Abstract
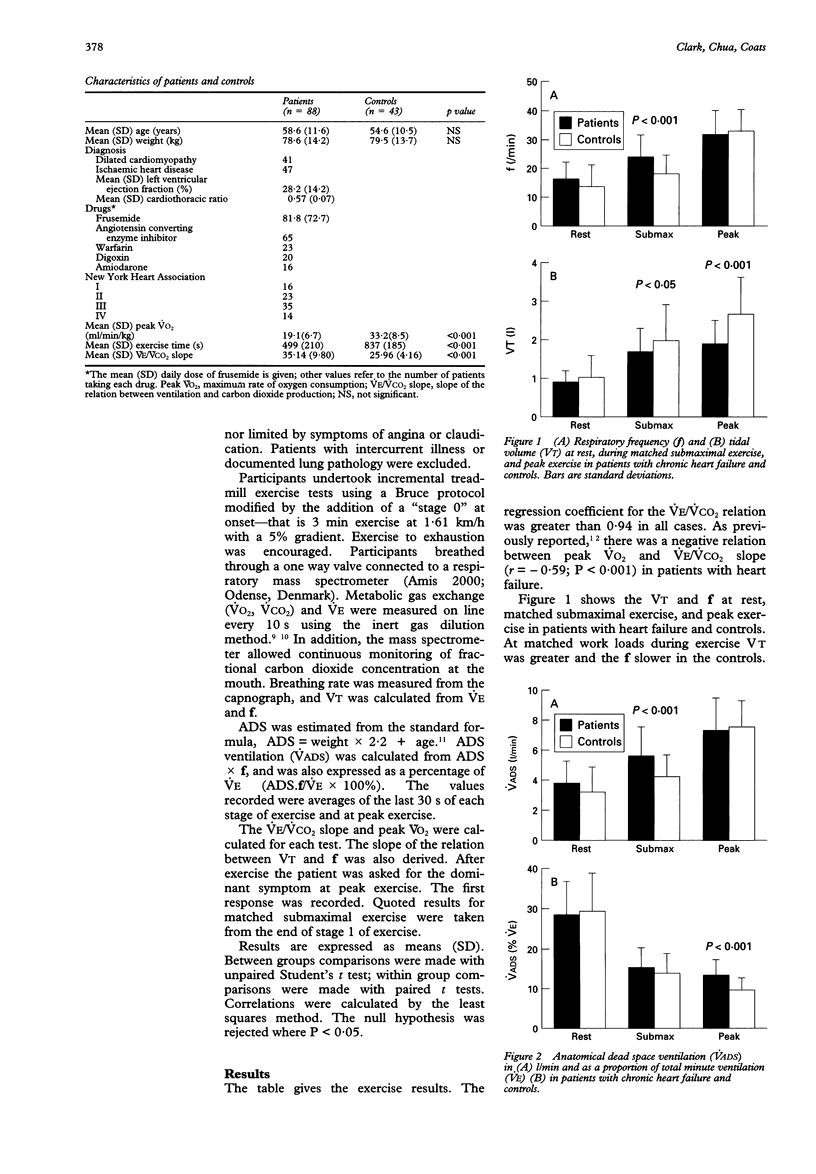
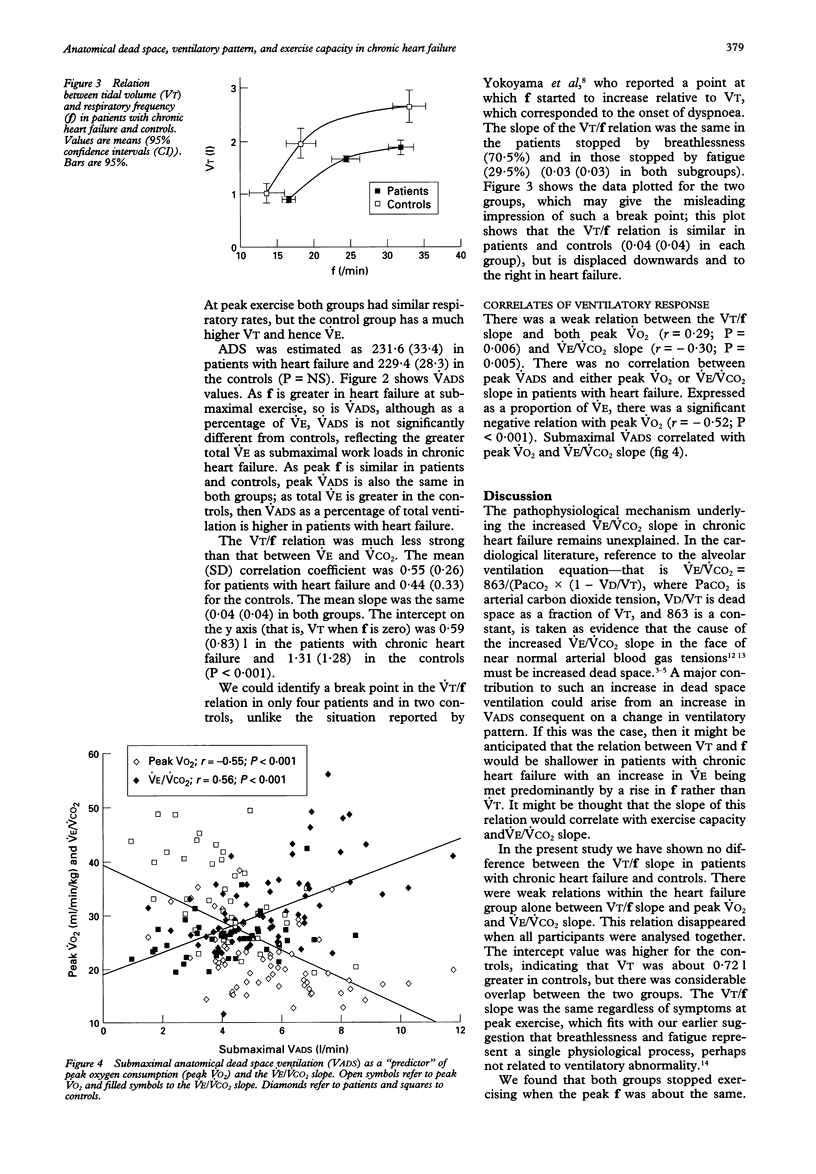
BACKGROUND--Patients with chronic heart failure have an excessive ventilatory response to exercise, characterised by an increase in the slope of the relation between ventilation and carbon dioxide production (VE/VCO2 slope). Patients have an altered respiratory pattern with an increased respiratory rate (f) at a given tidal volume (VT), which may result in increased anatomical dead space ventilation. METHODS--The ventilatory responses in 88 patients with chronic heart failure and 43 age matched controls during maximal incremental treadmill exercise were analysed. Peak oxygen consumption (VO2), VE/VCO2 slope, and the slope of the relation between f and VT were derived. Anatomical dead space was estimated from a standard formula and anatomical dead space ventilation calculated. RESULTS--Peak VO2 was greater (mean (SD)) (33.2 (8.5) v 19.4 (6.7) ml/min/kg; P < 0.001) and the VE/VCO2 slope lower in the controls (25.96 (4.16) v 35.14 (9.80); P < 0.001). During matched submaximal exercise VT was higher (1.97 (0.92) v 1.68 (0.62) 1; P < 0.05) and flower in the controls (18.23 (6.48) v 24.28 (7.58); P < 0.001). At peak exercise there was no difference in f, but VT was higher in the controls (2.66 (0.97) v 1.90 (0.61) 1; P < 0.001). The VT/f slope was the same (0.04 (0.04)) in both groups. The intercept of the relation was greater for the control group (1.31 (1.28) v 0.59 (0.83); P < 0.001). Anatomical dead space ventilation was lower in the controls at submaximal work load (4.17 (1.56) v 5.58 (1.93) l/min; P < 0.001). At peak exercise anatomical dead space ventilation was the same in both groups, but was lower expressed as a percentage of total VE in the control group (9.8 (3.3) v 13.5 (4.0); P < 0.001). There were weak relations within the heart failure group alone between VT/f slope and peak VO2 and VE/VCO2 slope. CONCLUSIONS--The relation between anatomical dead space ventilation and VE/VCO2 slope is expected: as f increases, so do VE/VCO2 slope and anatomical dead space ventilation. The VT/f slope was the same in patients with chronic heart failure and controls, so change in respiratory pattern cannot explain the increase in VE/VCO2 slope. The stimulus causing the increased f has yet to be identified.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buller N. P., Poole-Wilson P. A. Mechanism of the increased ventilatory response to exercise in patients with chronic heart failure. Br Heart J. 1990 May;63(5):281–283. doi: 10.1136/hrt.63.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. L., Coats A. J. Relationship between ventilation and carbon dioxide production in normal subjects with induced changes in anatomical dead space. Eur J Clin Invest. 1993 Jul;23(7):428–432. doi: 10.1111/j.1365-2362.1993.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Clark A. L., Coats A. J. Usefulness of arterial blood gas estimations during exercise in patients with chronic heart failure. Br Heart J. 1994 Jun;71(6):528–530. doi: 10.1136/hrt.71.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N. J., Denison D. M. The measurement of metabolic gas exchange and minute volume by mass spectrometry alone. Respir Physiol. 1979 Feb;36(2):261–267. doi: 10.1016/0034-5687(79)90029-x. [DOI] [PubMed] [Google Scholar]
- Davies S. W., Emery T. M., Watling M. I., Wannamethee G., Lipkin D. P. A critical threshold of exercise capacity in the ventilatory response to exercise in heart failure. Br Heart J. 1991 Apr;65(4):179–183. doi: 10.1136/hrt.65.4.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkin D. P., Perrins J., Poole-Wilson P. A. Respiratory gas exchange in the assessment of patients with impaired ventricular function. Br Heart J. 1985 Sep;54(3):321–328. doi: 10.1136/hrt.54.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metra M., Dei Cas L., Panina G., Visioli O. Exercise hyperventilation chronic congestive heart failure, and its relation to functional capacity and hemodynamics. Am J Cardiol. 1992 Sep 1;70(6):622–628. doi: 10.1016/0002-9149(92)90202-a. [DOI] [PubMed] [Google Scholar]
- Myers J., Salleh A., Buchanan N., Smith D., Neutel J., Bowes E., Froelicher V. F. Ventilatory mechanisms of exercise intolerance in chronic heart failure. Am Heart J. 1992 Sep;124(3):710–719. doi: 10.1016/0002-8703(92)90282-z. [DOI] [PubMed] [Google Scholar]
- Rubin S. A., Brown H. V., Swan H. J. Arterial oxygenation and arterial oxygen transport in chronic myocardial failure at rest, during exercise and after hydralazine treatment. Circulation. 1982 Jul;66(1):143–148. doi: 10.1161/01.cir.66.1.143. [DOI] [PubMed] [Google Scholar]
- Rubin S. A., Brown H. V. Ventilation and gas exchange during exercise in severe chronic heart failure. Am Rev Respir Dis. 1984 Feb;129(2 Pt 2):S63–S64. doi: 10.1164/arrd.1984.129.2P2.S63. [DOI] [PubMed] [Google Scholar]
- Sullivan M. J., Higginbotham M. B., Cobb F. R. Increased exercise ventilation in patients with chronic heart failure: intact ventilatory control despite hemodynamic and pulmonary abnormalities. Circulation. 1988 Mar;77(3):552–559. doi: 10.1161/01.cir.77.3.552. [DOI] [PubMed] [Google Scholar]
- Weber K. T., Kinasewitz G. T., Janicki J. S., Fishman A. P. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation. 1982 Jun;65(6):1213–1223. doi: 10.1161/01.cir.65.6.1213. [DOI] [PubMed] [Google Scholar]
- Yokoyama H., Sato H., Hori M., Takeda H., Kamada T. A characteristic change in ventilation mode during exertional dyspnea in patients with chronic heart failure. Chest. 1994 Oct;106(4):1007–1013. doi: 10.1378/chest.106.4.1007. [DOI] [PubMed] [Google Scholar]



