Abstract
A 65-year-old Japanese male with type 2 diabetes mellitus was admitted to our hospital with a productive cough and worsening dyspnea. He had started receiving vildagliptin, which is one of the dipeptideylpeptidase-4 (DPP-4) inhibitors, several days before the appearance of his symptoms. Laboratory findings revealed markedly elevated levels of immunoglobulin E and Krebs von den Lungen-6. Chest computed tomography revealed ground-glass opacity with irregular reticulation throughout both lungs. Biopsy specimens by transbronchial lung biopsy showed subacute interstitial pneumonia and an organizing pneumonia pattern with acute alveolar injury. The drug lymphocyte stimulation test showed a positive result for vildagliptin. Withdrawal of vildagliptin and administration of glucocorticoid treatment improved his respiratory condition and radiological findings. Therefore, we diagnosed the patient with vildagliptin-induced interstitial pneumonia based on both his clinical course and pathological findings. Interstitial pneumonia as a side effect of vildagliptin is rare. It may be necessary to monitor the respiratory condition of patients upon administration of DPP-4 inhibitors until further evidence is obtained.
Keywords: Drug-induced lung injury, DPP-4 inhibitor, Vildagliptin
Abbreviations: BAL, bronchoalveolar lavage; CT, computed tomography; DLST, drug lymphocyte stimulation tests; DPP-4, dipeptideylpeptidase-4; FVC, forced vital capacity; IgE, immunoglobulin E; IPAF, interstitial pneumonia with autoimmune features; KL-6, Krebs von den Lungen-6; PFT, pulmonary function testing; TBLB, transbronchial lung biopsy; T2DM, type 2 diabetes mellitus
1. Introduction
Type 2 diabetes mellitus (T2DM) is one of the most challenging health-care problems, and novel therapeutic strategies are necessary. Vildagliptin, one of the dipeptidyl peptidase (DPP)-4 inhibitors, is an oral anti hyperglycemic agent that enhances insulin secretion in a glucose-dependent manner and has been widely used in the management of T2DM. The known side effects of vildagliptin are hypersensitivity reactions, including skin disorders, hepatic toxicity and so forth [1]. Drug-induced lung injury including interstitial pneumonia associated with vildagliptin has rarely been reported. We here describe, to the best of our knowledge, the first case of interstitial pneumonia in a Japanese patient receiving vildagliptin.
2. Case report
A 65-year-old Japanese male was admitted to our hospital with a productive cough and progressive dyspnea. His comorbidities were hypertension and T2DM; therefore, he regularly received some medications. Several days before the appearance of his chief complaints, vilidagliptin (100 mg/day) was started for uncontrolled T2DM. His respiratory condition gradually worsened over about two weeks.
On hospital admission, his vital signs were as follows: body temperature 35.8 °C, blood pressure 120/79 mmHg, heart rate 66 bpm, and oxygen saturation 98% under 2 L/min of oxygen. On physical examination, the patient had fine crackles in both lung fields on chest auscultation. There were no physical signs suggestive of collagen vascular diseases. The laboratory tests showed high levels of serum immunoglobulin E (IgE: 4216 mg/dl, normal range<400 mg/dl) and Krebs von den Lungen-6 (KL-6: 9781 U/ml, normal range<500 U/ml). Examination of autoantibody titers, including anti-nuclear antibody, anti-ribonucleoprotein antibody, anti-smith antibody, anti-Ro/SSA antibodies and anti-La/SSB antibodies, as well as anti centromere antibody, anti-topoisomeraseI antibody, anti t-RNA synthetase antibody and serum complement, demonstrated that all were within normal range. Arterial blood gas analysis on 2 L/min of oxygen revealed respiratory alkalosis (pH: 7.450, PaO2: 111.6 Torr, PaCO2: 32.1 Torr, HCO3−: 21.8 mmol/L). A chest radiograph showed reduction of bilateral lung-volume and reticular shadows in all lung fields (Fig. 1a). Chest computed tomography (CT) demonstrated extensive ground-glass opacity (GGO) with associated irregular reticulation throughout both lungs. The distribution of interstitial shadows was peribronchovascular and basal dominant (Fig. 1b and c). Pulmonary function test (PFT) revealed a restrictive defect; forced vital capacity (FVC) was 43.2% of the predicted value. Flexible bronchoscopy showed normal airway anatomy. Bronchoalveolar lavage (BAL) fluid revealed inflammatory changes with a cell differential count of 23% macrophages, 57% lymphocytes, 5% neutrophils, and 12% eosinophils. Microbiological studies of BAL fluid were negative. Transbronchial lung biopsy (TBLB) was performed and biopsy samples were obtained from both the left upper and lower lobes. Biopsy specimens of the lung showed atypical and multinucleated regenerative alveolar epithelial cells, and infiltration of eosinophils, lymphocytes and plasma cells was observed (Fig. 2a). Interstitial fibrosis was seen both in the alveoli and around the thickened alveolar walls (Fig. 2b). These findings of TBLB specimens were consistent with subacute interstitial pneumonia, which is an organizing pneumonia with an acute alveolar injury pattern. In addition, the drug lymphocyte stimulation test (DLST) for vildagliptin was positive. From these results, we diagnosed the patient as having vildagliptin-induced interstitial pneumonia.
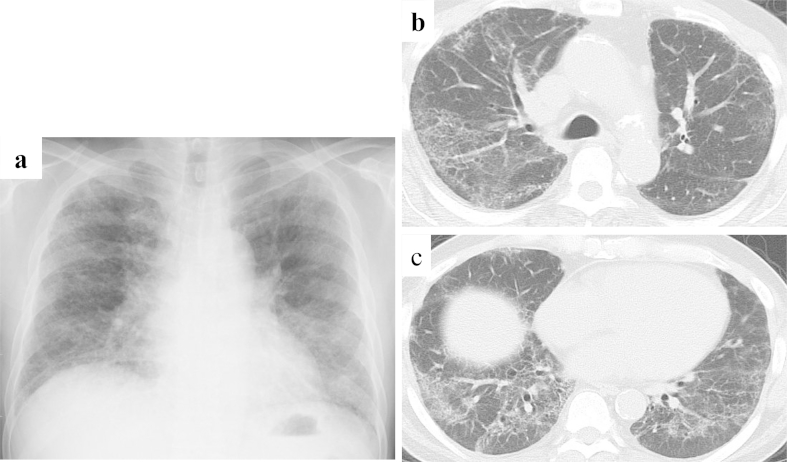
Fig. 1.
(a) Chest X-ray picture on admission. Reduction of lung volume and reticular shadows were observed bilaterally. (b and c) Chest computed tomography showed extensive ground-glass opacity including irregular reticular opacity in both lung fields. The distribution of interstitial shadows was peribronchovascular and basal dominant.
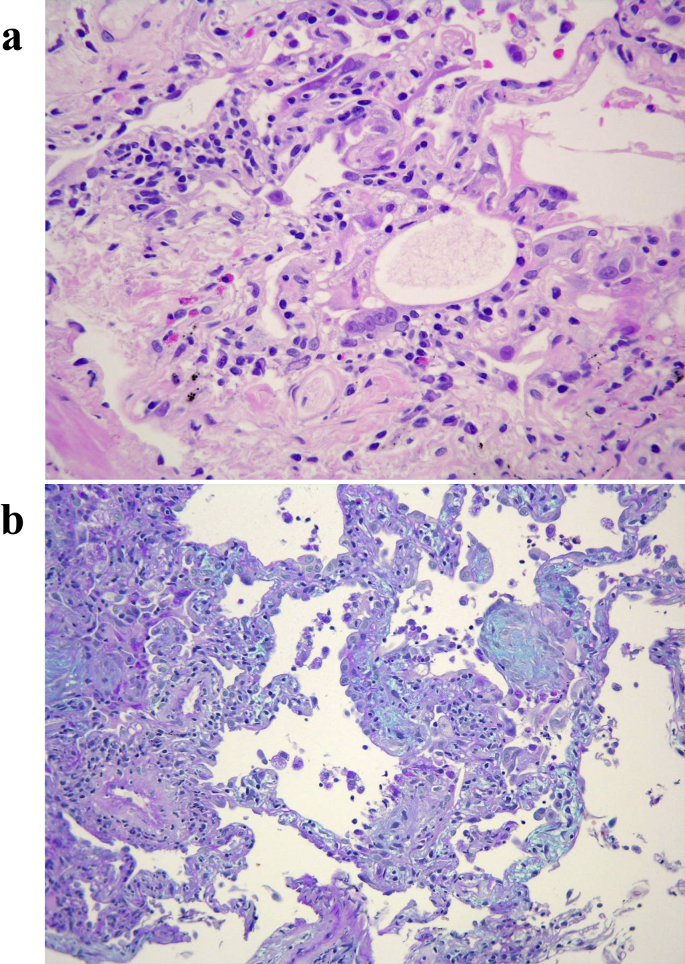
Fig. 2.
Pathological findings of biopsy specimens. (a) Atypical and multinucleated regenerating alveolar epithelial cells are found. Eosinophils, lymphocytes and plasma cells have infiltrated the lungs (Hematoxylin and Eosin staining). (b) Dense air space aggregates are present and stained blue, which indicated the subacute phase of the disease (Alcian-blue-PAS staining).
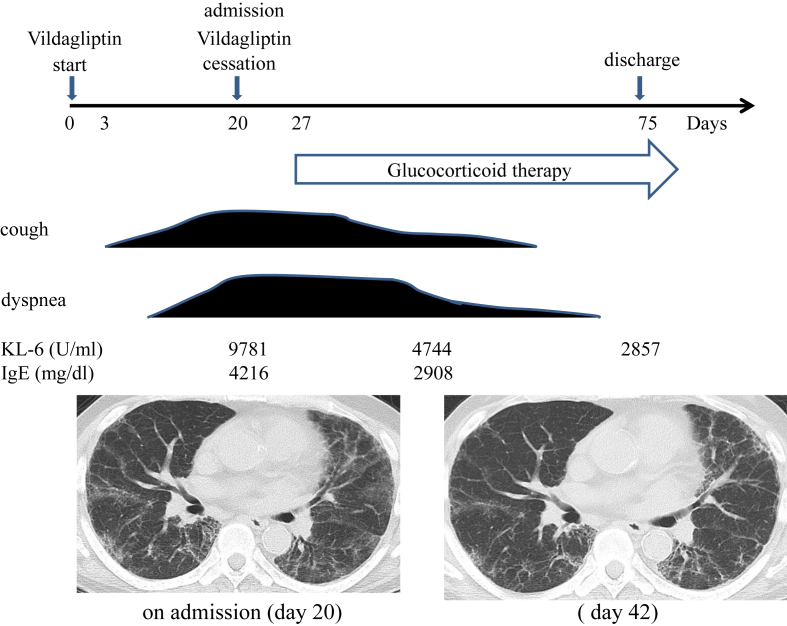
Vildagliptin was discontinued on the day that the patient was admitted (day 20) and glucocorticoid therapy was initiated on day 27 (Fig. 3). The respiratory condition and GGO findings on chest CT gradually improved after glucocorticoid treatment was started. The patient was discharged on day 75. The patient continues to take his regular his regular medications except vildagliptin, and has had no recurrence.
Fig. 3.
Clinical course of the patient. After cessation of vildagliptin and initiation of glucocorticoid therapy, the patient's respiratory symptoms and GGO findings on chest CT gradually improved. Serum KL-6 and IgE levels also improved.
3. Discussion
We herein described a very rare case of vildagliptin-induced interstitial pneumonia. To the best of our knowledge, there is no case report of vildagliptin-induced interstitial pneumonia in PubMed, U.S. Food and Drug Administration (FDA) and Pneumotox®. According to the ICHUSHI website, the database of Japanese articles, one case of pneumonitis induced by vildagliptin has been reported in the Japanese literature [2]. In addition, safety information by the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan suggested the association between vildagliptin and lung injury among Japanese people [3].
Little is known about the mechanism by which DPP-4 inhibitors including vildagliptin induce lung injury. DPP-4, also identified as CD26 antigen, is a type II trans-membrane glycoprotein that is constitutively expressed on activated lymphocytes and in lung parenchyma including endothelial cells and fibroblasts [4]. The effect of CD26/DPP-4 inhibitors on lung inflammation and fibrosis has not been fully investigated.
Stephan et al. reported [5] that continuous DPP-4 inhibition by oral administration could aggravate allergic inflammation of the airway; however, topical inhibition of DPP-4 by aerosolization had a beneficial effect on allergic symptoms in mice. On the other hand, Jungraithmay et al. [6] reported that CD26/DPP-4 inhibition recruited regenerative stem cells to the lungs and beneficially influenced ischemia-reperfusion lung injury after lung transplantation in mice. They demonstrated that DPP-4 inhibition increased the level of stromal cell-derived factor-1 (SDF-1) in plasma and the lungs together with up-regulation of its receptor CXC4. The SDF-1-CXCR4 axis improved engraftment of stem cells following tissue injury [6]. Recently, Sada et al. [7] reported a case of sarcoid-like lung granulomas following the administration of vildagliptin. The granuloma lesions disappeared after discontinuation of vildagliptin. The relationship between DPP-4 inhibition and granuloma formation in the lungs is unclear. The effect of a DPP-4 inhibitor on inflammation or immune system of the lungs is unclear and further studies are necessary.
As is well known, there is no specific clinical, radiological, or pathological feature for the diagnosis of drug-induced lung injury [8]. In the present case, it is clear that respiratory symptoms had been recognized several days after administration of vildagliptin and gradually worsened over two weeks. His symptoms and radiological findings recovered following the withdrawal of vildagliptin and administration of glucocorticoid. Pathological findings of lung specimens obtained by TBLB showed sub-acute interstitial pneumonia and organizing pneumonia with an acute alveolar injury pattern consistent with drug-induced lung injury. Of course the findings of chest CT in this case may be almost indistinguishable from those of interstitial pneumonia with autoimmune features (IPAF) but the pathologic findings were decisively different from those of IPAF. That is to say, infiltration of eosinophils and acute alveolar injury pattern were observed. These findings do not meet the diagnostic criteria for nonspecific interstitial pneumonia (NSIP) [9], [10]. Moreover, the result of the DLST for vildagliptin was positive. The DLST is not particularly accurate, and compelling evidence about the sensitivity and specificity of diagnosing drug-induced lung (DILD) injury has not been obtained. Therefore, it is indisputable that DILD injury should be diagnosed by exclusion of other diseases based on the patient's clinical course, laboratory data and imaging results [11]. Therefore, we diagnosed the present case with vildagliptin-induced interstitial pneumonia. Although the drug re-challenging test is recommended if possible, we did not believe that re-challenging of vildagliptin was safe in the present case.
In conclusion, we reported a very rare case of vildagliptin-induced interstitial pneumonia. To the best of our knowledge, this is the first report of vildagliptin-induced interstitial pneumonia that was diagnosed based on both the patient's clinical course and pathological findings.
Our purpose of reporting the present case is to alert physicians that they may encounter such unexpected adverse events in patients undergoing vildagliptin therapy. Accumulation of cases and further research are necessary in order to determine the mechanism of lung injury and predictable risk factors.
Conflicts of interest
No conflicts of interest exist with any companies/organizations.
References
- 1.Karagiannis T., Boura P., Tsapas A. Safety of dipeptidyl peptidase 4 inhibitors: a perspective review. Ther. Adv. Drug Saf. 2014;5:138–146. doi: 10.1177/2042098614523031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yajima T., Jingu D. A case of drug-induced pneumonitiss caused by vildagliptin. AJRS. 2015;4:176–218. [Google Scholar]
- 3.The issue of Pharmaceuticals and Medical Devices Agency (PMDA) safety information. 2013;305:17–18. [Google Scholar]
- 4.De Meester I., Korom S., Van Damme J. CD26, let it cut or cut it down. Immunol. Today. 1999;20:367–375. doi: 10.1016/s0167-5699(99)01486-3. [DOI] [PubMed] [Google Scholar]
- 5.Stephan M., Suhling H., Schade J. Effects of dipeptidyl peptidase-4 inhibition in an animal model of experimental asthma: a matter of dose, route, and time. Physiol. Rep. 2013;1:e00095. doi: 10.1002/phy2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jungraithmayr W., De Meester I., Matheeussen V. CD26/DPP-4 inhibition recruits regenerative stem cells via stromal cell-derived factor-1 and beneficially influences ischaemia-reperfusion injury in mouse lung transplantation. Eur. J. Cardiothorac. Surg. 2012;41:1166–1173. doi: 10.1093/ejcts/ezr180. [DOI] [PubMed] [Google Scholar]
- 7.Sada K., Wada J., Morinaga H. Sarcoid-like lung granulomas in a hemodialysiss patient treated with a dipeptidyl peptidese-4 inhibitor. Clin. Kidney J. 2014;7:182–185. doi: 10.1093/ckj/sft172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camus P., Fanton A., Bonniaud P. Interstitial lung disease induced by drugs and radiation. Respiration. 2004;71:301–326. doi: 10.1159/000079633. [DOI] [PubMed] [Google Scholar]
- 9.Fischer A., Antoniou K.M., Brown K.K. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015;46:976–987. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 10.Travis William D., Hunninghake Gary, King Talmadge E., Jr. Idiopathic nonspecific interstitial pneumonia report of an American Thoracic Society project. Am. J. Respir. Crit. Care Med. 2008;177:1338–1347. doi: 10.1164/rccm.200611-1685OC. [DOI] [PubMed] [Google Scholar]
- 11.Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir. Res. 2012;13:39. doi: 10.1186/1465-9921-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]