shrinkage with return of near normal diameter by day 90 has been reported [20].
Whether regeneration of the elastic lamellae, in an induced porcine AAA also is possible after smooth muscle recovery, remains to be investigated by an even longer follow-up time than 6 weeks. However, since spontaneous elastic recovery does not seem possible within the first 6 weeks, we believe that the infused PGG has exercised an immediate stabilizing effect on the damaged fibers and not resulted in any accelerated healing. Therefore, PGG may only stabilize the aortic wall and prevent or delay further accelerated aneurysmal dilatation due to progressive elastic deterioration and not reverse the disease itself.
As our AAA model doesn't display chronic inflammation, further investigations into the natural history/pathogenesis of AAAs and the potential treatment options that involves these inflammatory mechanisms, and then translating the findings to human conditions using this model may be biased, as it does not look to be pathogenically identical. However, as Jason et al. [11] demonstrated that PGG binds directly to the elastic lamellae and hereby strengthens the aneurysm wall itself without affecting the inflammatory response, and as we have demonstrated that PGG also impairs the early AAA development in our model without being dependent on an inflammatory infiltrate, we believe that PGG exerts its effect independently of these inflammatory mechanisms. Consequently, we believe that endovascular PGG infusion impairs the early development of AAAs in our model by preserving the integrity of elastic lamellae, making the aortic wall resistant to both enzymatic degradation and increased shear stress, and that PDD therefore might be considered tested in humans, despite our models general lack of applicability to human AAAs regarding the inflammatory pathophysiology.
However, questions to be answered before that; Although PGG is found naturally as a polyphenolic tannin in e.g. pomegranates, whose juice/seeds is known for a wide range of therapeutic/preventive properties [21], it is not known what side effects may occur when it in its pure form is added directly into the circulation (will it stiffen the entire arterial system or cause any organ damage etc.), therefore further studies are needed to clarify these issues. As a consequence, PGG may not be given as a systemic drug, and has to be delivered locally. Today, drug-coated balloons (DCBs) and drug-eluting stents (DESs) exists for the treatment of atherosclerotic peripheral and coronary artery disease [22], [23], and such devises could potentially be developed for aortic dimensions. Tensiometric measurements of human AAA walls may then be needed to clarify at which pressure the drug will be eluted. The next question would be, whether 30 min of perfusion with PGG is needed or whether it can be reduced considerably, as 30 min will require some “clamping time” and thus result in hemodynamic cardiac changes causing increased risks of complications. In addition, will it diffuse through a mural thrombus? We believe so, as the physiological transport in the aortic wall, owing to the pressure generated in the bloodstream, occurs mainly by radial/orthogonal hydraulic conductance, resulting in a centrifugal convection that transports soluble substances from the blood/lumen outwards through the arterial wall to the adventitia [24], a transport which is probably enhanced at the site of an AAA due to the damaged endothelial and medial layers. Such convection of PGG through a thrombus is most likely plausible due to the high permeability of macromolecules through the thrombus due to its network of interconnected canaliculi [25].
Ethical approval
The procedure was conducted under local project license J.nr. 2014-15-2934-01031 in conformity with the Danish legislations regarding animal welfare and experimental surgery. During the operative procedure and the 28 days' observation period, the pigs were housed at the DCA – Danish Centre for Food and Agriculture, Aarhus University Foulum.
Sources of funding
Health Research Fund of Central Denmark Region. The Danish Heart Foundation, grant number 10-04-R78-A2817-22602. The funding sources had no involvement in the in study design, data collection, analysis, interpretation of data or in the writing or the decision to submit this article for publication.
Author contribution
Brian O. Kloster: Study design, data collection, data analysis, and writing. Jes S. lindholt: Study design, data analysis and writing. Lars Lund: Study design, and writing.
Conflicts of interest
The authors declare no conflicts of interest.
Guarantor
Brian O. Kloster.
Disclosure statement
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the Health Research Fund of Central Denmark Region and The Danish Heart Foundation grant number 10-04-R78-A2817-22602. The funding sources had no involvement in the in study design, collection, analysis, interpretation of data or in the writing or the decision to submit this article for publication. Pathologist Gorm Soendergaard is thanked for the histological analysis.
Contributor Information
Brian O. Kloster, Email: Brian.Kloster@ki.au.dk.
Lars Lund, Email: lars.lund@rsyd.dk.
Jes S. Lindholt, Email: Jes.Sanddal.Lindholt@rsyd.dk.
References
- 1.Murray C.J.L. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015 January 10;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashton H.A., Buxton M.J., Day N.E., Kim L.G., Marteau T.M., Scott R.A. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360(9345):1531–1539. doi: 10.1016/s0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- 3.Lindholt J.S., Juul S., Fasting H., Henneberg E.W. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ. 2005;330:750–753. doi: 10.1136/bmj.38369.620162.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grøndal N., Søgaard R., Lindholt J.S. Baseline prevalence of abdominal aortic aneurysm, peripheral arterial disease and hypertension in men aged 65–74 years from a population screening study (VIVA trial) Br. J. Surg. 2015 Jul;102(8):902–906. doi: 10.1002/bjs.9825. [DOI] [PubMed] [Google Scholar]
- 5.Salem M.K., Rayt H.S., Hussey G., Rafelt S., Nelson C.P. Should asian men be included in abdominal aortic aneurysm screening programmes? Eur. J. Vasc. Endovasc. Surg. 2009;38:748–749. doi: 10.1016/j.ejvs.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch A.T., Haskal Z.J., Hertzer N.R., Bakal C.W., Creager M.A., Halperin J.L. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American association for vascular surgery/society for vascular surgery, society for cardiovascular angiography and interventions, society for vascular medicine and biology, society of interventional radiology, and the ACC/AHA task force on practice guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease): endorsed by the American association of cardiovascular and pulmonary rehabilitation; national heart, lung, and blood institute; society for vascular nursing; trans Atlantic inter-society consensus; and vascular disease foundation. Circulation. 2006 Mar 21;113(11):463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 7.Moll F.L., Powell J.T., Fraedrich G., Verzini F., Haulon S., Waltham M. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur. J. Vasc. Endovasc. Surg. 2011;41:1–58. doi: 10.1016/j.ejvs.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Schlösser F.J.V., MJD Tangelder, Verhagen H.J.M., GJMG van der Heijden, Muhs B.E., van der Graaf Y. Growth predictors and prognosis of small abdominal aortic aneurysms. J. Vasc. Surg. 2008;47:1127–1133. doi: 10.1016/j.jvs.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 9.Cohen J.R., Mandell C., Chang J.B., Wise L. Elastin metabolism of the infrarenal aorta. J. Vasc. Surg. 1988:210–214. doi: 10.1067/mva.1988.avs0070210. [DOI] [PubMed] [Google Scholar]
- 10.Diehm N., Dick F., Schaffner T., Schmidli J., Kalka C., Di Santo S. Novel insight into the pathobiology of abdominal aortic aneurysm and potential future treatment concepts. Prog. Cardiovasc. Dis. 2007 Nov-Dec;50(3):209–217. doi: 10.1016/j.pcad.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Isenburg Jason C., Simionescu Dan T., Starcher Barry C., Vyavahare Narendra R. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation. 2007;115:1729–1737. doi: 10.1161/CIRCULATIONAHA.106.672873. [DOI] [PubMed] [Google Scholar]
- 12.Kloster B.O., Lund L., Lindholt J.S. Induction of continuous expanding infrarenal aortic aneurysms in a large porcine animal model. Ann. Med. Surg. 2015;4(1):30–35. doi: 10.1016/j.amsu.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindeman J.H., Abdul-Hussien H., Schaapherder A.F., Van Bockel J.H., Von der Thüsen J.H., Roelen D.L. Enhanced expression and activation of pro-inflammatory transcription factors distinguish aneurys- mal from atherosclerotic aorta: IL-6- and IL-8-dominated inflammatory responses prevail in the human aneurysm. Clin. Sci. (Lond.) 2008;114:687–697. doi: 10.1042/CS20070352. [DOI] [PubMed] [Google Scholar]
- 14.Abdul-Hussien H., Hanemaaijer R., Kleemann R., Verhaaren B.F.J., van Bockel J.H. The pathophysiology of abdominal aortic aneurysm growth: corresponding and discordant inflammatory and proteolytic processes in abdominal aortic and popliteal artery aneurysms. J. Vasc. Surg. 2010;51:1479–1487. doi: 10.1016/j.jvs.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 15.Lindholt J.S., Shi G.P. Chronic inflammation, immune response, and infection in abdominal aortic aneurysms. Eur. J. Endovasc. Surg. 2006;31:453–463. doi: 10.1016/j.ejvs.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Hynecek Robert L., DeRubertis Brian G., Trocciola Susan M., Zhang Honglei, Prince Martin R. The creation of an infrarenal aneurysm within the native abdominal aorta of swine. Surgery. 2007;142:143–149. doi: 10.1016/j.surg.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Bi Y., Zhong H., Xu K., Qi X., Zhang Z., Wu G., Han X. Novel experimental model of enlarging abdominal aortic aneurysm in rabbits. J. Vasc. Surg. 2015 Oct;62(4) doi: 10.1016/j.jvs.2014.02.062. [DOI] [PubMed] [Google Scholar]
- 18.Bi Y., Xu K., Zhong H., Qi X., Zhang Z., Ni Y.J. A novel in vivo rabbit model of abdominal aortic aneurysm induced by periarterial incubation of papain. Vasc. Interv. Radiol. 2012 Nov;23(11):1529–1536. doi: 10.1016/j.jvir.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Bi Y., Zhong H., Xu K., Ni Y., Qi X., Zhang Z., Li W. Performance of a modified rabbit model of abdominal aortic aneurysm induced by topical application of porcine elastase: 5-month follow-up study. Eur. J. Vasc. Endovasc. Surg. 2013 Feb;45(2) doi: 10.1016/j.ejvs.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Origuchi N., Shigematsu H., Izumiyama N., Nakamura K., Toku A., Muto T. Aneurysm induced by periarterial application of elastase heals spontaneously. Int. Angiol. 1998;17 113e9. [PubMed] [Google Scholar]
- 21.Zarfeshany A., Asgary S., Javanmard S.H. Potent health effects of pomegranate. Adv. Biomed. Res. 2014;3:100. doi: 10.4103/2277-9175.129371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeller T., Rastan A., Macharzina R., Beschorner U., Noory E. Novel approaches to the management of advanced peripheral artery disease: perspectives on drug-coated balloons, drug-eluting stents, and bioresorbable scaffolds. Curr. Cardiol. Rep. 2015 Sep;17(9):624. doi: 10.1007/s11886-015-0624-6. [DOI] [PubMed] [Google Scholar]
- 23.Stefanini Giulio G., Holmes David R., Jr. Drug-eluting coronary-artery stents. N. Engl. J. Med. 2013;368:254–265. doi: 10.1056/NEJMra1210816. [DOI] [PubMed] [Google Scholar]
- 24.Lacolley Patrick. Véronique Regnault, Antonino Nicoletti, Zhenlin Li, Jean-Baptiste Michel. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res. 2012 Jul 15;95(2):194–204. doi: 10.1093/cvr/cvs135. [DOI] [PubMed] [Google Scholar]
- 25.Adolph R., Vorp D.A., Steed D.L., Webster M.W., Kameneva M.V., Watkins S.C. Cellular content and permeability of intraluminal thrombus in abdominal aortic aneurysm. J. Vasc. Surg. 1997;25:916–926. doi: 10.1016/s0741-5214(97)70223-4. [DOI] [PubMed] [Google Scholar]