Abstract
Abnormalities in motor skills have been regarded as part of the symptomatology characterizing autism spectrum disorder (ASD). It has been estimated that 80% of subjects with autism display “motor dyspraxia” or clumsiness that are not readily identified in a routine neurological examination. In this study we used behavioral measures, event-related potentials (ERP), and lateralized readiness potential (LRP) to study cognitive and motor preparation deficits contributing to the dyspraxia of autism. A modified Posner cueing task was used to analyze motor preparation abnormalities in children with autism and in typically developing children (N=30/per group). In this task, subjects engage in preparing motor response based on a visual cue, and then execute a motor movement based on the subsequent imperative stimulus. The experimental conditions, such as the validity of the cue and the spatial location of the target stimuli were manipulated to influence motor response selection, preparation, and execution. Reaction time and accuracy benefited from validly cued targets in both groups, while main effects of target spatial position were more obvious in the autism group. The main ERP findings were prolonged and more negative early frontal potentials in the ASD in incongruent trials in both types of spatial location. The LRP amplitude was larger in incongruent trials and had stronger effect in the children with ASD. These effects were better expressed at the earlier stages of LRP, specifically those related to response selection, and showed difficulties at the cognitive phase of stimulus processing rather that at the motor execution stage. The LRP measures at different stages reflect the chronology of cognitive aspects of movement preparation and are sensitive to manipulations of cue correctness, thus representing very useful biomarker in autism dyspraxia research. Future studies may use more advance and diverse manipulations of movement preparation demands in testing more refined specifics of dyspraxia symptoms to investigate functional connectivity abnormalities underlying motor skills deficits in autism.
Keywords: Autism, reaction time, event-related potential, lateralized readiness potential, dyspraxia, motor response preparation, spatial attention
Introduction
Ever since early publications, abnormalities in motor skills have been regarded as part of the symptomatology characterizing autism as a unique syndrome (Asperger, 1944; Kanner, 1943). It has been estimated that 80% of subjects with autism display “motor dyspraxia,” or clumsiness (Weimer, Schatz, Lincoln, Ballantyne, and Trauner, 2001). These motor abnormalities are not readily identified in a routine neurological examination and prevalence rates depend on the number and types of symptoms examined. Furthermore, the presence of delayed motor skills at the age of 2 is the clearest distinguishing factor for children who continue meeting diagnostic criteria for Autism Spectrum Disorder (ASD) at four years of age (Sutera et al., 2007). In the majority of examined patients, motor abnormalities are observed early in infancy and are present throughout life, though the symptoms tend to become less severe with age (Ming et al., 2007). Underlying the motor skill deficits in ASD is dyspraxia, defined as impairments in ability to plan, organize, and execute movements in the absence of any known physical and/or neurological conditions. In dyspraxia, movement and coordination difficulties can involve both fine (e.g., writing, tying shoelaces) and gross motor skills (e.g., jumping, hoping). The motor deficits tend to be debilitating, since they can interfere with daily activities and academic achievement. Dyspraxia has also been linked to difficulties with sequencing and language, as well as in maintaining attention (Dziuk et al., 2007; Iverson and Braddock, 2011; MacNeil and Mostofsky, 2012).
The neurological basis of dyspraxia in autism is not well understood. Subjects with ASD perform worse than controls on a modified Romberg's test, which involves a tandem gait and in repetitive finger-thumb apposition, but do not show deficits in tasks involving visual-motor integration (Weimer et al., 2001). It seems, by default, that the underlying cause for dyspraxia in ASD is either cortical or subcortical (including defects of corticocortical connectivity) (Fuentes, Mostofsky, and Bastian, 2011). Neuropathological studies of dyspraxic patients with conditions such as corticobasal degeneration, Alzheimer's disease, Pick's disease have usually emphasized cortical disturbances (Kawamura and Mochizuki, 1999). The emphasis on cortical deficits is instilled in one of the classifications for apraxia (complete loss of motor abilities), which is categorized into motor, sensory, and conduction forms, in a similar manner to aphasia (Rowland and Pedley, 2010). Prior neurological studies have revealed several cortical abnormalities in patients with autism. Bias in the corticocortical connectivity in autism was first hypothesized by Belmonte and colleagues (Belmonte et al., 2004). Physiological studies have confirmed the presence of local bias and global performance impairment in autistic individuals (Just,Cherkassky, Keller, Kana, and Minshew, 2007; Koshino et al., 2005; Mottron et al., 2003; Wang, Mottron, Peng, Berthiaume, and Dawson, 2007). Anatomical findings suggestive of diminished neuronal cell soma size and increased outer radiate white matter seem to validate the presence of supernumerary short corticocortical projections (Casanova et al., 2006, 2009; Herbert et al., 2004). Unbiased stereological quantification has shown an excess number of thin axons in the white matter just underneath the cortex in autistic individuals (Zikopoulos and Barbas, 2010). In contrast, anatomical and structural studies suggestive of a diminished corpus callosum indicate a reduction in the total number of longer corticocortical projections, despite larger brain volume (Casanova et al., 2009).
The abnormalities in structural and functional cortical connectivity may negatively affect executive functions, an umbrella term for various complex cognitive processes (task-switching, planning of actions, response error monitoring, etc). The literature converges on the view that successful performance on tests of executive function is critically dependent on the frontal cortex. However, recent theories suggest that this view is simplistic and subcortical regions and networks (e.g., fronto-striatal network) may also be critically involved (Elliott, 2003). The deficits in flexible control of cognition and impairments of motor function seen in patients with autism are consistent with a disturbance of central executive functions. Several theories consider executive deficit as one of the major symptoms of autism (Hill, 2004; Hill and Frith, 2003; Ozonoff, 1997; Ozonoff,Strayer, McMahon, and Filloux, 1994). Impairment of executive functions involved in action planning, motor initiation, and output performance monitoring have also been implicated in ASD. The role of cognitive and motor processes is usually investigated using reaction time (RT) tests where manipulation of stimuli is employed to affect motor response preparation and execution stages.
The process of generating a motor response passes through a number of consecutive stages from preparation to execution. The frontal cortex cooperates with subcortical structures, but seems to play the leading role in motor control during tasks which require a motor response. Several authors have suggested a hierarchical organization of response control with a higher-level role for the prefrontal cortex (Fuster, 1997, 2001; Hoshiyama et al., 1997; Shallice and Burgess, 1991). Between the leading activity of the prefrontal cortex and the final commands from the primary motor cortex (M1), there are some preparatory processes performed by the premotor (PM) and supplementary motor area (SMA). The primary motor cortex is the last cortical level where motor activity can be modulated. The frontal lobes control voluntary actions through the planning (prefrontal cortex and associated basal ganglia circuits), preparation (SMA and PM and their basal ganglia and cerebellum loops), and execution of movements (primary motor and sub-cortical areas) (Faw, 2003; Fuster, 1997; Jahanshahi and Hallet, 2003). The SMA is more involved in self-paced voluntary movements and in preparation of movements based on internal cues held in memory, while the PM areas seem more involved in selection of movements based on external cues or prompts (Faw, 2003; Fuster, 1999; Passingham, 1995).
The somatic motor system controls bodily reactions to situations and interactions with environment. Posterior parts of somatic motor system involve parietal lobe somato-sensory and visual processing areas. The primary motor area is the principle activator of the pyramidal motor system which produces voluntary motor responses by projecting to motor neurons in the brainstem and spinal cord (Faw, 2003; Fuster, 1999). The PM area has control over limb movements based on external cues, while the contribution of SMA is based on internal cues or working memory (Passingham, 1995). The PM and SMA areas participate in a bilateral extra-pyramidal motor system, along with contra-lateral motor responses of the pyramidal system, helping coordinate broader bodily responses for more precise movement. The dorso-lateral prefrontal cortex (DLPFC) contributes to what can be called a “movement readiness potential”, which originates approximately 1 s prior to movement. The PM and SMA areas show their “readiness” potential later (500–600 ms prior to action), thus preparing extra-pyramidal motor commands, while the motor cortex sends basic pyramidal commands (Fuster, 1999). Both left and right DLPFC can enact voluntary actions contralaterally, but the left DLPFC seems to be more definite initiator of voluntary actions (Cummings, 1998; Spatt and Goldberg, 1997). The left DLPFC is most important in programming strategies, control of executive functions, and motor responses (Geschwind and Iacoboni, 1999).
Visual spatial perception abilities enable the processing of spatial information by exploring stimuli in the visual field, detecting their spatial position, and the relationships between them. These processes are fundamental to the development of several specific visuo-spatial abilities, such as spatial attention, orientation, memory, and spatial imagery (Piazza, Fumarola, Chinello, and Melcher, 2011; Smith and Chatterjee, 2008). The spatial attention and cognition abilities are essential for the development of spatial representations, allowing the individual to perform complex spatial tasks and to acquire academic skills (e.g. reading, geometry, and numerical skills). These spatial attention and perception skills are also important for proper visuo-motor coordination and other processes directly related to dyspraxia symptoms in ASD.
In this study we used behavioral measures (RT, response error rate), electrocortical event-related potentials (ERP), and premotor cortical potentials to study cognitive and motor preparation deficits contributing to the dyspraxia of autism. One of the main electrocortical indices used in the study was the lateralized readiness potential (LRP), which is a slow negative electroencephalographic (EEG) activity which precedes self-initiated or externally triggered movement. The LRP represents the cortical mechanisms that transpire in preparation for intended movement (Eimer, 1998; Platz et al., 2000; Wascher et al., 1997).
A modified visual Posner cueing task (Posner, 1980) was selected as most appropriate test to analyze motor preparation abnormalities in autism and to compare with normative responses in typically developing subjects. Psychophysiological studies that supplement reaction time (RT) measures with electrophysiological measures such as ERP and LRP can help to directly assess precuing effects within the Stimulus–Response mapping and processing chain (Coles, 1989; Eimer, 1998; Leuthold et al., 2004; Praamstra, Schmitz, Freund, and Schnitzler, 1999).. The LRP enables the determination of the point in time at which the activation of the motor cortex controlling one hand surpasses the activation of the motor cortex controlling the other side and is sensitive to covert aspects of movement preparation (Gratton, Sirevaag, Eriksen, and Donchin, 1988).
The purpose of our study was to investigate RT, accuracy, dense-array ERPs, and LRP in a Posner cued spatial attention task in children with ASD and in typically developing children. The study was aimed to understand the abnormal neural and functional mechanisms underlying visual spatial attention, motor preparation, and execution abnormalities in autism. It was hypothesized that children with ASD would perform worse than controls on cued spatial attention task, as reflected by RT and response error rates. Furthermore, manipulation of stimuli, such as incongruent cuing and higher stimulus complexity would have a greater impact on performance and physiological measures in ASD than in controls.
Materials and Methods
Research design and methods
Participants
The participants in this study included 30 children diagnosed with autism spectrum disorder (ASD), and 30 typically developing children (CNT). Participants with ASD (age range 11 to 21 years) were recruited through the Weisskopf Child Evaluation Center (WCEC) and the Autism Center at the University of Louisville. Diagnosis was made according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) (APA, 2000) and further ascertained with the Autism Diagnostic Interview – Revised (ADI-R) (LeCouteur, Lord, and Rutter, 2003). Participants referred with a diagnosis of ASD were given the Autism Diagnostic Observation Schedule (ADOS, Lord et al., 1989). Examination of participants entailed a pediatrician who took a developmental history and physical exam and a referral to an ASD specialist certified in the use of ADOS and ADI-R assessment tools. Cutoff scores for ADOS were Communication: 3, Social Interaction: 6, and Social/Communication Total: 10. Standard diagnostic cutoffs were utilized with the ADI-R. All subjects had normal hearing based on past hearing screens. Participants either had normal vision or wore corrective lenses. Participants with a history of seizure disorder, significant hearing or visual impairment, a brain abnormality conclusive from neuroimaging studies, or an identified genetic disorder were excluded from the study. All participants were high-functioning persons with ASD with full scale IQ > 80 assessed using the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler, 2003) to ensure adequate cooperation during procedures and testing. Participants in the ASD group had no comorbid or causative conditions (chromosomal, neurologic, infectious), history of seizures, perinatal brain injury, or head trauma, and were considered to be in good general health.
Candidates within the neurotypical control group (CNT) were screened for neurologic, psychiatric, and medical disorders. Additionally, their family history was screened for the same conditions. Inclusion criteria for comparison individuals included: good general physical health, no regular use of psychotropic medications, good performance at school and/or on the job, and good peer relationships based on self-report or staff observations during testing. Exclusion criteria for control subjects included: first-, second-, or third degree relative diagnosed with ASD, personal and/or family history of other psychiatric disorders (excluding dyslexia), perinatal brain injury, and head trauma. Subjects in the CNT group were recruited through advertisements in the local media. Participants within the control and autism groups were matched by age, full scale IQ, and socioeconomic status of their family. Socioeconomic status was compared based on parent education and annual household income, and were similar for the two groups. Mean age and male to female ratio in the ASD group (15.63 ± 3.85 year olds, 8 girls, N=30) was similar to the control group (15.76 ± 3.89 year olds, 7 girls, N=30).
Participating subjects and their parents (or legal guardians) were provided with full information about the study including the purpose, requirements, responsibilities, reimbursement, risks, benefits, alternatives, and role of the local Institutional Review Board (IRB). The consent and assent forms approved by the IRB were reviewed and explained to all subjects who expressed interest to participate. All questions were answered before consent signature was requested. If the individual agreed to participate, she/he signed and dated the consent and/or assent form and received a copy countersigned by the investigator who obtained consent. All participants were reimbursed ($25 per visit) for their time and parking costs.
Experimental procedure: Cued Posner spatial attention task
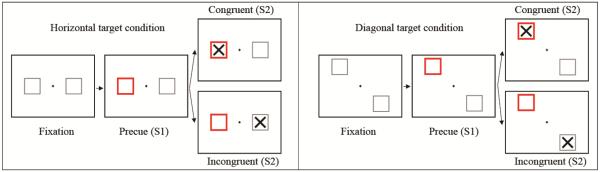
A modified visual Posner cueing task (Figure 1) was used as assessment of spatial attention and motor preparation/response. In all experiments, subjects were seated in an adjustable chair with their chin in a chinrest. The chinrest was placed so that subject's eyes were 50 cm from the center of the 17 inch monitor screen. Breaks are provided approximately every 5 min so subjects can rest their eyes. For the task, subjects are instructed to respond to a visual stimulus (X) appearing on either left or right side of a monitor screen, by pressing a button with either left or right hand. For 1 sec. before the onset of each stimulus, subjects are cued (red outline) to the location of the upcoming stimulus, allowing for preparation of a motor response to that side. In 20% of the trials the cue was incongruent, meaning it appeared on the incorrect side of the screen. The study consisted of 2 blocks – A “horizontal” block where stimuli appeared either on the left or right side of the screen, and a more difficult “diagonal” block, where cues and stimuli appeared in the corners of the screen. The task for programmed using E-prime experimental control system, and presented on a 17” inch computer monitor 50 cm away. The procedure lasted a total of 20 minutes, including the time needed for task instruction and practice, and the actual task (10 minutes; 200 trials). For each trial, behavioral response measures (RT and error rate) were collected, in addition to EEG waveform which was used for ERP and LRP analysis.
Figure 1.
Cued Posner spatial attention task. The subject attends to the small fixation cross at the center of the screen, with a box to either side. As a cue, one of the boxes is highlighted in red color. A large target cross appears inside one of the boxes (with 1000 ms S1–S2 inter-stimulus interval), and the test subject indicates which box by pressing a button with the corresponding hand. In 80 % of trials, the target cross will appear in the box cued in prior step (valid cue, congruent), while 20 % of the time it will appear in the other box (invalid cue, incongruent). The horizontal condition is shown at the left, while diagonal condition at the right.
EEG data acquisition and signal processing
Raw EEG data was acquired with a 128 channel Geodesic EEG System 200 (Electrical Geodesics Inc., Eugene, Oregon) consisting of Sensor Net electrodes, Net Amps amplifiers, and Net Station version 4.0.1 software. EEG data were sampled at 500 Hz, 0.1 Hz–100 Hz analog filtered, referenced to the vertex. The Sensor Net is a lightweight elastic thread structure containing Ag-AgCl electrodes housed in a synthetic sponge on a pedestal. The sponges were soaked in a KCl solution to render them conductive. Stimulus-locked EEG data were segmented off-line around the critical stimulus events (pre-cue baseline, cue, and target stimulus). Datasets were digitally screened for artifacts, and following additional visual inspection, contaminated trials were removed using built-in artifact rejection tools. EEG channels with high impedance or visually detectable artifacts (e.g., channel drift, gross movement, etc.) were identified using Net Station event marker tools in `on-line' mode and removed in the `off-line' mode using Net Station Waveform Tools (NSWT). Remaining data were sorted by condition and averaged to create the ERP. Averaged ERP data were passed through a 20 Hz digital lowpass filter to remove residual high-frequency noise, baseline corrected, and re-referenced into an average reference frame. EEG data were segmented off-line into 2000 ms epochs spanning 200 ms pre-cue baseline, 1000 ms cue period, and 800 ms post-S2 (imperative) stimulus. The critical stimulus events in the task were: (1) correctly cued targets in horizontal position (40%), (2) incorrectly cued targets in horizontal position (10%); (3) correctly cued targets in horizontal position (40%), (4) incorrectly cued targets in horizontal position (10%).
Event-Related Potentials (ERP)
Stimulus-locked ERP were recorded from electrodes in the region-of-interest (ROI) which included the frontal and centro-parietal loci. The ERP data was segmented for each stimulus condition and averaged across subjects in each group. For each condition, the amplitude and latency of ERP peaks were analyzed: N100, P200, N200, and P300, in the temporal window following the presentation of the target stimulus. More detailed description of ERP analysis used can be found in our previous publications (Sokhadze et al., 2009, 2010).
LRP recording and analysis
The LRP is computed on the basis of potentials recorded over the right and left motor cortices prior to and during the execution of a motor response with a particular hand. The LRPs were derived from a pair of electrodes, overlying the left and right motor cortex. Electrodes were positioned at C3' and C4' sites located 1 cm anterior of C3 and C4 by the 10–20 International EEG system. For condition, ERP waveforms recorded over the primary cortex on the same side as the responding hand are subtracted from the motor cortex in the contralateral hemisphere. This was based on averaging method described in Coles (1989). These subtractions are performed for the left and right hand, and averaged to produce the LRP waveform.
The resulting waveform represents the difference in activation between the left and right motor cortex. LRP reflects the lateralization of slow motor ERP activity observed prior to movement onset that is assumed to be related to a central activation of a one-handed response (Eimer, 1998) with a negative deflection of the LRP representing preparation of the correct hand, and positive deflection representing incorrect preparation (as during trials with incongruent cues). Typically, LRP-based mental chronometry helps to determine exact point in time when sensory information starts affect motor processing and response execution. The LRP can be computed on the basis of stimulus-locked average waveforms or as a response-locked average. We used stimulus-locked LRP method and since the interval length between cue and stimulus was set at 1 s for all trials. We computed mean LRP and integrated LRP values for 2 windows: Early (600 –1000 ms post-cue) and late (1000 ms–1400 ms post-cue). For the baseline correction we used 1200 ms from the S2 target stimulus.
Statistics
The primary statistical analysis was the repeated measures ANOVA (rANOVA), with dependent variables being those described above. Each ERP component was analyzed for pre-selected ROI and time window. The rANOVA design for all dependent ERP/EEG variables included within-subject factors Stimulus Congruence (congruent, incongruent) × Stimulus Spatial Location (horizontal, diagonal) X Hemisphere (left, right), and between-subject factor being Group (ASD, CNT). For LRP we used similar analysis design except that was added factor of LRP Stage (early, late). In all ANOVAs, Greenhouse-Geisser corrected p-values were employed where appropriate. For estimation of the effect size and power (Murphy and Myors, 2004) we used Partial Eta Squared (η2) and observed power (π) computed using α=0.05. IBM SPSS 19.0 and Sigma Stat 3.1 statistical packages were used for the analysis of data.
Results
Behavioral responses
Reaction Time and Accuracy
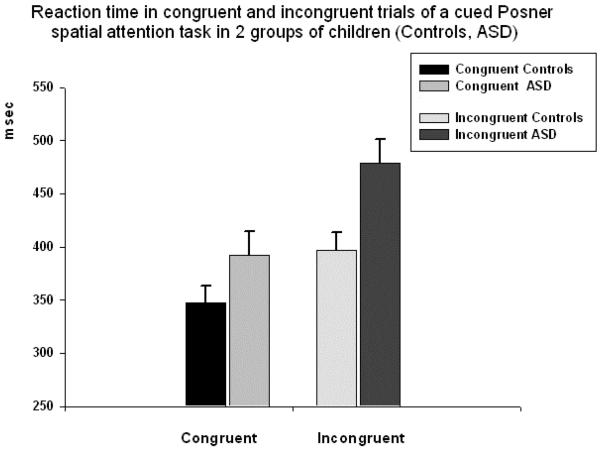
Reaction time (RT) to targets was significantly different between two groups of subjects in all conditions. Congruence (congruent, incongruent) had strong main effect on RT (F1,58=69.5, p<0.001, η2=0.56, observed power (π)=0.99). Main effect of the target spatial position (horizontal, diagonal) was also significant (F1,58=8.47, p=0.005, η2=0.13, π=0.81). Congruence (congruent, incongruent) X Group (ASD, CNT) interaction was significant (F1,58=5.92, p=0.018, η2=0.10, π=0.67). RT in both horizontal and diagonal congruent and incongruent trials was longer in the ASD group, but difference was more pronounced in incongruent trials (see Fig. 2). In particular, in horizontal incongruent condition difference was even more significant than in the diagonal one (horizontal: 481 ± 148 ms in ASD vs. 375 ± 104 ms in CNT, F1,58=9.80, p=0.003; diagonal: 489 ± 141 ms in ASD vs. 404 ± 117 ms in CNT, F1,58=6.03, p=0.017).
Figure 2.
Reaction time (RT, mean ± standard error [SE]) in congruent and incongruent trials of a cued Posner spatial attention task in 2 groups of children (Controls, ASD). Congruence (congruent, incongruent) had strong main effect on RT (F1,58=69.5, p<0.001, η2=0.56, observed power (π)=0.99). Congruence X Group interaction was moderately significant (F1,58=5.92, p=0.018, η2=0.10, π =0.67).
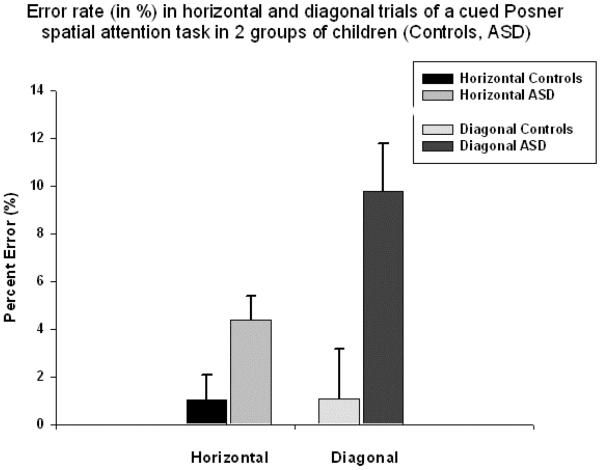
A difference in total error rate in the ASD and CNT groups was significant in all four conditions. However, only spatial location of the target had main effect on error rate ((F1,58=7.23, p<0.009, η2=0.12, π=0.75). In error rate measure analysis only Spatial location (horizontal, diagonal) X Group (ASD, CNT) interaction was significant (F1,58=6.94, p=0.011, η2=0.11, π=0.73). In the CNT group difference in error rate was not affected by the target location, but it was proportionally higher in diagonal condition in the ASD group (Fig. 3) regardless of congruence (diagonal congruent: 1.28 ± 2.20 percent in CNT vs. 9.83 ± 16.5 percent in ASD, F1,58=7.51, p=0.008; diagonal incongruent: 1.04 ± 3.18 percent in CNT vs. 4.31 ± 7.1 percent in ASD, F1,58=4.77, p=0.033).
Figure 3.
Error rate (in percent, mean ± SE) in congruent and incongruent trials of a cued Posner spatial attention task in 2 groups of children (Controls, ASD). Spatial location of the target had main effect on error rate (F1,58=7.23, p=0.009, η2=0.12, π =0.75). Spatial location X Group interaction was significant (F1,58=6.94, p=0.011, η2=0.11, π =0.73). In the CNT group difference in error rate was not affected by the target location, but it was higher in diagonal condition in the ASD group regardless of congruence.
ERP to imperative stimulus
Frontal and centro-parietal ERPs
Frontal N100 amplitude. In all conditions, except of the horizontal incongruent one, the amplitude of the frontal N100 ERP component was higher in the ASD group (e.g., diagonal congruent: −2.41 ± 2.99 μV in ASD vs. −0.54 ± 1.38 μV in CNT, F1,58=10.78, p=0.002; while in diagonal incongruent: −2.84 ± 2.56 μV in ASD vs. −0.79 ± 1.65 μV in CNT, F1,58=7.65, p=0.008; and in horizontal congruent : −2.16 ± 2.37 μV in ASD vs. −0.83 ± 0.87 μV in CNT, F1,58=7.65, p=0.008). Spatial location X Group interaction effect was marginally reaching significance level (F1,58=4.31, p=0.043, η2=0.08, π=0.53, well below desired level of π = 0.80). The amplitude of N100 was more negative in the ASD group in horizontal rather than in the diagonal target position trials. This effect was had higher observed power and better effect size at the right hemisphere (F1,58=7.04, p=0.011, η2=0.13, π=0.74).
Frontal N100 latency. Only Congruence factor had main effect on latency of the N100 component (F1,58=6.72, p=0.012). Congruence X Group (F1,58=6.35, p=0.015; η2=0.11, π=0.71) effect was significant. Even Spatial Location X Congruence X Group (F1,58=5.10, p=0.028, η2=0.09, π=0.56) interactions was close to marginal significance, though it was not sufficiently well powered and effect was very weak. Similar to the effect observed for the amplitude, the latency of N100 was more affected in the horizontal conditions being prolonged in the ASD group, while incongruence resulted in delayed latency of the N100 in the ASD group with less significant effects in the CNT group. For instance, in the diagonal incongruent condition latency was significantly longer in the ASD group (162 ± 26 ms in ASD vs. 147 ± 26 ms in CNT, F1,58=4.72, p=0.034).
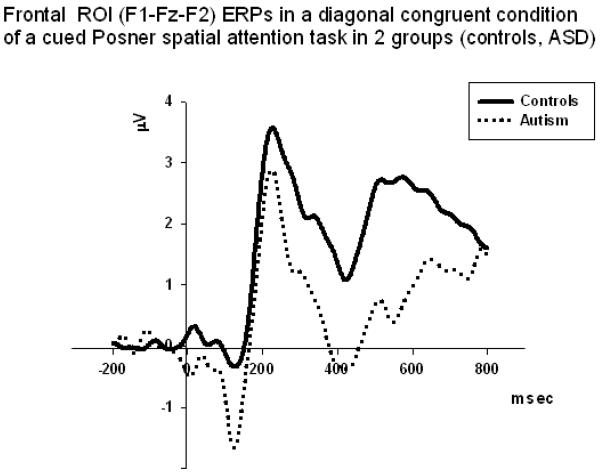
Frontal N200 and P200 amplitude. Group differences effects were observed only in the diagonal target trials (congruent: F1,58=7.34, p=0.009; incongruent: F1,58=7.05, p=0.011). Spatial Location X Group interaction effect was marginal (F1,58=4.33, p=0.043) and was not powered well (i.e., η2=0.089, π=0.56). The amplitude of N200 was more negative in diagonal rather than horizontal target positions. No group differences were found for P200 amplitude.
Frontal N200 latency did not show any statistically significant group differences or any interactions. Congruence had moderate main effect on the latency of P200 (F1,58=5.31, p=0.026, η2=0.11, π=0.61). In particular, the latency of P200 was prolonged in ASD as compared to CNT group in both incongruent diagonal (F1,58=5.75, p=0.021) and incongruent horizontal (F1,58=4.71, p=0.035) conditions. There were not observed any interactions for the frontal P200 latencies.
Centro-parietal P300 (P3b). There were not found any statistically significant group differences for P3b amplitude and latency, though the Congruence factor had main effect on P3b amplitude (F1,58=5.34, p=0.025) and even more on P3b latency (F1,58=9.03, p=0.004).
Lateralized Readiness Potential (LRP)
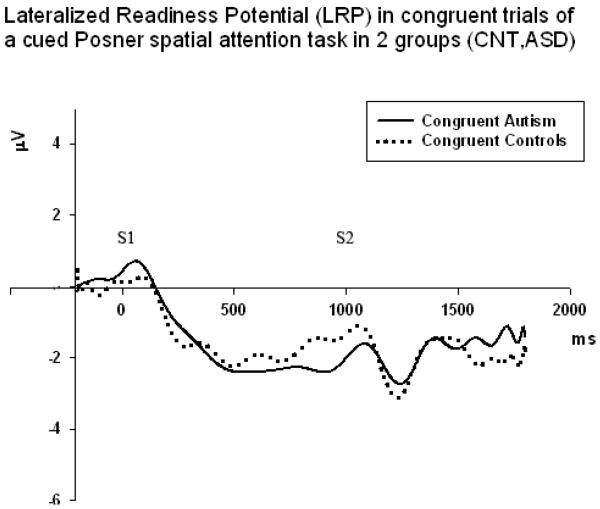
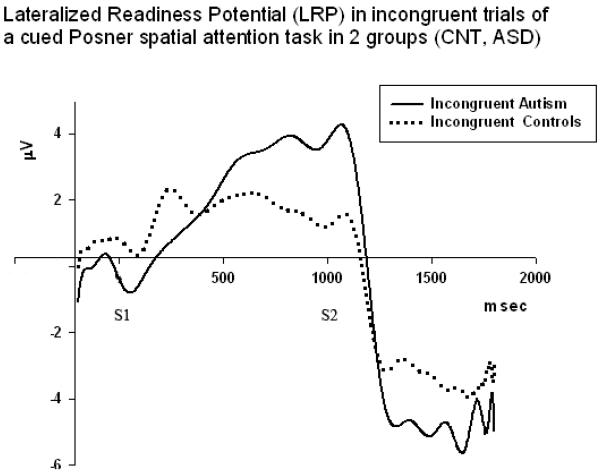
The Stage of LRP (early – 600–1000 ms, late- 1000–1400 ms) had very strong main effect on amplitude (F1,58=35.06, p<0.001, η2=0.42, π=0.99). Congruence had also similarly strong main effect on LRP amplitude (F1,58=39.76, p<0.001, η2=0.45, π=0.99). However, main effect of target Cue Position (horizontal, diagonal) was not reaching significance. Congruence X Group interaction was statistically significant and effect was strong (F1,58=8.49, p=0.005, η2=0.15, π=0.81). Paired sample t-test showed that group difference was statistically significant in incongruent condition at the early LRP stage (1.94 ± 3.29 μV, t=3.27, df=29, p=0.006). Late LRP group differences were not significant for both congruent and incongruent conditions.LRP Stage (early, late) × Congruence (congruent, incongruent) X Group (ASD, CNT) interaction effect was marginally reaching significance, but was very weak in term of effect size and observed power (F1,58=4.11, p=0.048, η2=0.08, π=0.51). Figures 7 and 8 illustrate LRP dynamics in both groups in congruent and incongruent trials.
Figure 7.
LRP waveforms in congruent conditions in 2 groups (Controls, ASD, N=30/per group). Congruence had strong main effect on LRP amplitude (F1,58=39.76, p<0.001, η2=0.45, π=0.99).
Figure 8.
LRP waveforms in incongruent conditions in 2 groups (Controls, ASD, N=30/per group). Congruence X Group interaction was statistically significant and effect had strong power (F1,58=8.49, p=0.005, η2=0.15, π=0.81).
Discussion
In this study behavioral (RT, error rate) and electrophysiological cortical activity (frontal ERP, motor strip LRP) measures were used to investigate the effects of cuing in a visual Posner cueing spatial attention task in ASD and neurotypical children. Both groups had lower RTs and less errors for trials with congruent cues, compared to trials with incongruent cues. In addition, main effects of target spatial position were more obvious in the ASD group. The main ERP findings were prolonged and more negative early frontal potentials (N100, P200) in the ASD in incongruent trials in both types of spatial location. The LRP amplitude was larger in incongruent trials and had stronger effect in the children with ASD. These effects were better expressed at the earlier stages of LRP, specifically those related to response selection, and showed difficulties at the cognitive phase of stimulus processing rather that at the motor execution stage.
In the present study subjects were cued to the spatial location of the upcoming target stimulus, and responded faster and more accurately when the cue was congruent. This is consistent with previous studies using similar experimental paradigms. (Golob, Pratt, and Starr, 2002). Our study showed expected differences in RT, ERP, and LRP between ASD and control groups. In our study the attentional selectivity processes are more negatively affected by congruence and, to a lesser extent, by spatial location in ASD group, compared to CNT group.
A review of motor skills and motor abilities in autism outlines that altered motor behavior is commonly reported and is highly prevalent, though etiology of this phenomenon remains unclear (Gowen and Hamilton, 2013). It is important to take into account that motor abnormalities in autism are observed even in infancy and are apparent during childhood and adolescence (Brian et al., 2008; Ming et al., 2007; Provost, Lopez, and Heimerl, 2007; van Waelvede, Oostra, Dewitte, Van Den Broeck, and Jongmans, 2010). It is very likely that abnormalities of motor control development and dyspraxia can exert further negative consequences on different daily motor skills in children with ASD, and it is very probable that motor control training in early childhood could decrease severity of dyspraxia symptoms in ASD in later life (Sutera et al., 2007). Early recognition of dyspraxia is essential, as early intervention can lead to better outcomes. If dyspraxia in autism is diagnosed early enough, a variety of therapies could be employed to improve motor performance, and increase self-perceived competence. This may have secondary beneficial effects such as increased participation in community activities, while avoiding complications due to superimposed learning problems, low self-esteem, and repeated injuries (Gibbs, Appleton, and Appleton, 2007; Pless, Carlsson, Sundelin, and Persson, 2001).
Distinguishing imitation deficits and dyspraxia is an important area of research in autism that may inform understanding of neural mechanisms underlying imitation skills deficiency (Stieglitz Ham et al., 2011). This is especially important considering faulty “mirror neuron system” (MNS) hypothesis that according to MNS and imitation role proponents might be responsible for a cascading effect resulting in lack of emotional responsiveness and empathy, deficient joint attention, theory of mind, and other self-other mapping impairments observed in autism (Oberman and Ramachandran, 2007, Oberman, Ramachandran, and Pineda, 2008; Ramachandran and Oberman, 2006; Rizzolatti and Craighero, 2004; Williams,Whiten, Suddendorf, and Perrett, 2001). Recently the mirror neuron system and imitation deficit hypothesis was criticized on a ground of numerous discrepancies, emphasizing that it cannot account for majority of the patterns of motor deficits observed in children with ASD and adult patients with autism (Fan, Decety, Yang, Liu, and Cheng, 2010; Hamilton,Brindley, and Frith, 2007; Hamilton, 2008; Leighton,Bird, Charman, and Heyes, 2008; Mostofsky et al., 2006; Stieglitz Ham et al., 2011). Findings from our study help delineate the importance of abnormalities in neural networks defined by longer corticocortical projections in ASD. Abnormalities of imitation, in this regard, should be subsumed under a generalized praxis disorder, not a defect in mirror neurons (Mostofsky et al., 2006).
More in-depth analysis of cognitive processes accompanying motor preparation deficits in ASD may provide better understanding of underlying neurobiological abnormalities of this disorder. In autism, it is thought that there are brain regions that store “movement representations” (i.e., sequence of motor actions) and help in initiation of program in the premotor cortices to transcode them into primary motor cortex for execution (Dowell, Mahone, and Mostofsky, 2009; Heilman and Rothi, 1993). Application of ERP and LRP measures helps in breakdown of stages of this process at early and late stages of movement preparation and executions. This approach is helpful in understanding potential contributors of dyspraxia in autism, such as impaired storage of motor representations, impaired transcoding of these representations into motor programs, impairment in execution of motor acts at the level of the primary motor cortex (Jansiewicz et al., 2006), or a combination of these deficits.
Though there are no definitive medical tests for autism, it has been proposed that autism specific biomarkers could be used to aid in early diagnosis or in evaluation of outcomes (Dowell et al., 2009; Gidley Larson and Mostofsky, 2006; Mostofsky et al., 2006). For instance, using the LRP in conjunction with behavioral/neurological evaluations can be useful in evaluation of dyspraxia in autism. Using quantifiable measures of neural activity could add construct validity to diagnostic screening and serve as better targets for treatment.
Dipole source modeling and localization studies of similar RT tasks demonstrated contribution of higher-order motor areas (e.g., SMA, cingulate motor area [CMA]) to such effector-specific preparation of movement (Leuthold and Jentzsch, 2001). It was suggested motor preparation involves two phases, the first phase involving the assembly and selection of a motor program (dipole in the SMA, and pre-SMA areas), while the second involves implementation of the motor command (dipole in lateral pre-motor [PMA] and primary motor [MI] areas) (Ulrich, Leuthold, and Sommer, 1998). In general, the present study showed that the joint application of behavioral (RT, accuracy) and electrophysiological (ERP and LRP) measures in a cued spatial attention task provides better understanding about the functional organization of movement preparation within the cortical systems involved in motor activity, and helps in deeper understanding of specificity of deficits of motor preparation in children with autism. It can be concluded that individuals with ASD are experiencing more difficulties at the stages of changing an assembled and selected motor program during preparation to movement. In our experimental task, the rare presentations of incongruently cued trials placed more demands on proper executive functioning to inhibit invalidly cued preparation process and initiate motor preparation of the opposite hand.
There is a distinction between a specific cognitive deficit and motor slowness, as movement execution may be impaired in ASD for reason of cognition deficit. The delayed response may be due to difficulties in selecting the direction of the motor act, or difficulties in timely initiation of the selected motor act. These two possible sources of RT delay cannot be delineated by behavioral response measures alone. In this study we used LRPs to study the distinction between measures of cognition and execution. Analysis of LRPs is an important tool in the field of psychophysiology research, as it measures timing of motor preparation process, and estimates response tendencies when interferences are used (Coles, 1989; EImer, 1998; Gratton et al., 1988; Wascher et al., 1997). The LRP amplitude reflects the chronology of cognitive aspects of motor preparation and is sensitive to interferences (i.e., manipulations of cue correctness), and could be a potentially useful biomarker in autism dyspraxia research.
Future research studies may use more advance and diverse manipulations of movement preparation demands in testing more refined specifics of dyspraxia symptoms to investigate structural and functional connectivity abnormalities underlying motor skills deficits observed in autism. Various processes of motor act selection and preparation have been studied by analyzing behavioral response measures in behavioral paradigms (e.g., Dowell et al., 2009). Application of electrocortical measures such as ERPs and LRPs could complement such behavioral observations and shred the light on the role of connectivity abnormalities (e.g., local over-connectivity at the background of distal under-connectivity) in autism. Further studies using functional MRI (fMRI) or combination of fMRI with concurrent EEG recordings would further clarify how dysfunctional activity in specific prefrontal and premotor cortices contributes to dyspraxia in autism.
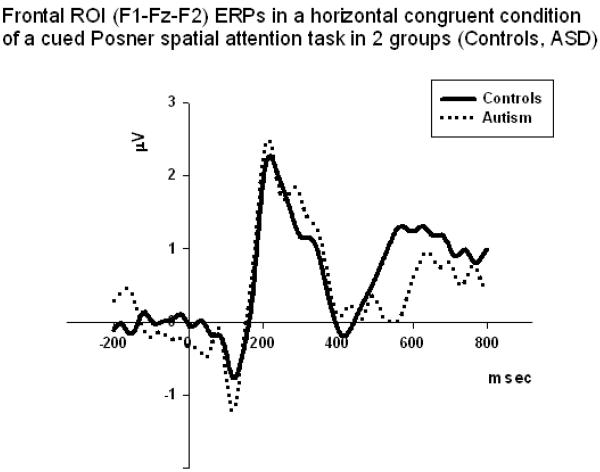
Figure 4.
Frontal ERPs (average across F1, Fz, and F2) in a horizontal congruent condition of a cued Posner spatial attention task in 2 groups (Controls, ASD). The amplitude of N100 was more negative in the ASD group in horizontal rather than in the diagonal target position trials. In horizontal congruent condition group difference was statistically significant (F1,58=7.65, p=0.008).
Figure 5.
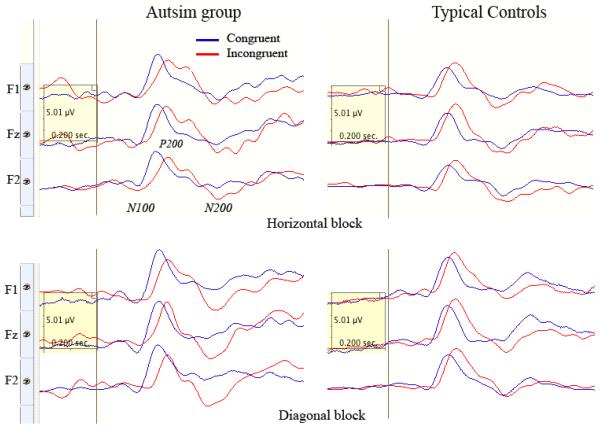
Frontal ERPs (F1, Fz, F2) in a horizontal incongruent condition of a cued Posner spatial attention task in 2 groups (Controls, ASD). The amplitudes of N100 and also N200 components were more negative in the ASD group in this condition.
Figure 6.
Frontal ERPs (F1, Fz, F2) in congruent (dark blue) and incongruent (light red) trials of horizontal (up) and diagonal (down) target conditions in 2 groups (Controls, ASD, N=30/per group). Congruence had moderate main effect on the latency of P200 (F1,58=5.31, p=0.026, η2=0.11, π =0.61). In particular, the latency of P200 was prolonged in ASD as compared to CNT group in incongruent diagonal (F1,58=5.75, p=0.021) condition.
Acknowledgments
The study was partially supported by National Institutes of Health Eureka R01 grant MH86784 to Manuel F. Casanova and by a pilot research grant from Autism Research Institute (San Diego, CA) to Estate M. Sokhadze.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Press; Washington, D.C.: 2000. text revision. [Google Scholar]
- Asperger H. Die “Autistischen Psychopathen” im Kindesalter. Archiv für Psychiatrie und Nervenkrankheiten. 1944;117:76–136. [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. The Journal of Neuroscience. 2004;24(2):9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian J, Bryson SE, Garon N, Roberts, Smith IM, Szatmari P, Zwaigenbaum L. Clinical assessment of autism in high-risk 18-month-olds. Autism. 2008;12(5):433–456. doi: 10.1177/1362361308094500. [DOI] [PubMed] [Google Scholar]
- Brunia CH. Neural aspects of anticipatory behavior. Acta Psychologica. 1999;101(2–3):213–242. doi: 10.1016/s0001-6918(99)00006-2. [DOI] [PubMed] [Google Scholar]
- Brunia CH, van Boxtel GJ. Wait and see. International Journal of Psychophysiology. 2001;43(1):59–75. doi: 10.1016/s0167-8760(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Steinbusch HW, Hof PR, Trippe J, Stone J, Schmitz C. Minicolumnar abnormalities in autism. Acta Neuropathologica. 2006;112(3):287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Casanova MF, El-Baz A, Mott M, Mannheim G, Hassan H, Fahmi R, Giedd J, Rumsey JM, Switala AE, Farag A. Reduced gyral window and corpus callosum size in autism: Possible macroscopic correlates of a minicolumnopathy. Journal of Autism and Developmental Disorders. 2009;39(5):751–764. doi: 10.1007/s10803-008-0681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles MG. Modern mind-brain reading: Psychophysiology, physiology, and cognition. Psychophysiology. 1989;26(3):251–269. doi: 10.1111/j.1469-8986.1989.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Cui RQ, Huter D, Egkher A, Lang W, Lindinger G, Deecke L. High resolution DC-EEG mapping of the Bereitschaftspotential preceding simple or complex bimanual sequential finger movement. Experimental Brain Researh. 2000;134(1):49–57. doi: 10.1007/s002210000449. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior: Commentary. Journal of Psychosomatic Research. 1998;44:627–628. doi: 10.1016/s0022-3999(98)00034-8. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Lalouschek W, Dirnberger G, Walla P, Lindinger G, Asenbaum S, Brucke T, Lang W. A medial to lateral shift in pre-movement cortical activity in hemi-Parkinson's disease. Clinical Neurophysiology. 2001;112(4):608–618. doi: 10.1016/s1388-2457(01)00467-9. [DOI] [PubMed] [Google Scholar]
- Donkers FC, van Boxtel GJ. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and Cognition. 2004;56(2):165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Dowell LR, Mahone EM, Mostofsky SH. Association of postural knowledge and basic motor skill with dispraxia in autism: Implication for abnormalities in disturbed connectivity and motor learning. Neuropsychology. 2009;23(5):563–570. doi: 10.1037/a0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziuk MA, Gidley Larson JC, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: Association with motor, social, and communicative deficits. Developmental Medicine and Child Neurology. 2007;49(10):734–739. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Elliott R. Executive functions and their disorders. British Medical Bulletin. 2003;65:49–59. doi: 10.1093/bmb/65.1.49. [DOI] [PubMed] [Google Scholar]
- Eimer M. The lateralized readiness potential as an on-line measure of central response activation processes. Behavior Research Methods, Instruments, & Computers. 1998;30(1):146–156. [Google Scholar]
- Fan YT, Decety J, Yang CY, Liu JL, Cheng Y. Unbroken mirror neurons in autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2010;51(9):981–988. doi: 10.1111/j.1469-7610.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- Faw B. Pre-frontal executive committee for perception, working memory, attention, long-term memory, motor control, and thinking: A tutorial review. Consciousness and Cognition. 2003;12:83–139. doi: 10.1016/s1053-8100(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Fuentes CT, Mostofsky SH, Bastian AJ. No proprioceptive deficits in autism despite movement-related sensory and execution impairments. Journal of Autism and Developmental Disorders. 2011;41(10):1352–1361. doi: 10.1007/s10803-010-1161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: anatomy, physiology, and neuropsychology of the rontal lobe. 3rd ed. Lippincott-Raven; Philadelphia: 1997. [Google Scholar]
- Fuster JM. Cognitive functions of the frontal lobes. In: Miller BL, Cummings JL, editors. Human frontal lobes. Guilford; New York: 1999. pp. 187–195. [Google Scholar]
- Fuster JM. The prefrontal cortex- an update: Time is of the essence. Neuron. 2001;30(2):319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nature Neuroscience. 2000;3(5):516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Iacoboni M. Structural and functional asymmetries of the human frontal lobes. In: Miller BL, Cummings JL, editors. Human frontal lobes. Guilford; New York: 1999. pp. 45–70. [Google Scholar]
- Gibbs J, Appleton J, Appleton R. Dyspraxia or developmental coordination disorder. Unraveling the enigma. Archives of Disease in Childhood. 2007;92(6):534–539. doi: 10.1136/adc.2005.088054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidley Larson JC, Mostofsky SH. Motor deficits in autism. In: Tuchman R, Rapin I, editors. Autism: a neurological disorder of early brain development. MacKeith Press; London: 2006. pp. 231–247. [Google Scholar]
- Golob EJ, Pratt H, Starr A. Preparatory slow potentials and event-related potentials in an auditory cued attention task. Clinical Neurophysiology. 2002;113:1544–1557. doi: 10.1016/s1388-2457(02)00220-1. [DOI] [PubMed] [Google Scholar]
- Gowen E, Hamilton A. Motor abilities in autism: A review using a computational context. Journal of Autism and Developmental Disorders. 2013;43(2):323–344. doi: 10.1007/s10803-012-1574-0. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Sirevaag EJ, Eriksen CW, Donchin E. Pre- and poststimulus activation of response channels: A psychophysiological analysis. Journal of Experimental Psychology. Human Perception and Performance. 1988;14(3):331–344. doi: 10.1037//0096-1523.14.3.331. [DOI] [PubMed] [Google Scholar]
- Hamilton A. Emulation and mimicry for social interaction: A theoretical approach to imitation in autism. Quarterly Journal of Experimental Psychology. 2008;61:101–115. doi: 10.1080/17470210701508798. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Brindley R, Frith U. Imitation and action understanding in autistic spectrum disorders: How valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia. 2007;45:1859–1868. doi: 10.1016/j.neuropsychologia.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Haswell C, Izawa J, Dowell L, Mostofsky S, Shadmehr R. Representation of internal models of action in the autistic brain. Nature Neuroscience. 2009;12(8):970–972. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Rothi LJ. Apraxia. In: Heilman K, Valstein E, editors. Clinical Neuropsychology. 3rd ed. Oxford University Press; New York: 1993. pp. 141–163. [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Annals of Neurology. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hill EL. Evaluating the theory of executive dysfunction in autism. Developmental Review. 2004;24:189–233. [Google Scholar]
- Hill EL, Frith U. Understanding autism: Insights from mind and brain. Philosophical Transactions of the Royal Society London, B. 2003;358:281–289. doi: 10.1098/rstb.2002.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiyama M, Kakigi R, Berg P, Koyama S, Kitamura Y, Shimojo M. Identification of motor and sensory brain activities during unilateral finger movement: Spatiotemporal source analysis of movement-associated magnetic fields. Experimental Brain Research. 1997;115:6–14. doi: 10.1007/pl00005685. [DOI] [PubMed] [Google Scholar]
- Iverson JM, Braddock BA. Links between language, gesture, and motor skill in children with language impairment. Journal of Speech, Language, and Hearing Research. 2011;54(1):72–86. doi: 10.1044/1092-4388(2010/08-0197). [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Hallet M. Bereitschaftspotential: movement-related cortical potentials. Kluwer Academic; New York: 2003. [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain. 1995;118(4):913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH. Motor signs distinguish children with high functioning autism and Asperger's syndrome from controls. Journal of Autism and Developmental Disorders. 2006;36:613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Jahashahi M, Jueptner M, Passingham R, Brooks DJ. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain. 2000;123(6):1216–1228. doi: 10.1093/brain/123.6.1216. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an fMRI study of an executive function task and corpus callosum morphomtery. Cerebral Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kawamura M, Mochizuki S. Primary progressive apraxia. Neuropathology. 1999;19:249–258. [Google Scholar]
- Kopp B, Rist F, Mattler U. N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology. 1996;33(3):282–294. doi: 10.1111/j.1469-8986.1996.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24(3):810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Lord C, Rutter M. The autism diagnostic interview - revised (ADI-R) Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Leighton J, Bird G, Charman T, Heyes C. Weak imitative performance is not due to a functional `mirroring' deficit in adults with autism spectrum disorders. Neuropsychologia. 2008;46:1041–1049. doi: 10.1016/j.neuropsychologia.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Leuthold H, Sommer W, Ulrich R. Preparing for action: Inferences from CNV and LRP. Journal of Psychophysiology. 2004;18:77–88. [Google Scholar]
- Leuthold H, Jentzsch I. Neural correlates of advance movement preparation: A dipole source analysis approach. Brain Research. Cognitive Brain Research. 2001;12(2):207–224. doi: 10.1016/s0926-6410(01)00052-0. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders. 1989;19(2):185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- MacNeil LK, Mostofsky SH. Specificity of dyspraxia in children with autism. Neuropsychology. 2012;26(2):165–171. doi: 10.1037/a0026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain & Development. 2007;29(9):565–570. doi: 10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. Journal of the International Neuropsychological Society. 2006;12(3):314–326. doi: 10.1017/s1355617706060437. [DOI] [PubMed] [Google Scholar]
- Mottron L, Burack JA, Iarocci G, Belleville S, Enns JT. Locally oriented perception with intact global processing among adolescents with high-functioning autism: Evidence from multiple paradigms. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2003;44(6):904–913. doi: 10.1111/1469-7610.00174. [DOI] [PubMed] [Google Scholar]
- Muller RA, Pierce K, Ambrose JB, Allen G, Courchesne E. Atypical patterns of cerebral motor activation in autism: A functional magnetic resonance study. Biological Psychiatry. 2001;49(8):665–676. doi: 10.1016/s0006-3223(00)01004-0. [DOI] [PubMed] [Google Scholar]
- Murphy KR, Myors B. Statistical power analysis. A simple and general model for traditional and modern hypotheses tests. 2nd ed. Lawrence Erlbaum Associates; Mahwah, NJ: 2004. [Google Scholar]
- Näätänen R, Gaillard AW, Mäntysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychologica. 1978;42(4):313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Ramachandran VS, Pineda JA. Modulation of mu suppression in children with autism spectrum disorder in response to familiar or unfamiliar stimuli: The mirror neuron hypothesis. Neuropsychologia. 2008;46(5):1558–1565. doi: 10.1016/j.neuropsychologia.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Ramachandran VM. The simulating social mind: The role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychological Bulletin. 2007;133:310–327. doi: 10.1037/0033-2909.133.2.310. [DOI] [PubMed] [Google Scholar]
- Ozonoff S. Casual mechanisms of autism: unifying perspectives from an information-processing framework. In: Cohen DJ, Volkmar FR, editors. Handbook of autism and pervasive developmental disorders. John Wiley; New York: 1997. pp. 868–879. [Google Scholar]
- Ozonoff S, Strayer DL, McMahon WM, Filloux F. Executive function abilities in autism and Tourette syndrome: An information processing approach. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1994;35(6):1015–1132. doi: 10.1111/j.1469-7610.1994.tb01807.x. [DOI] [PubMed] [Google Scholar]
- Passingham R. Frontal lobes and voluntary action. Oxford University Press; Oxford: 1995. [Google Scholar]
- Platz T, Kim IH, Pintschovious H, Winter T, Kieselbach A, Villringer K, Kurth R, Mauritz KH. Multimodal EEG analysis in man suggests impairment-specific changes in movement-related electric brain activity after stroke. Brain. 2000;123(12):2475–2490. doi: 10.1093/brain/123.12.2475. [DOI] [PubMed] [Google Scholar]
- Piazza M, Fumarola A, Chinello A, Melcher D. Subitizing reflects visuo-spatial object individuation capacity. Cognition. 2011;121:147–153. doi: 10.1016/j.cognition.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Pless M, Carlsson M, Sundelin C, Persson K. Pre-school children with developmental co-ordination disorder: Self-perceived competence and group motor skill intervention. Acta Paediatrica. 2001;90(5):532–538. [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: An integrative review. Biological Psychology. 1995;41(2):103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Medicine. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Schmitz F, Freund HJ, Schnitzler A. Magneto-encephalographic correlates of the lateralized readiness potential. Brain Research. Cognitive Brain Research. 1999;8(2):77–85. doi: 10.1016/s0926-6410(99)00008-7. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Stegeman DF, Cools AR, Horstink MW. Reliance on external cues for movement initiation in Parkinson's disease. Evidence from movement-related potentials. Brain. 1998;121(1):167–177. doi: 10.1093/brain/121.1.167. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Meyer A, Cools A, Horstink MW, Stegeman DF. Movement preparation in Parkinson's disease. Time course and distribution of movement-related potentials in a movement precueing task. Brain. 1996;119(5):1689–1704. doi: 10.1093/brain/119.5.1689. [DOI] [PubMed] [Google Scholar]
- Pritchard WS. Psychophysiology of P300. Psychological Bulletin. 1981;89:506–540. [PubMed] [Google Scholar]
- Provost B, Lopez BR, Heimerl S. A comparison of motor delays in young children: Autism spectrum disorder, developmental delay, and developmental concerns. Journal of Autism and Developmental Disorders. 2007;37:321–328. doi: 10.1007/s10803-006-0170-6. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Oberman LM. Broken Mirrors: A theory of autism. Scientific American. 2006;295(5):62–69. doi: 10.1038/scientificamerican1106-62. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rowland LP, Pedley TA. Meritt's neurology. 12th ed. Lippincot Williams and Wilkins; Philadelphia, PA: 2010. [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Smith SE, Chatterjee A. Visuospatial attention in children. Archives of Neurology. 2008;65:1284–1288. doi: 10.1001/archneur.65.10.1284. [DOI] [PubMed] [Google Scholar]
- Spatt J, Goldenberg G. Speed of motor execution and apraxia. Journal of Clinical and Experimental Neuropsychology. 1997;19:850–856. doi: 10.1080/01688639708403765. [DOI] [PubMed] [Google Scholar]
- Stieglitz Ham H, Bartolo A, Corley M, Rajendran G, Szabo A, Swanson S. Exploring the relationship between gestural recognition and imitation: evidence of dyspraxia in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2011;41(1):1–12. doi: 10.1007/s10803-010-1011-1. [DOI] [PubMed] [Google Scholar]
- Sutera S, Pandey J, Esser EL, Rosenthal MA, Wilson LB, Barton M, Green J, Hodgson S, Robins DL, Dumont-Mathieu T, Fein D. Predictors of optimal outcome in toddlers diagnosed with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(1):98–107. doi: 10.1007/s10803-006-0340-6. [DOI] [PubMed] [Google Scholar]
- Thaler DE, Rolls ET, Passingham RE. Neuronal activity of the supplementary motor area (SMA) during internally and externally triggered wrist movement. Neuroscience Letters. 1988;93(2–3):264–269. doi: 10.1016/0304-3940(88)90093-6. [DOI] [PubMed] [Google Scholar]
- Ulrich R, Leuthold W, Sommer W. Motor programming of response force and movement direction. Psychophysiology. 1998;35:721–728. [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring process in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14(4):593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Van Waevelde H, Oostra A, Dewitte G, Van Den Broeck C, Jongmans MJ. Stability of motor problems in young children with or at risk of autism spectrum disorder, ADHD, and or developmental coordination disorder. Developmental Medicine and Child Neurology. 2010;52(8):e174–178. doi: 10.1111/j.1469-8749.2009.03606.x. [DOI] [PubMed] [Google Scholar]
- Vergeler R. Malfunctions of central control of movement studied with slow brain potentials in neurological patients. Journal of Psychophysiology. 2004;18(2):105–120. [Google Scholar]
- Wang L, Mottron L, Peng D, Berthiaume C, Dawson M. Local bias and local-to-global interference without global deficit: A robust finding in autism under various conditions of attention, exposure of attention, exposure time, and visual angle. Cognitive Neuropsychology. 2007;24(5):550–574. doi: 10.1080/13546800701417096. [DOI] [PubMed] [Google Scholar]
- Wascher E, Verleger R, Vieregge P, Jaskowski P, Koch S, Kompf D. Responses to cued signals in Parkinson's disease. Distinguishing between disorders of cognition and of activation. Brain. 1997;120(8):1355–1375. doi: 10.1093/brain/120.8.1355. [DOI] [PubMed] [Google Scholar]
- West R. Neural correlates of cognitive control and conflict detection in the Stroop and digit-location tasks. Neuropsychologia. 2003;41(8):1122–1135. doi: 10.1016/s0028-3932(02)00297-x. [DOI] [PubMed] [Google Scholar]
- West R, Bowry R, McConville C. Sensitivity of medial frontal cortex to response and nonresponse conflict. Psychophysiology. 2004;41(5):739–748. doi: 10.1111/j.1469-8986.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- Wiese H, Stude P, Nebel K, de Greiff A, Forsting M, Diener HC, Keidel M. Movement preparation in self-initiated versus externally triggered movements: An event-related fMRI-study. Neuroscience Letters. 2004;371(2–3):220–225. doi: 10.1016/j.neulet.2004.08.078. [DOI] [PubMed] [Google Scholar]
- Wijers AA, Mulder G, Gunter TC, Smid HGOM. Brain potential analysis of selective attention. In: Neumann O, Sanders AF, editors. Handbook of perception and action. 3. Attention. Academic Press; Tullamore, Ireland: 1996. pp. 333–387. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 4th ed. Harcourt Assessment, Inc; San Antonio, TX: 2003. [Google Scholar]
- Weimer AK, Schatz A, Lincoln A, Ballantyne AO, Trauner DA. “Motor” impairment in Asperger syndrome: Evidence for a deficit in propioception. Journal of Developmental and Behavioral Pediatrics. 2001;22(2):92–101. doi: 10.1097/00004703-200104000-00002. [DOI] [PubMed] [Google Scholar]
- Williams J, Whiten A, Suddendorf T, Perrett D. Imitation, mirror neurons, and autism. Neuroscience and Biobehavioral Reviews. 2001;25:577–596. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Changes in prefrontal axons may disrupt the network in autism. The Journal of Neuroscience. 2010;30(44):14595–14609. doi: 10.1523/JNEUROSCI.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]