Manipulations of plasma cholesterol concentrations have been the mainstay of experimental atherosclerosis research for many decades. Prior to the introduction of the widespread use of genetic manipulations, atherosclerosis research primarily relied on dietary manipulations to produce hypercholesterolemic states.1 This was easily achieved in some species, such as rabbits, by the addition of cholesterol to the diet.2 While many animals have been used in the development of atherosclerosis studies, there has been a dramatic increase in focusing on the use of mice to determine mechanisms of the disease. Like many species, mice do not readily respond to elevations of dietary cholesterol to generate a hypercholesterolemic state. The use of mice for atherosclerosis research was pioneered by Dr. Beverly Paigen’s landmark studies described the appearance and quantification of lesions in the mouse aorta after mice were fed a diet containing high content of cholesterol and cholate.3,4
The application of mice to atherosclerosis studies was enhanced dramatically by manipulation of genes relevant to lipoprotein metabolism. For example, mice deficient in apoE are hypercholesterolemic and develop atherosclerotic lesions even when fed normal laboratory diets.5,6 The extent of hypercholesterolemia and atherosclerosis are enhanced by feeding diets that mimic the fat contents of some fast food companies.7 Another widely used model is the LDL receptor knockout mouse, and in this case, the use of a high-cholesterol/high-fat diet is required to achieve hypercholesterolemia and atherosclerosis.8 For both strains of mice, numerous studies have demonstrated that atherosclerosis develops in several vascular areas, particularly in the aortic root and innominate artery, although superficial atherosclerotic lesions can be found in focal areas throughout the aorta.9,10
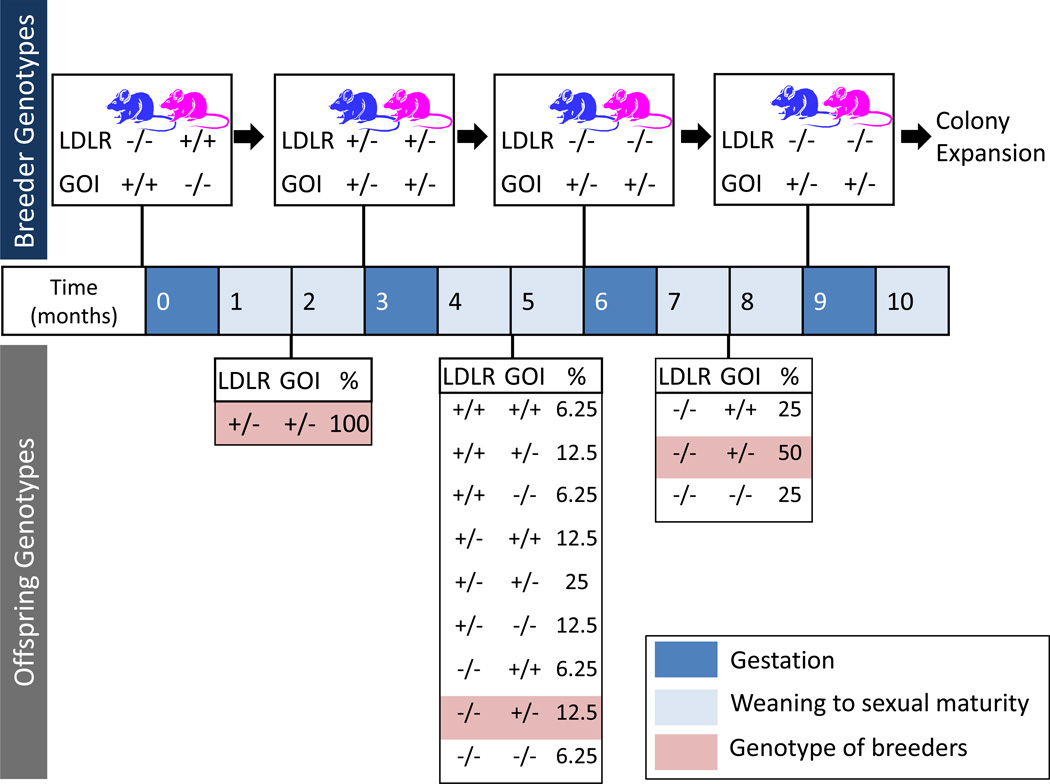
Since the introduction of these genetically altered animals, there has been an impressive number of potential targets identified that potentially reduce lesion size and their proclivity to rupture.11 Identification of some novel mechanisms in the development of atherosclerosis that led to a proliferation of potential therapeutic targets has been based on the development of mice with compound genetic deficiencies. The availability of these mice has been a great boost to both academic and pharmaceutical industries to develop potential new therapies. However, there are two major constraints to the use of compound deficient mice, i.e., time and money. As illustrated in Figure 1, the development of genetically deficient animals is a process that commonly takes in a range of two years to generate colonies of sufficient sizes for the determination of atherosclerosis. The C57BL/6 genetic background is commonly used, and this mouse strain has a reputation as being poor breeders. Even when breeding schemes are optimized, the development of compound deficient animals consumes considerable costs, including colony housing personnel to perform management and genotyping.
Figure 1. Provisional estimate for developing LDL receptor (LDLR) −/− background in mice to determine the effects of a gene of interest (GOI) on atherosclerosis.
The proposed scheme assumes availability of appropriate genotypes in the offspring to optimize the breeding pairs. However, the wide array of genotypes in the 2nd generation frequently leads to further cycles of breeding. This stage also requires considerable effects for genotyping. In optimal circumstances, a minimum of 3 breeding cycles are needed to develop parental lines for the study mice. The optimal genotypes of the parental breeders are represented about the time line. The potential genotypes of the offspring are listed below the time line and the frequency expressed as percentage occurrence. The gestation time for most mice strains is approximately 19 days and sexual maturity is achieved within 2 months of the age.
As a mode of circumventing the need to develop mice in a hypercholesterolemic background to perform atherosclerosis studies, two recent studies have demonstrated the ability to acutely promote high plasma cholesterol concentrations using an adeno-associated viral vector (AAV) expression of a mutant form of proprotein convertase subtilisin/kexin type 9 (PCSK9).12,13 PCSK9 has evoked intense interest in recent years following the discovery that mutations of PCSK9 were the basis for some forms of autosomal dominant hypercholesterolemia.14 PCSK9 regulates plasma cholesterol concentrations through recognition of the extracellular domain of LDL receptors, which then accelerates its intracellular degradation. Several PCSK9 mutants have been identified in humans, including the gain-of-function mutant used in these 2 recent reports.12,13 Both reports12,13 used AAVs as a delivery mechanism to promote chronic expression of gain-of-function mutants that were either human D374Y or mouse D377Y. Both studies demonstrated that the combination of either mutant of PSCK9 expression and feeding fat-enriched diets leads to pronounced hypercholesterolemia and atherosclerosis. The lipoprotein cholesterol distribution in the presence of mutant PCKS9 resembled profiles generated using plasma from LDL receptor −/− mice fed fat-enriched diets. Therefore, both studies demonstrated the ability to develop a phenotype akin to LDL receptor deficiency in mice without the substantial time and effort of breeding mice into genetically deficient in LDL receptors.
Many facilities can develop AAV at reasonable cost, and at the infection rate used in these two publications, the cost of the amount of AAV needed to infect mice is minimal. Also, there are no major biosafety concerns using AAVs. Therefore, there can be considerable savings in negating the needs to purchase apoE −/− or LDL receptor −/− mice. Because apoE −/− and LDL receptor −/− mice are only available commercially in a C57BL/6 background, this approach will also facilitate the studies that use different strains to search for genes that modify atherosclerotic lesion formation.
While it is assumed that mice infected with mutant PCSK9 is mimicking responses in LDL receptor −/− mice, the effects of PCSK9 could extent beyond effects on LDL receptors. For example, PCSK9 is also known to interact with other members of the LDL receptor gene family, including VLDL receptor and LRP1. These broader effects have the potential to promote differences between mice expressing mutant PCSK9 and those genetically lacking LDL receptors, which should be investigated extensively in future studies.
The ability to acutely develop hypercholesterolemia in mice represents a major benefit in the use of mouse models to study mechanisms of atherosclerosis. It may also have applicability to other areas. For example, a commonly used model for development of abdominal aortic aneurysms (AAAs) is infusion of AngII into hypercholesterolemic mice.15 While AngII-induced AAAs can be generated in normolipidemic mice, the incidence is much lower than in hypercholesterolemic mice. As with atherosclerosis studies, the development of compound deficient animals has been a major impediment to execution of AngII-induced AAA studies. Therefore, validation of the approach of injecting an AAV expressing PCSK9 would be a valuable addition to the literature.
Overall, the recent two studies12,13 demonstrate that persistent expression of a gain-of-function mutant of PCSK9 leads to chronic hypercholesterolemia in mice and subsequent atherosclerosis. We predict that there will be a rapid assimilation of this approach into atherosclerosis studies, which will greatly accelerate the rate of discoveries on atherosclerosis mechanisms while diminishing costs.
Acknowledgments
Sources of Funding
Aortic aneurysm research in the Daugherty lab is supported by HL107319. The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures
None.
REFERENCES
- 1.Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan J, Kitajima S, Watanabe T, Xu J, Zhang J, Liu E, Chen YE. Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol Ther. 2014 doi: 10.1016/j.pharmthera.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985;57:65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- 4.Paigen B, Morrow A, Holmes P, Mitchell D, Williams R. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987;68:231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 5.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 6.Plump AS, Smith JD, Hayek T, Aaltosetala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein-E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 7.Zhang SH, Reddick RL, Burkey B, Maeda N. Diet-induced atherosclerosis in mice heterozygous and homozygous for apolipoprotein E gene disruption. J Clin Invest. 1994;94:937–945. doi: 10.1172/JCI117460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E - Evaluation of lesional development and progression. Arterioscler Thromb Vacsc Biol. 1994;14:141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler Thromb Vasc Biol. 2000;20:2587–2592. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- 11.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 12.Roche-Molina M, Sanz-Rosa D, Cruz FM, Garcia-Prieto J, Lopez S, Abia R, Muriana FJ, Fuster V, Ibanez B, Bernal JA. Induction of Sustained Hypercholesterolemia by Single Adeno-Associated Virus-Mediated Gene Transfer of Mutant hPCSK9. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.114.303617. [DOI] [PubMed] [Google Scholar]
- 13.Bjorklund MM, Hollensen AK, Hagensen MK, Dagnaes-Hansen F, Christoffersen C, Mikkelsen JG, Bentzon JF. Induction of atherosclerosis in mice and hamsters without germline genetic engineering. Circ Res. 2014;114:1684–1689. doi: 10.1161/CIRCRESAHA.114.302937. [DOI] [PubMed] [Google Scholar]
- 14.Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 15.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]