Figure 2.
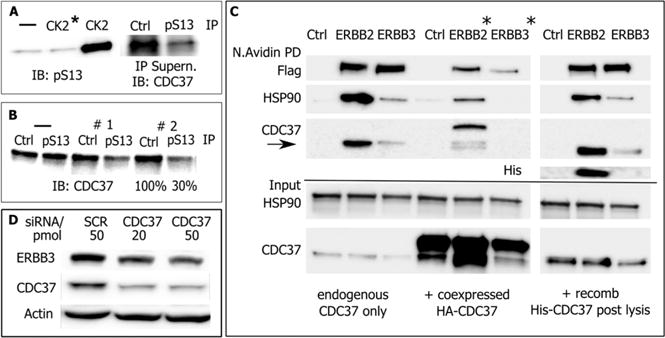
Evaluation of E. coli expressed CDC37. (A) (Left) In vitro phosphorylation of E. coli expressed His-CDC37/Ser13 probed by phosphorylation-site-specific antibody (IB: pSer). The control reaction with heat-deactivated CK2 is marked (*). (Right) Estimate of phosphorylation efficiency for in vitro phosphorylated E. coli expressed CDC37. pSer13/CDC37 was removed by three consecutive immunoprecipitations using immobilized pSer13 antibody or resin control. Approximately 85% of CDC37 was removed from the supernatant, and the remainder was detected by CDC37 antibody. (B) Estimate of phosphorylation efficiency of endogenous CDC37 in MCF7 cells. Two sequential immunoprecipitations with pSer13/CDC37 antibody remove approximately 70% from cell lysate. (C) Client binding preference of endogenous mammalian and in vitro phosphorylated, E. coli expressed CDC37. Recombinant, in vivo biotin tagged ERBB2 and ERBB3 receptors were analyzed for coimmunoprecipitated endogenous CDC37 (left). Cotransfected HA-tagged CDC37 and smaller size endogenous CDC37 (center) or E. coli expressed and in vitro phosphorylated CDC37 (right). E. coli expressed and endogenous CDC37 show the same client binding preferences. Note that ERBB2/3 levels in cotransfection experiments (*) are differentially impacted by the addition of endogenous CDC37. (D) Despite low binding in pull-down studies, the dependence of ERBB3 on CDC37 during early maturation is reflected in its sensitivity to CDC37 knockdown by siRNA.
