Abstract
Streptococcus pneumoniae is an important human pathogen. To cause disease, it must first colonize the nasopharynx. The widespread use of pneumococcal-conjugate vaccines which target the capsular polysaccharide has led to decreased nasopharyngeal carriage of vaccine serotypes, but a concomitant increase in carriage of non-vaccine serotypes and nonencapsulated S. pneumoniae (NESp). Some NESp express pneumococcal surface protein K (PspK), a virulence factor shown to contribute to nasopharyngeal colonization.
We present the case of a child with chronic adenoiditis caused by a PspK+ NESp. We tested the pneumococcal isolate, designated C144.66, for antimicrobial resistance, the presence of the pspK gene and the expression of PspK. Sequence typing and genome sequencing were performed. C144.66 was found to be resistant to erythromycin and displayed intermediate resistance to penicillin and trimethoprim/sulfamethoxazole. C144.66 has the pspK gene in place of the capsule locus. Additionally, PspK expression was confirmed by flow cytometry. NESp are a growing concern as an emerging human pathogen, as current pneumococcal vaccines do not confer immunity against them. An inability to vaccinate against NESp may result in increased carriage and associated pathology.
Keywords: Streptococcus pneumoniae, Chronic adenoiditis, Pneumococcal surface protein K, Nonencapsulated Streptococcus pneumoniae (NESp)
Introduction
Streptococcus pneumoniae is the most common bacterial cause of acute otitis media (AOM) and pneumonia in children [1], [2]. The pneumococcus is an important cause of bacterial rhinosinusitis as well as invasive disease such as sepsis and meningitis [3]. Historically, pneumococci that cause disease are encapsulated. Therefore, the 13-valent pneumococcal conjugate polysaccharide vaccine (PCV) and the 23-valent pneumococcal polysaccharide vaccine (PPV) target the specific capsular serotypes known to cause the majority of human disease [4]. Introduction of the PCV and PPV has led to decreased nasopharyngeal colonization of pneumococcal vaccine serotypes in humans, with a concomitant increase in isolation of both non-vaccine capsular serotypes and nonencapsulated S. pneumoniae (NESp) [5], [6]. There has, additionally, been an increase in prevalence of disease caused by NESp [7], [8], [9], [10], [11], [12], [13], [14], [15].
Some NESp isolates express pneumococcal surface protein K (PspK) [12]. PspK has been shown to aid colonization of the nasopharynx [16]. We have previously shown that PspK increases adhesion of NESp to human epithelial cells and enhances AOM in the chinchilla model [17]. Expression of PspK in an avirulent NESp was sufficient to induce pathogenicity, and deletion of PspK from a virulent strain decreased the bacterial burden in chinchillas [17]. We present a case of a child with chronic adenoiditis found to have PspK+ NESp from a sinus culture at adenoidectomy. To our knowledge, this is the first report of a PspK+ NESp isolate from the United States.
Case report
A 2-year-old male presented to otolaryngology clinic for evaluation of chronic adenoiditis. Patient had a 6 month history of nasal congestion with a clear mucosal discharge and was a chronic mouth breather since birth. Of note, patient was refractory to several trials of antimicrobials. Enlarged adenoids were appreciated on physical exam. The patient's family was counseled and elected to undergo adenoidectomy, which was completed without complication. During the procedure, bilateral maxillary sinuses were aspirated, both revealing sanguineous fluid that was sent for aerobic and anaerobic culture. The sinuses were then irrigated with saline. The right sinus culture returned with no growth after 48 h, and the left sinus culture had moderate growth of S. pneumoniae. Anaerobic cultures showed no growth. After 24 h observation, the patient was discharged. After discharge, the patient was lost to follow up.
Antimicrobial sensitivities of the isolate, complete with minimum inhibitory concentrations, showed erythromycin resistance, as well as intermediate resistance to both penicillin G and trimethoprim/sulfamethoxazole (Table 1).
Table 1.
Antimicrobial susceptibility of S. pneumoniae C144.66.
| Antibiotic | Resistance |
|---|---|
| Erythromycin | Resistant |
| Penicillin G | Intermediate |
| Trimethoprim/sulfamethoxazole | Intermediate |
| Levofloxacin | Susceptible |
| Tetracycline | Susceptible |
| Vancomycin | Susceptible |
| Cefotaxime | Susceptible |
| Ceftriaxone | Susceptible |
| Chloramphenicol | Susceptible |
The clinical isolate was designated C144.66. Genomic DNA was isolated from C144.66 using Qiagen DNeasy kit. Polymerase chain reaction amplification was performed with primers for pspK, LSM826 (5′-CCCGGGGCATGAATAATAAGAATATCA-3′) and LSM827 (5′-GAATTCGCCTAATTTTTATGTTTAACAAATG-3′) and for the conserved capsule gene cpsA (forward: 5′-GCAGTACAGCAGTTTGTTGGACTGACC-3′ and reverse: 5′-GAATATTTTCATTATCAGTCCCAGTC-3′). PCR, Western blot analysis, and flow cytometry were completed through standard methods [16], [18]. Polymerase chain reaction analysis compared to an encapsulated strain showed that C144.66 lacked the highly conserved first gene in the capsule locus, cpsA (Fig. 1). Further PCR analysis demonstrated the presence of pspK. Expression of PspK was confirmed through Western blot (data not shown), and flow cytometry comparing C144.66 to a known PspK positive strain (Fig. 2).
Fig. 1.
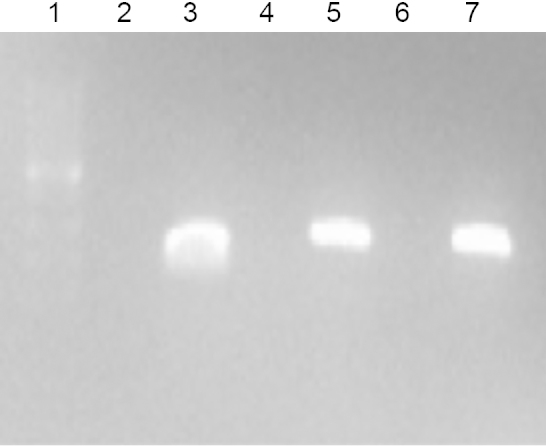
PCR analysis of C144.66. PCR analysis of strains MNZ11 (cpsA−, pspK+) D39 (cpsA+, pspK−), and C144.66 (cpsA−, pspK+), respectively, for cpsA (lanes 2–4) and pspK (lanes 5–7). Lane 1 is a 1-Kb DNA ladder.
Fig. 2.
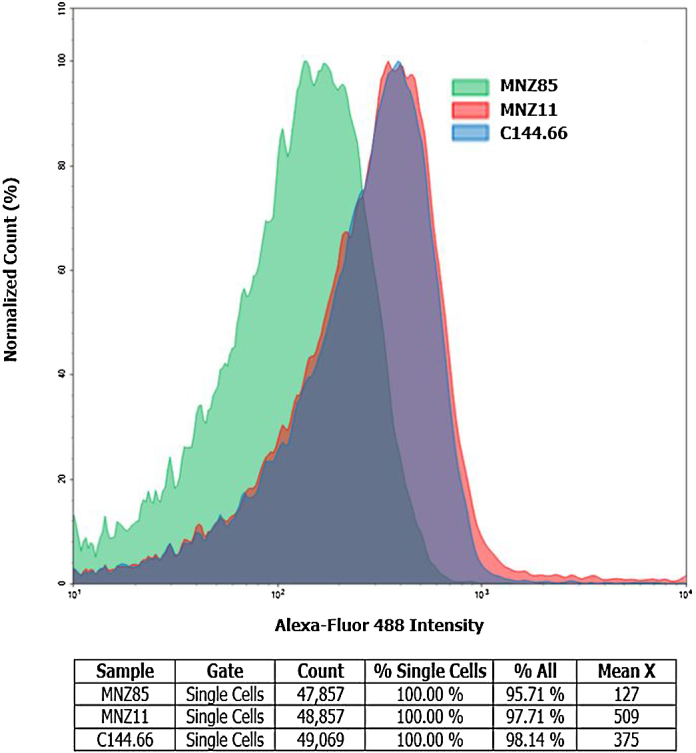
Flow cytometry for the presence of PspK. For analysis 1 × 107 log phase pneumococci were incubated with a mouse anti-PspK antiserum. A biotinylated anti-mouse secondary antibody was detected by streptavidin conjugated to Alex Flour 488. A representative histogram is shown. The negative control is MNZ85 which lacks PspK.
To further characterize C144.66, multilocus sequence typing was performed as previously described [19]. Additionally, genome sequencing was carried out on C144.66. Multiplexed paired-end libraries (2× 150 bp) were prepared using a Nextera XT DNA sample preparation kit (Illumina). Sequencing was done with an Illumina MiSeq. CLC Genomics Workbench v6.0 software was used for quality trimming of the reads and de novo assembly. Rapid Annotations using Subsystems Technology (RAST) pipeline determined gene prediction and annotation for the assembled contigs.
The sequence type (ST) of C144.66 was 9570. A draft genome sequence of C144.66 was generated to provide further information about the strain. Both approaches confirmed that C144.66 was a PspK+ NESp.
We previously demonstrated that C144.66 can cause AOM in the chinchilla model [17]. To extend our observation, we used a mouse model of colonization. C57/BL6 mice were anesthetized with isoflurane and infected by inoculation with 1 × 107 colony forming units of C144.66 into the nasal passage. After 5 days, the mice were euthanized. Nasopharyngeal washes as well as nasal passages and middle ears were collected to estimate the bacterial load by plating on blood agar with 200 μg/mL gentamicin. All mice were found to have nasopharyngeal colonization by C144.66; additionally, C144.66 was found to have ascended to the middle ear in a subset of the mice (Fig. 3).
Fig. 3.
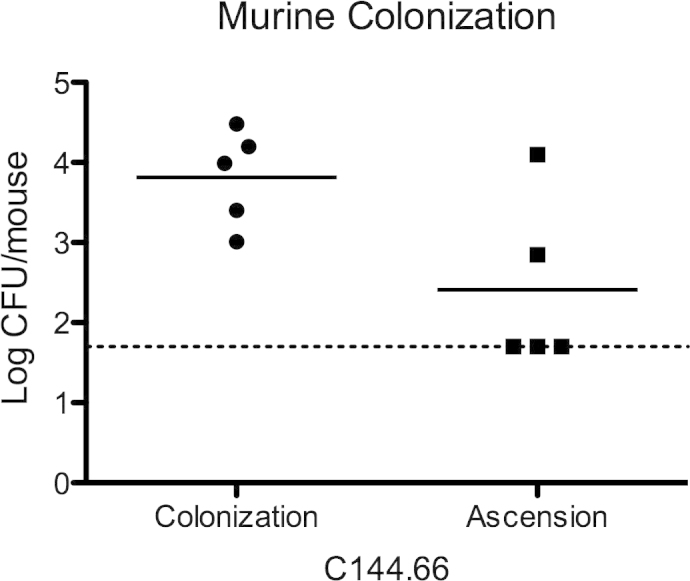
Colonization and ascension of C144.66 in a murine model. After intranasal inoculation of C144.66, colonization was achieved in all mice. Ascension into the middle ear occurred in two of the five mice. The dash line is the lower limit of detection.
Conclusion
This case of chronic adenoiditis caused by NESp reinforces the growing concern of infection caused by pneumococcal strains not covered by the current vaccines. With widespread use of PCV and PPV, human nasopharyngeal colonization by NESp will likely continue to increase.
Since pneumococcal colonization is a prerequisite for disease, it is likely that with increased carriage of NESp there will be increased disease burden. A recent study from Japan found that 6.4% of AOM isolates were NESp with 4.7% of the NESp being PspK positive [20]. AOM is the most common indication for antimicrobial prescription for children in the United States, and has a financial burden in excess of $2.8 billion, a number likely to grow with increasing infections with non-vaccine serotypes and NESp [21]. After widespread use of the 7-valent PCV (prior to the introduction of the 13-valent PCV), new serotypes, including capsular serotype 19A, emerged; some strains were multidrug resistant [22], [23]. Studies have shown that NESp can contain multiple antimicrobial resistance genes [24], [25], [26]. Thus, co-colonization of NESp with encapsulated pneumococci could lead to increased antimicrobial resistance among encapsulated pneumococci. Additionally, there is concern for the emergence of novel serotypes as well as proliferation of NESp with novel proteins, such as PspK, which are not covered by currently available pneumococcal vaccines.
Acknowledgements
We acknowledge Ms. Jessica Friley for her help with animal studies and Ms. Haley Pipkins for technical support. The research was supported in part by funds from the Medical Scholars Research Program of the University of Mississippi Medical Center and institutional research funds.
References
- 1.Kaur R., Adlowitz D.G., Casey J.R., Zeng M., Pichichero M.E. Simultaneous assay for four bacterial species including Alloiococcus otitidis using multiplex-PCR in children with culture negative acute otitis media. Pediatr Infect Dis J. 2010;29:741–745. doi: 10.1097/INF.0b013e3181d9e639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelton S.I., Hammerschlag M.R. Overcoming current obstacles in the management of bacterial community-acquired pneumonia in ambulatory children. Clin Pediatr (Phila) 2005;44:1–17. doi: 10.1177/000992280504400101. [DOI] [PubMed] [Google Scholar]
- 3.Musher D.M. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin Infect Dis. 1992;14:801–807. doi: 10.1093/clinids/14.4.801. [DOI] [PubMed] [Google Scholar]
- 4.Tan T.Q. Pediatric invasive pneumococcal disease in the United States in the era of pneumococcal conjugate vaccines. Clin Microbiol Rev. 2012;25:409–419. doi: 10.1128/CMR.00018-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang S.S., Platt R., Rifas-Shiman S.L., Pelton S.I., Goldmann D., Finkelstein J.A. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics. 2005;116:e408–e413. doi: 10.1542/peds.2004-2338. [DOI] [PubMed] [Google Scholar]
- 6.Flasche S., Van Hoek A.J., Sheasby E., Waight P., Andrews N., Sheppard C. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med. 2011;8:e1001017. doi: 10.1371/journal.pmed.1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ing J., Mason E.O., Kaplan S.L., Lamberth L.B., Revell P.A., Luna R.A. Characterization of nontypeable and atypical Streptococcus pneumoniae pediatric isolates from 1994 to 2010. J Clin Microbiol. 2012;50:1326–1330. doi: 10.1128/JCM.05182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacapa R., Bliss S.J., Larzelere-Hinton F., Eagle K.J., McGinty D.J., Parkinson A.J. Changing epidemiology of invasive pneumococcal disease among White Mountain Apache persons in the era of the pneumococcal conjugate vaccine. Clin Infect Dis. 2008;47:476–484. doi: 10.1086/590001. [DOI] [PubMed] [Google Scholar]
- 9.Melchiorre S., Camilli R., Pietrantoni A., Moschioni M., Berti F., Del Grosso M. Point mutations in wchA are responsible for the non-typability of two invasive Streptococcus pneumoniae isolates. Microbiology. 2011;158:338–344. doi: 10.1099/mic.0.054270-0. [DOI] [PubMed] [Google Scholar]
- 10.Norcross E.W., Tullos N.A., Taylor S.D., Sanders M.E., Marquart M.E. Assessment of Streptococcus pneumoniae capsule in conjunctivitis and keratitis in vivo neuraminidase activity increases in nonencapsulated pneumococci following conjunctival infection. Curr Eye Res. 2010;35:787–798. doi: 10.3109/02713683.2010.492462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okade H., Funatsu T., Eto M., Furuya Y., Mizunaga S., Nomura N. Impact of the pneumococcal conjugate vaccine on serotype distribution and susceptibility trends of pediatric non-invasive Streptococcus pneumoniae isolates in Tokai, Japan over a 5-year period. J Infect Chemother. 2014;20:423–428. doi: 10.1016/j.jiac.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Park I.H., Kim K.-H., Andrade A.L., Briles D.E., McDaniel L.S., Nahm M.H. Nontypeable pneumococci can be divided into multiple cps types, including one type expressing the novel gene pspK. MBio. 2012;3 doi: 10.1128/mBio.00035-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park I.H., Geno K.A., Sherwood L.K., Nahm M.H., Beall B. Population-based analysis of invasive nontypeable pneumococci reveals that most have defective capsule synthesis genes. PLOS ONE. 2014;9:e97825. doi: 10.1371/journal.pone.0097825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porat N., Greenberg D., Givon-Lavi N., Shuval D.S., Trefler R., Segev O. The important role of nontypable Streptococcus pneumoniae international clones in acute conjunctivitis. J Infect Dis. 2006;194:689–696. doi: 10.1086/506453. [DOI] [PubMed] [Google Scholar]
- 15.Keller L.E., Robinson D.A., McDaniel L.S. Nonencapsulated Streptococcus pneumoniae: emergence and pathogenesis. mBio. 2016;7(2):e01792–e017915. doi: 10.1128/mBio.01792-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller L.E., Jones C.V., Thornton J.A., Sanders M.E., Swiatlo E., Nahm M.H. PspK of Streptococcus pneumoniae increases adherence to epithelial cells and enhances nasopharyngeal colonization. Infect Immun. 2013;81:173–181. doi: 10.1128/IAI.00755-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller L.E., Friley J., Dixit C., Nahm M.H., McDaniel L.S. Nonencapsulated Streptococcus pneumoniae cause acute otitis media in the chinchilla that is enhanced by pneumococcal surface protein K. Open Forum Infect Dis. 2014;1:ofu037. doi: 10.1093/ofid/ofu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDaniel L.S., Sheffield J.S., Delucchi P., Briles D.E. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular type. Infect Immun. 1991;59:222–228. doi: 10.1128/iai.59.1.222-228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enright M.C., Spratt B.G. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144(Pt 1):3049–3060. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 20.Hotomi M., Nakajima K., Hiraoka M., Nahm M.H., Yamanaka N. Molecular epidemiology of nonencapsulated Streptococcus pneumoniae among Japanese children with acute otitis media. J Infect Chemother. 2016;22:72–77. doi: 10.1016/j.jiac.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Soni A.P. Stat Br #228; 2008. Ear infections (Otitis media) in children (0–17): use and expenditures, 2006; p. 5. [Google Scholar]
- 22.Xu Q., Pichichero M.E., Casey J.R., Zeng M. Novel type of Streptococcus pneumoniae causing multidrug-resistant acute otitis media in children. Emerg Infect Dis. 2009;15:547–551. doi: 10.3201/eid1504.071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pichichero M.E., Casey J.R. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298:1772–1778. doi: 10.1001/jama.298.15.1772. [DOI] [PubMed] [Google Scholar]
- 24.Sánchez-Tatay D., Arroyo L.A., Tarragó D., Lirola M.J., Porras A., Fenoll A. Antibiotic susceptibility and molecular epidemiology of nasopharyngeal pneumococci from Spanish children. Clin Microbiol Infect. 2008;14:797–801. doi: 10.1111/j.1469-0691.2008.02025.x. [DOI] [PubMed] [Google Scholar]
- 25.Andrade A.L.S., Franco C.M., Lamaro-Cardoso J., André M.C.D.P.B., Oliveira L.L.G., Kipnis A. Non-typeable Streptococcus pneumoniae carriage isolates genetically similar to invasive and carriage isolates expressing capsular type 14 in Brazilian infants. J Infect. 2010;61:314–322. doi: 10.1016/j.jinf.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chewapreecha C., Harris S.R., Croucher N.J., Turner C., Marttinen P., Cheng L. Dense genomic sampling identifies highways of pneumococcal recombination. Nat Genet. 2014;46:305–309. doi: 10.1038/ng.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]


