Abstract
Background
Fibromuscular Dysplasia (FMD) is an non-atherosclerotic disease associated with hypertension, headache, dissection, stroke, and aneurysm. The etiology is unknown but hypothesized to involve genetic and environmental components. Previous studies suggest a possible overlap of FMD with other connective tissue diseases that present with dissections and aneurysms. The aim of this study was to investigate the prevalence of connective tissue physical features in FMD.
Methods and Results
142 FMD patients were consecutively enrolled at a single referral center (97.9% female, 92.3% had multifocal FMD). Data are reported for 139 female patients. Moderately severe myopia (29.1%), high palate (33.1%), dental crowding (29.7%), and early onset arthritis (15.6%) were prevalent features. Classic connective features such as hypertelorism, cleft palate, and hypermobility were uncommon. Frequency of systemic connective tissue features was compared between FMD patients with a high vascular risk profile (having had ≥1 dissection and/or ≥2 aneurysms) and those with a standard vascular risk profile. History of spontaneous pneumothorax (5.9% high risk vs. 0% standard risk) and atrophic scarring (17.3% high risk vs. 6.8% standard risk) were significantly more prevalent in the high risk group, p<0.05. High palate was observed in 43.1% of the high risk group vs. 27.3% in the standard risk group, p=0.055.
Conclusions
In a cohort of women with FMD, there was a prevalence of moderately severe myopia, high palate, dental crowding, and early onset osteoarthritis. However, a characteristic phenotype was not discovered. Several connective tissue features such as high palate and pneumothorax were more prominent among FMD patients with a high vascular risk profile.
Introduction
Fibromuscular dysplasia (FMD) is a non-atherosclerotic, non-inflammatory vascular disease which causes arterial stenosis, aneurysm, dissection, and/or tortuosity.(1,2) The etiology of FMD is unknown but hypothesized to involve both genetic and environmental components. Importantly, >90% of FMD patients are women.(1,3) Male patients with FMD may have a more aggressive vascular course with high risk of aneurysm and dissection.(4) Previous studies suggest a possible overlap of FMD with other connective tissue diseases that present with arterial and aortic dissections and aneurysms such as Marfan syndrome, Loeys-Dietz syndrome (TGFβ pathway disorders), and vascular Ehlers-Danlos syndrome (EDS).(5–8) Several case reports describe patients presenting with phenotypic connective tissue abnormalities and vascular lesions consistent with FMD.(5–7) However, the prevalence of defined genetic mutations associated with connective tissue diseases was negligible in a cohort of clinically confirmed FMD patients at our institution who underwent genetic testing.(5) Of interest, two patients in this cohort were found to have novel point mutations in TGF-β receptor type 1 gene that were classified as variants of undetermined clinical significance as pathogenicity could not be firmly established.(5) Both of these patients had a history of arterial dissection and had aortic ectasia or aneurysm. Recently, Ganesh and Morissette and colleagues described systemic connective tissue features in a small cohort of 47 patients with multifocal FMD.(9) A majority of patients (95.7%) presented with physical examination findings suggestive of mild connective tissue dysplasia. They also found elevated secretion of TGF-β1 and TGF-β2 by skin fibroblasts derived from FMD patients as well as elevated levels of circulating TGF-β1 and TGF-β2 compared to age and sex matched non-FMD controls.(9) However, this relatively small cohort of patients with a high prevalence of stroke and aneurysm seen at the National Institute on Aging (NIA) may have been subject to referral bias and may not be reflective of a more generalized FMD patient population.(1,9)
We sought to characterize and determine the prevalence of systemic connective tissue physical features among a large and diverse cohort of patients with clinically established FMD. Secondly, we sought to determine whether systemic connective tissue features were more prevalent among FMD patients with a high vascular risk profile of arterial aneurysm and dissection. We hypothesized that FMD patients with a high vascular risk profile would have more connective tissue physical features compared to FMD patients with a standard vascular risk profile.
Methods
Study Population
Patients with a diagnosis of FMD, as confirmed by a vascular medicine specialist in the Cleveland Clinic FMD program, and enrolled in an institutional FMD biorepository protocol were approached for enrollment in this study. Inclusion criteria were: age ≥ 18 years, confirmed (by imaging) diagnosis of FMD, and ability to undergo a non-invasive biometric connective tissue physical assessment. Patients were excluded if they had a molecularly confirmed diagnosis of another connective tissue disorder (i.e., patients with prior genetic testing confirming a known causative mutation consistent with vascular EDS, Loeys-Dietz syndrome/TGFβ pathway disorders, or Marfan syndrome). Enrollment began 11/1/2013 and concluded 7/3/2014 and 91.6% of patients approached consented for enrollment for a total enrollment of 142 patients. The study was approved by the Cleveland Clinic Institutional Review Board.
Connective Tissue Physical Feature Assessment
A brief medical history was completed assessing for complications associated with connective tissue features: history of joint dislocation, non-traumatic bone fracture, hernia, scoliosis, cleft palate, club foot, moderately severe myopia (defined as diopter correction greater than −3), early onset arthritis (defined as onset before age 50), and post-partum hemorrhage requiring blood transfusion. Dental crowding was defined by history of tooth extraction for crowding excluding wisdom tooth extraction. Height, weight, and body mass index (BMI) were recorded. A connective tissue physical feature assessment was performed. To maintain consistency, each connective tissue assessment was performed by the same examiner (SO) using standardized techniques for morphologic measurements.(10) The primary connective tissue physical features examined in our cohort were based on selected components of the systemic score of the revised Ghent Nosology (for Marfan syndrome), Beighton score of hyperextensibility, and other features identified in connective tissue disorders with a vascular phenotype similar to FMD.(11,12) Methodology for assessment of connective tissue parameters and definitions of abnormalities are further outlined in the Appendix.
FMD and Vascular Risk Profile Assessment
Distribution of vessel involvement with FMD, history of arterial dissection, arterial aneurysm, and type of FMD (multifocal or focal) were abstracted from the medical record and medical imaging review and confirmed by an experienced clinician in the Cleveland Clinic FMD program. High vascular risk profile was defined as a history of ≥1 arterial dissection and/or ≥2 arterial aneurysms. The high risk categorization was defined by the study investigators based upon clinical experience and reported findings of the United States FMD Registry(3). The high risk definition of ≥1 arterial dissection was based on the previously reported high risk prevalence of arterial dissections (~20%) among the FMD population and the relatively rare occurrence of dissections in the general population.(3) Since aortic and arterial aneurysms occur with some frequency in the general population, as well as up to 20% of patients with FMD, the high risk vascular profile was categorized as ≥2 arterial aneurysms as this would be an uncommon finding in both the general and FMD patient population.(3) In addition, modern non-invasive imaging techniques have allowed for detection of small, asymptomatic aneurysms that may not be of clinical significance.(3,13)
Statistical analysis
Summary statistics are presented as mean (standard deviation) for normally distributed continuous variables, median (IQR) for nonparametric continuous variables, and number (%) for categorical variables. Two sample t-tests or nonparametric equivalents were performed to examine differences in continuous variables; χ2 testing was used to test differences in proportions. Historical control data, when available, was abstracted from the medical literature.(14–21) Given the low number of male patients recruited and potential for sex related differences in physical examination features, only data for the female cohort are presented. A p-value < 0.05 was considered statistically significant. All statistics were analyzed using JMP Pro 10 and SAS 9.3 (SAS Institute, Inc., Cary, North Carolina).
Results
Baseline characteristics, demographics, and morphology
142 patients were enrolled, 97.9% of whom were female (N=139). Among female patients, 92.1% had multifocal (string of beads type) FMD.(1) Baseline characteristics of the female cohort (N=139) are displayed in Table 1. Median age of at the time of participation in the study was 54 years (IQR 47–62 years) and median age at diagnosis was 48 years (IQR 43–57). All but one patient were within two standard deviations of the average height of the American population.(22) The majority of patients were of normal BMI (median 23.5 kg/m2).(22) All but one patient (99.3%) had undergone both renal and carotid artery imaging during diagnostic evaluation. Nearly three-quarters of the patients had multi-vessel involvement with more than one arterial bed involved. Multi-vessel involvement may be underestimated since not all arterial beds were imaged for every patient. Extracranial carotid FMD was the most common arterial bed involved followed closely by renal FMD (Table 2). Fifty one (36.7%) of patients were categorized into the high vascular risk group.
Table 1.
Baseline characteristics of female FMD cohort, N=139 and by high risk and standard risk subsets
| Total (N=139) | High risk (=51) | Standard risk (N=88) | |
|---|---|---|---|
| Sex (female) | 139 (100.0%) | 51 (100%) | 88 (100%) |
| Age (years), median (IQR) | 54 (47–62) | 51 (46–59) | 56 (47–63) |
| Race (white) | 124 (89.2%) | 49 (96.1%)† | 75 (85.2%)† |
| Height (cm), mean ± SD | 165.1 ± 7.1 | 166.2 ± 6.7 | 164.5±7.2 |
| BMI (kg/m2), median (IQR) | 23.5 (20.8–28.2) | 23.7 (21.6–29.4) | 23.4 (20.8–26.7) |
| FMD type=multifocal | 128 (92.1%) | 50 (98.0%)* | 78 (88.6%)* |
| History of dissection | 44 (31.7%) | 44 (86.3%)^ | 0^ |
| History of aneurysm | 30 (21.7%) | 14 (28.0%) | 16 (18.2%) |
High risk group defined as history of arterial dissection and/or 2 or more arterial aneurysms
p-value high risk vs. standard risk = 0.047
p-value high risk vs. standard risk = 0.055
p-value high risk vs. standard risk <0.001
Table 2.
Distribution of vessel involvement* among female cohort, N=139
| Renal arteries | 92/138 (66.7%) |
| Extracranial carotid arteries | 109/139 (78.4%) |
| Extracranial vertebral arteries | 42/110 (38.2%) |
| Intracranial arteries | 20/127 (15.7%) |
| Mesenteric arteries | 15/133 (11.3%) |
| Aortaƚ | 7/92 (7.6%) |
| Lower Extremity | 19/32 (59.4%) |
| Multi-vessel FMD involvement | 100/139 (71.9%) |
Vessel involvement defined as imaging identification of vascular abnormality including typical FMD arterial lesions (multifocal or focal), aneurysm, and/or dissection
Primarily manifest as aortic aneurysm
Table 3 displays the frequency of systemic connective tissue features obtained from the medical history of the FMD cohort compared to controls. Compared to historical control data derived from populations similar to the study cohort, there were few features that were more prevalent in the study cohort. Features among the FMD patients more prevalent than reported among controls included early-onset osteoarthritis (15.6%) and moderately severe myopia (29.1%). A history of spontaneous pneumothorax was reported in 2.2% of the FMD cohort. A history of peripartum hemorrhage requiring transfusion occurred in 3.4% of the FMD cohort.
Table 3.
Prevalence of medical history of systemic connective tissue features in female FMD cohort compared to historical control data as available.
| Categorical Variable | FMD patients number/total assessed* | % FMD patients with feature | %Historical Controls (Reference) | Available Control Population |
|---|---|---|---|---|
| Nearsightedness | 95/138 | 68.8% | 34.2%(17) 28.1%(18) |
German female (n=6,897) age 35–74 years American females (n=2354) age 40–84 years |
| Diopters < −3 | 37/127 | 29.1% | -- | |
| Dental crowding | 41/138 | 29.7% | 7.1–59.2%(19) | Caucasian children age 3.5–16 years |
| Scoliosis | 37/137 | 27.0% | 36%(24) | American females age 50–84 |
| Early onset osteoarthritis | 21/135 | 15.6% | 5.20%(25) | American females 25–74 years, cumulative prevalence for 25–54 years with knee OA |
| Hernia | 13/139 | 9.4% | -- | |
| Joint dislocation | 17/139 | 12.2% | -- | |
| Without obvious trauma | 8/17 | 5.7%† | -- | |
| Ever broken bone | 76/139 | 54.7% | -- | |
| Without obvious trauma | 8/76 | 5.7%† | -- | |
| Transfusion peripartum | 4/117 | 3.4% | 1.4%(26) 1.0%(27) |
Premenopausal American females N=10,134 N=59,282 |
| Spontaneous pneumothorax | 3/138 | 2.2% | -- | |
| Club foot | 0/139 | 0.0% | 1–2/1000(20) | US Live Births 1999–2004 |
| Cleft Palate | 0/139 | 0.0% | 6.28%(20) | US Live Births 1999–2004 |
Denominator reflects total number assessed. Data was excluded if subjects were unsure of clinical history or unable to perform physical maneuvers
Percent of the total cohort
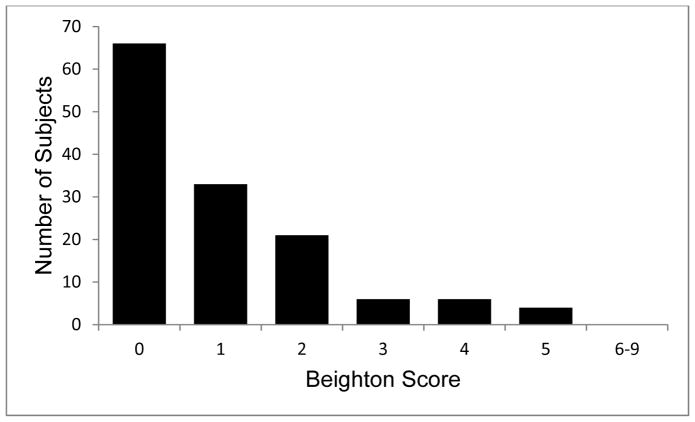
Table 4 displays the systemic connective tissue features identified on physical examination of the FMD cohort. Of note, pectus deformity was present in 7.2% of female FMD patients and equally divided between pectus excavatum and pectus carinatum. Palatal abnormalities (high palate or tori) were observed in 56.1% with high palate present in one-third of the cohort. Facial morphologic characteristics were measured. No patients demonstrated hypertelorism. Median near interpupillary (IP) distance measured 53.8mm (IQR 52.0–56.0) and median far IP distance was 58.5mm (IQR 57.0–61.0) for the entire study cohort. Median head circumference measured 55.0cm (IQR 54.0cm–56.25cm). Other physical features observed in connective tissue disorders such as bifid uvula, cleft palate, or club foot were not identified. Only one patient had an abnormal uvula, a uvula with a midline raphe. FMD patients in the cohort did not demonstrate significant hypermobility. Median Beighton score was 1 (IQR 0–2) and only 4 patients (2.9%) had a Beighton score ≥5/9. See Figure 1 for distribution of Beighton scores for the entire cohort. Compared to published studies of the prevalence of systemic connective tissue features in Marfan syndrome and overlapping connective tissue disease (OCTD), the FMD cohort had lower prevalence of positive wrist and thumb sign (arachnodactyly), pectus deformities, skin striae, and pes planus (Supplemental Table 1).(14) Several physical features were nearly as prevalent in the FMD cohort as in published series of Marfan syndrome and OCTD, namely myopia, and high palate.
Table 4.
Prevalence of systemic connective tissue features on physical examination in female FMD cohort compared to historical control data as available.
| Categorical Variable | FMD patients number/total assessed* | % FMD patients with feature | %Historical Controls (Reference) | Available Control Population |
|---|---|---|---|---|
| High palate | 46/139 | 33.1% | -- | |
| Tori palatinus | 26/139 | 18.7% | 22.8% (white)(15) 12.2 (black)(15) |
Skull analysis of Modern Americans, n=328 |
| Tori mandibularis | 28/139 | 20.1% | 38.8% (white)(15) 24.8 (black)(15) |
Skull analysis of Modern Americans, n=328 |
| Wrist and Thumb sign | 9/139 | 6.5% | -- | |
| Wrist sign | 24/139 | 17.3% | -- | |
| Thumb sign | 10/138 | 7.2% | -- | |
| Dystrophic scarring | 24/139 | 17.3% | -- | |
| Keloid | 13/139 | 9.4% | -- | |
| Atrophic | 15/139 | 10.8% | -- | |
| Skin striae | 8/139 | 5.8% | -- | |
| Pes Planus | 18/139 | 12.9% | 8%(16) 16.3% (white)(28) 38.3% (black)(28) |
Framingham Foot Substudy Cohort (n=2.089) 55% Female, mean age 65 years American cohort (n=1691), mean age 69 years, 68% Female, 31% African American |
| Hindfoot deformity | 13/138 | 9.4% | -- | |
| Pectus | 10/139 | 7.2% | ||
| Pectus excavatum | 5/139 | 3.6% | 1/1000(21) | Newborn cohort (n=52,000) 45% white, 47% black |
| Pectus carinatum | 5/139 | 3.6% | 1/1500(21) | Newborn cohort (n=52,000) 45% white, 47% black |
| Uvula abnormality | 1/139 | 0.7% | -- |
Denominator reflects total number assessed. Data was excluded if subjects were unsure of clinical history or unable to perform physical maneuvers
Figure 1.
Distribution of Beighton score for hypermobility in cohort of female FMD patients (N=136 patient completing assessment). Median score=1, IQR (0–2). High Beighton consistent with hypermobility defined as score ≥5/9
Comparison of connective tissue features between high risk and standard vascular risk profile
The study cohort was divided among high vascular risk (N=51) and standard vascular risk subjects (N=88) (Table 1).. Age, height, and BMI did not significantly differ between the groups. Patients in the high risk group were more likely to be white and more likely to have multifocal FMD than those in the standard risk group. No significant differences were observed in IP distance between high risk and standard risk groups (near IP distance 53.5mm vs. 54.0mm, p=0.450; far IP distance 58.5mm vs. 59mm, respectively, p=0.693). No significant difference was observed in median head circumference between high and standard risk groups (55.0cm vs. 55.0cm, p=0.261).
A comparison of 20 connective tissue features between high risk and standard risk groups is shown in Table 5. High vascular risk profile was associated with an increased prevalence of high palate (43.1% high risk vs. 27.3% standard risk, p=0.055). Spontaneous pneumothorax was observed in three patients in the high risk group and none in the standard risk group (5.9% high risk vs. 0 standard risk, p=0.049). Atrophic scarring was more prevalent in the high risk group (17.6% high risk vs. 6.8% standard risk, p=0.047). There was no statistically significant difference in the percentage of patients with hypermobility (5.9% high risk vs. 1.1% standard risk, p=0.140), the prevalence of pectus deformity (7.8% high risk vs. 6.8% standard risk, p=1.0), or the prevalence of moderately severe myopia (26.1% high risk vs. 30.9% standard risk, p=0.569), however this study may have been underpowered to detect small differences between the groups.
Table 5.
Comparison of 20 select connective tissue physical features in female FMD cohort by vascular risk profile
| Connective Tissue Finding | number/total* (%), n=139 | High risk †, n=51 | Standard Risk, n=88 | p-value |
|---|---|---|---|---|
| Nontraumatic joint dislocation | 8/138 (5.8) | 3/50 (6.0) | 5/88 (5.7) | 0.294 |
| Nontraumatic fracture | 8/137 (5.8) | 1/49 (2.0) | 7/88 (8.0) | 0.258 |
| Early onset osteoarthritis | 21/135 (15.6) | 5/49 (10.2) | 16/86 (18.6) | 0.200 |
| Hernia | 13/139 (9.4) | 6/51 (11.8) | 7/88 (8.0) | 0.549 |
| Scoliosis | 37/137 (27.0) | 10/50 (20.0) | 27/87 (31.0) | 0.334 |
| Club foot | 0 (0) | 0 (0) | 0 (0) | -- |
| Cleft palate | 0 (0) | 0 (0) | 0 (0) | -- |
| Nearsightedness >−3 diopters | 37/127 (29.1) | 12/46 (26.1) | 25/81 (30.9) | 0.569 |
| Spontaneous pneumothorax | 3/138 (2.2) | 3/51 (5.9) | 0/87 (0) | 0.049 |
| Peripartum blood transfusion | 4/117 (3.4) | 1/43 (2.3) | 3/74 (4.1) | 1.000 |
| Dental crowding | 41/138 (29.7) | 13/50 (26.0) | 28/88 (31.8) | 0.318 |
| Beighton ≥5/9 | 4/139 (2.9) | 3/51 (5.9) | 1/88 (1.1) | 0.140 |
| Wrist and Thumb sign | 9/139 (6.5) | 4/51 (7.8) | 5/88 (5.7) | 0.725 |
| Pectus deformity | 10/139 (7.2) | 4/51 (7.8) | 6/88 (6.8) | 1.000 |
| Dystrophic scarring | 24/139 (17.3) | 11/51 (21.6) | 13/88 (14.8) | 0.307 |
| Atrophic scarring | 15/139(10.8) | 9/51 (17.6) | 6/88 (6.8) | 0.047 |
| Keloid scarring | 13/139 (9.4) | 4/51 (7.8) | 9/88 (10.2) | 0.768 |
| Hypertelorism | 0 (0) | 0 (0) | 0 (0) | -- |
| High Palate | 46/139 (33.1) | 22/51 (43.1) | 24/88 (27.3) | 0.055 |
| Uvula abnormality | 1/139 (0.7) | 0/51 (0) | 1/88 (1.1) | 1.000 |
| Pes planus and/or hindfoot deformity | 26/139 (18.7) | 11/51 (21.6) | 15/88 (17.0) | 0.510 |
| Skin striae | 8/139 (5.8) | 2/51 (3.9) | 6/88 (6.8) | 0.710 |
Denominator reflects total number assessed. Data was excluded if subjects were unsure of clinical history or unable to perform physical maneuvers
High risk defined as ≥1 arterial dissection and/or ≥2 arterial aneurysms
There was no significant difference in the distribution of Beighton scores between the high vascular risk and standard vascular risk group. The median Beighton score was 1 (IQR 0–2) for the standard risk group and 0.5 (IQR 0–2) for the high risk group. Neither group demonstrated significant hypermobility.
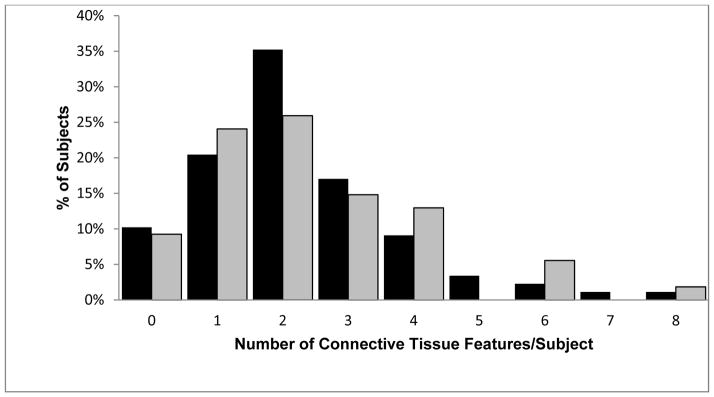
The median number of connective tissue features/patient (maximum 20) was 2 (IQR 1–3) for both groups. No significant difference in distribution of connective tissue scores was observed, p=0.955. 18.7% of the cohort demonstrated a connective tissue phenotype defined as ≥4 connective tissue features/patient. There was no difference in prevalence of the connective tissue phenotype (≥4 connective tissue features/patient) between high vascular risk and standard vascular risk patients (21.6% vs. 17.0% p=0.510).
Discussion
Fibromuscular dysplasia is an arterial disease that may lead to major vascular events including arterial dissection, symptomatic aneurysm, stroke, and myocardial infarction (due to coronary artery dissection). It has been hypothesized that FMD represents a systemic vasculopathy, although the genetic mechanism of this disease and the specific mechanisms of interaction with environmental factors remains unknown.(1) In our cohort of 139 female FMD patients, the prevalence of classical features of connective tissue disorders was lower than hypothesized. Only 18.7% of patients in our cohort demonstrated a connective tissue phenotype with ≥4/20 systemic connective tissue findings among those we assessed. No patients had a bifid uvula, cleft palate, or club foot deformity. Prevalence of many connective tissue features among the FMD patients were consistent with those of historical control series taken from a general female population.
A recent publication from Ganesh and Morissette and colleagues at the NIA suggested a potential role of the TGF-β signaling pathway in the pathogenesis of FMD.(9) The NIA study and previously published case reports described systemic connective tissue physical features among patients with FMD.(6,7,9) These studies report a high prevalence of joint hypermobility and skeletal abnormalities. Our study identified a lower prevalence of connective tissue findings. However there were a few features that stood out in our cohort of women with FMD. First, we identified early onset osteoarthritis (defined as a clinical diagnosis of arthritis with onset before age 50) in 15.6% of patients in the study cohort, a finding which is suggestive of a possible association with FMD. However, without a larger case-control study, true association cannot be proven. More research is needed to determine the relationship between FMD and early onset osteoarthritis. Early onset osteoarthritis may be associated with joint hypermobility in youth but may also explain the low Beighton scores observed in this cohort of middle aged women.(23) We also observed a relatively high prevalence of moderately severe myopia, defined as diopter correction >−3, (29.1%), dental crowding (29.7%), pectus deformity (carinatum or excavatum) (7.2%), and high arched palate (33.1%).
The findings in our FMD cohort contrast somewhat to those of the NIA cohort. Few patients had an elevated Beighton score (2.9% meeting the criteria for hypermobility) at the time of the study examination in our cohort whereas 57.4% demonstrated hypermobility in the NIA cohort(9). Ganesh and Morissette and colleagues found mild connective tissue dysplasia in 95.7% of subjects.(9) Our cohort demonstrated a much lower prevalence of connective tissue features. Pectus deformity was reported in 40.4% of the NIA cohort compared to 7.2% in our cohort. Higher prevalence of palate abnormalities (87.2% NIA vs 56.1% our cohort), pes planus (72.3% NIA vs 12.9% our cohort) and abnormal scars (57.4% NIA vs 17.3% our cohort) were also reported.(9) The NIA cohort authors do report that most of the physical exam findings were “mild and subclinical”.(9) The discrepancy between the findings of the NIA cohort and our data may be due to differences in the study populations. The NIA cohort demonstrated a much higher prevalence of aneurysms and fewer dissections. More than 60% of the NIA cohort had a history of aneurysm, which is three times the prevalence reported in the US FMD Registry cohort, and only 4.3% reported history of arterial dissection.(3,9) The prevalence of stroke/TIA was also very high in the NIA cohort (48.9%).(9) The NIA cohort may represent a unique subset of FMD patients self-referred to a national research institute whereas our study presents data derived from a population of women with predominantly multifocal FMD referred for clinical management. In addition, strict data definitions used for the physical assessment in our study erred against reporting subclinical findings.
Several connective tissue features tended to be more prevalent in the high vascular risk group: high palate, spontaneous pneumothorax, and atrophic scarring. Interestingly, some features classically associated with Loeys-Dietz syndrome/TGFβ pathway disorders (uvula abnormalities, scoliosis, club foot, and pes planus) were not more prevalent in the high risk group. More investigation as to the differences in biochemical profiles between those presenting with high vascular risk profiles and standard vascular risk profiles is needed, especially given the potential role of abnormal TGF-β signaling in FMD suggested by the NIA cohort.(9)
The fact that most FMD patients do not present with striking connective tissue dysmorphology highlights the importance of screening for FMD based on symptoms of vascular bed involvement rather than connective tissue features. Given the lack of striking dysmorphology in this FMD cohort, clinical suspicion for a distinct connective tissue genetic disease (i.e., other than FMD) should be high in a patient presenting with arterial dissections and aneurysms who demonstrates significant suggestive morphologic features on physical exam.
Strengths
This is the largest study investigating systemic connective tissue features among patients with FMD. Though patients were seen at an expert referral center, the FMD cohort in this study is representative of a clinical FMD patient population. The vascular bed involvement, number of dissections, number of aneurysms, and demographic characteristics align closely with the reported findings of the US Registry for FMD.(3) Patients were only enrolled within this study after the FMD diagnosis was confirmed by experts in the field of vascular medicine and FMD. Data definitions, history questionnaire, and physical examination criteria were meticulously researched and delineated prior to study enrollment. Vascular risk profiles (higher vs. standard risk) were established a priori. All examinations were performed by the same study investigator to eliminate variation in examination technique.
Limitations
The lack of a concurrent non-FMD control population is a significant limitation of this study. Historical control data from the published literature is imperfect especially since data definitions may not be consistent between examiners. This is particularly true for studies investigating qualitative data such as pectus deformity, abnormal scarring, and high palate. Every effort was made to utilize strict definitions and quantitative measurements when possible. An ideal control population could not be obtained within the study constraints as an optimal control population would have required complete vascular imaging to rule out subclinical and asymptomatic FMD lesions.
Other limitations include reliance on patient reported medical history and the inability to assess an exhaustive list of potential connective tissue features. A short physical examination aimed at assessing a diverse range of connective tissue features was developed. Despite best efforts to assess a broad range of features, it is possible that prevalent connective tissue features, such as small joint hypermobility, were missed as they were not studied. Finally, given the relatively uncommon nature of FMD and our subsequent modest sample size, the study may have been underpowered to detect statistically significant differences among all comparisons.
Conclusions
This study demonstrates a low prevalence of traditional connective tissue features in a cohort of female FMD patients. The prevalence of most connective tissue findings studied was consistent with published norms for adult females. However, we did identify several features that were more prevalent in FMD: early-onset osteoarthritis (before age 50), abnormalities of the palate (especially high arched palate), moderately severe myopia, pectus deformities, and dental crowding. Large joint hypermobility was not common in this cohort, although assessment may have been limited by the prevalence of early-onset arthritis.
These findings warrant further exploration and incorporation into genetic and biochemical analyses to further define the etiology of FMD and the possibility that this arterial disease represents a distinct connective tissue disorder with variable morphological, clinical, and vascular phenotypic manifestations.
Supplementary Material
Figure 2.
Comparison of number of connective tissue features between high risk (gray) and standard risk (solid) FMD patients. No significant difference, p=0.955. Median High Risk= 2 IQR (1–3), Median Standard Risk=2 IQR (1–3).
Acknowledgments
The authors acknowledge the help and assistance of Neil Poria.
Funding
Ms. O’Connor was funded by the American Heart Association Student Research Grant. Dr. Gornik and the Cleveland Clinic FMD Biorepository has been supported in part by the National Institutes of Health, National Center for Research Resources, CTSA 1UL1RR024989, Cleveland, Ohio.
Appendix A: Connective Tissue Physical Feature Assessment – Definitions
Hypertelorism: Near interpupillary (IP) distance >65mm or far IP >68mm(29)
High arch palate:palatal height > twice the height of canine teeth(10)
Abnormal uvula:bifid uvula, uvula with midline raphe, or uvula >twice normal width
Joint hypermobility: passive Beighton score ≥5/9(30)
Beighton Score: maneuvers: passive dorsiflexion of the little fingers beyond 90°, 1 point for each hand; passive apposition of the thumbs to the flexor aspect of the forearm, 1 point for each hand; hyperextension of the elbows beyond 10°, 1 point for each elbow; hyperextension of the knees beyond 10°, 1 point for each knee; and forward flexion of the trunk with knees fully extended so the palms of the hand rest flat on the floor, 1 point
Positive wrist sign (Walker-Murdoch sign): ability of the thumb to cover the entire fingernail of the fifth finger when wrapped around contralateral wrist with either/both hands.(31)
Positive thumb sign (Steinberg test): ability of entire phalanx of the adducted thumb to extend beyond the ulnar border of the palm with or without the assistance of the patient or examiner to achieve maximum adduction with either/both hands.(32)
Pectus deformities: protrusion of the sternum (carinatum) or inversion of the sternum (excavatum).(33)
Dystrophic scarring: one or more atrophic or keloid scars.
Skin striae: irregular areas of the skin that looked like bands, stripes, or lines, with an off-color hue; not associated with weight gain, pregnancy, or Cushing syndrome.
Pes planus: loss of the normal longitudinal arch of the medial foot, with dorsum height/foot length ratio <0.182.(34)
Hindfoot deformity (varus or valgus): eversion (valgus) or inversion (varus) of the calcaneus relative to the tibia was >10°.(35,36)
Footnotes
Disclosures
Dr. Gornik is a non-compensated member of the medical advisory board of the FMD Society of America, a non-profit organization. The other authors had no disclosures.
References
- 1.Olin JW, Gornik HL, Bacharach JM, Biller J, Fine LJ, Gray BH, et al. Fibromuscular Dysplasia: State of the Science and Critical Unanswered Questions: A Scientific Statement From the American Heart Association. Circulation. 2014 Mar 4;129(9):1048–78. doi: 10.1161/01.cir.0000442577.96802.8c. [DOI] [PubMed] [Google Scholar]
- 2.Sethi S, Lau J, Gustavson S, Olin J. The S curve: a novel morphological finding in the internal carotid artery in patients with fibromuscular dysplasia. Vasc Med. 2014;19(5):356–62. doi: 10.1177/1358863X14547122. [DOI] [PubMed] [Google Scholar]
- 3.Olin JW, Froehlich J, Gu X, Bacharach JM, Eagle K, Gray BH, et al. The United States Registry for Fibromuscular Dysplasia: Results in the First 447 Patients. Circulation. 2012 Jun 26;125(25):3182–90. doi: 10.1161/CIRCULATIONAHA.112.091223. [DOI] [PubMed] [Google Scholar]
- 4.Kim ESH, Olin JW, Froehlich JB, Gu X, Bacharach JM, Gray BH, et al. Clinical Manifestations of Fibromuscular Dysplasia Vary by Patient SexA Report of the United States Registry for Fibromuscular Dysplasia. J Am Coll Cardiol. 2013;61(21):2026–8. doi: 10.1016/j.jacc.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 5.Poloskey SL, Kim ES, Sanghani R, Al-Quthami AH, Arscott P, Moran R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med. 2012 Dec 1;17(6):371–8. doi: 10.1177/1358863X12459650. [DOI] [PubMed] [Google Scholar]
- 6.Russo L. Fibromuscular hyperplasia of the extracranial arteries: report of a case associated with intracranial aneurysm and skeletal deformities and a brief review of the literature. Mt Sinai J Med. 1973;40(1):60–7. [PubMed] [Google Scholar]
- 7.Alomari A, Alomari A. A rare association of fibromuscular dysplasia, renal agenesis, renal arteriovenous fistulae, and vertebral anomalies: Expanding the V in VACTERL association. Am J Med Genet. 2012;158A:2863–5. doi: 10.1002/ajmg.a.33523. [DOI] [PubMed] [Google Scholar]
- 8.Morissette RR, Ganesh S, Griswold B, Sloper L, McDonnell N. Redefining Fibromuscular Dysplasia of the Arteries as a TGF-β Pathway Disorder. Am Soc Hum Genet Annu Meet; 2012; Abstract. [Google Scholar]
- 9.Ganesh SK, Morissette R, Xu Z, Schoenhoff F, Griswold BF, Yang J, et al. Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF-β expression and connective tissue features. FASEB J. 2014;28:3313–24. doi: 10.1096/fj.14-251207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall JG, Gripp KW, Allanson JE, Slavotinek AM. Handbook of Physical Measurements. 2. New York, New York: Oxford University Press; 2007. [Google Scholar]
- 11.Beighton P, Paepe AD, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: Revised nosology, Villefranche, 1997. Am J Med Genet. 1998;77(1):31–7. doi: 10.1002/(sici)1096-8628(19980428)77:1<31::aid-ajmg8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Loeys BL, Dietz HC, Braverman AC, Callewaert BL, Backer JD, Devereux RB, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010 Jul 1;47(7):476–85. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 13.Norman P, Powell J. Abdominal Aortic Aneurysm The Prognosis in Women Is Worse Than in Men. Circulation. 2007;115:2865–9. doi: 10.1161/CIRCULATIONAHA.106.671859. [DOI] [PubMed] [Google Scholar]
- 14.Glesby MJ, Pyeritz RE. Association of mitral valve prolapse and systemic abnormalities of connective tissue. A phenotypic continuum. J Am Med Assoc. 1989;262:523–8. [PubMed] [Google Scholar]
- 15.Sonnier KE, Horning GM, Cohen ME. Palatal Tubercles, Palatal Tori, and Mandibular Tori: Prevalence and Anatomical Features in a U.S. Population. J Periodontol. 1999;70(3):329–36. doi: 10.1902/jop.1999.70.3.329. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen US, Dufour AB, Positano RG, Dines JS, Dodson CC, Gagnon DG, et al. The occurrence of ipsilateral or contralateral foot disorders and hand dominance: the Framingham foot study. J Am Podiatry Med Assoc. 2013;103(1):16–23. doi: 10.7547/1030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfram C, Höhn R, Kottler U, Wild P, Blettner M, Bühren J, et al. Prevalence of refractive errors in the European adult population: the Gutenberg Health Study (GHS) Br J Ophthalmol. 2014 Feb 10;98:857–61. doi: 10.1136/bjophthalmol-2013-304228. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Klein BE, Klein R, Moss SE. Refractive status in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1994 Dec 1;35(13):4344–7. [PubMed] [Google Scholar]
- 19.Mugonzibwa EA, Eskeli R, Laine-Alava MT, Kuijpers-Jagtman AM, Katsaros C. Spacing and crowding among African and Caucasian children. Orthod Craniofac Res. 2008;11(2):82–9. doi: 10.1111/j.1601-6343.2007.00416.x. [DOI] [PubMed] [Google Scholar]
- 20.Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS, et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999?2001. Birt Defects Res A Clin Mol Teratol. 2006;76(11):747–56. doi: 10.1002/bdra.20294. [DOI] [PubMed] [Google Scholar]
- 21.Chung CS, Myrianthopoulos NC. Factors affecting risks of congenital malformations. I. Analysis of epidemiologic factors in congenital malformations. Report from the Collaborative Perinatal Project. Birth Defects Orig Artic Ser. 1975;11(10):1–22. [PubMed] [Google Scholar]
- 22.McDowell M, Fryer C, Ogden C, Flegal K. Anthropoetric Reference Data for Children and Adults: United States, 2003–2006. Natl Health Stat Rep. 2008;10:1–48. [PubMed] [Google Scholar]
- 23.Kirk J, Ansell B, Bywaters E. The hypermobility syndrome. Musculoskeletal complaints associated with generalized joint hypermobility. Ann Rheum Dis. 1967;26:419–25. doi: 10.1136/ard.26.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwab F, Dubey A, Gamez L, Fegoun AE, Hwang K, Pagala M, et al. Adult Scoliosis: Prevalence, SF-36, and Nutritional Parameters in an Elderly Volunteer Population. Spine. 2005;30(9):1082–5. doi: 10.1097/01.brs.0000160842.43482.cd. [DOI] [PubMed] [Google Scholar]
- 25.Maurer K. Basic Data on Arthritis Knee, Hip, and Sacroiliac Joints in Adults ages 25–74 Years. Vital Health Stat Data Natl Heath Surv. 11(213):79–1661. [PubMed] [Google Scholar]
- 26.Dilla A, Waters J, Yazer M. Validation of Risk Stratification Criteria for Peripartum Hemorrhage. Obstet Gynecol. 2013;122(1):120–6. doi: 10.1097/AOG.0b013e3182941c78. [DOI] [PubMed] [Google Scholar]
- 27.Ehrenthal D, Chichester M, Cole O, Jiang X. Maternal risk factors for peripartum transfusion. J Womens Health. 2012;21(7):792–7. doi: 10.1089/jwh.2011.3248. [DOI] [PubMed] [Google Scholar]
- 28.Golightly Y, Hannan MT, Dufour AB, Jordan J. Racial differences in foot disorders and foot type: The Johnston County Osteoarthritis Project. Hoboken. 2012;64(11):1756–9. doi: 10.1002/acr.21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pointer JS. The interpupillary distance in adult Caucasian subjects, with reference to “readymade” reading spectacle centration. Ophthalmic Physiol Opt. 2012;32(4):324–31. doi: 10.1111/j.1475-1313.2012.00910.x. [DOI] [PubMed] [Google Scholar]
- 30.Russek L. Hypermobility Syndrome. Phys Ther. 1999;79(6):591–9. [PubMed] [Google Scholar]
- 31.Walker B, Murdoch J. The Wrist Sign: A Useful Physical Finding in the Marfan Syndrome. Acrhives Intern Med. 1970;126(2):276–7. doi: 10.1001/archinte.126.2.276. [DOI] [PubMed] [Google Scholar]
- 32.Cocco G. The “thumb and wrist sign” in Marfan syndrome. Heart. 2001;86:602. doi: 10.1136/heart.86.6.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fokin A, Steuerwald N, Ahrens W, Karen E, Allen K. Anatomic, Histologic, and Genetic Characteristics of Congenital Chest Wall Deformities. Semin Thorac Cardiovasc Surg. 2009;21:44–57. doi: 10.1053/j.semtcvs.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Williams DS, McClay IS. Measurements used to characterize the foot and medial longitudinal arch: reliability and validity. Phys Ther. 2000;80:864–71. [PubMed] [Google Scholar]
- 35.Barg A, Harris M, Henninger H, Amendola R, Saltzman C, Hintermann B, et al. Medial Distal Tibial Angle: Comparison between Weightbearing Mortise View and Hindfoot Alignment View. Foot Ankle Int. 2012;33:655–61. doi: 10.3113/FAI.2012.0655. [DOI] [PubMed] [Google Scholar]
- 36.Chang C, Miller F, Schuyler J. Dynamic Pedobarograph in Evaluation of Varus and Valgus Foot Deformities. J Pediatr Orthop. 2002;22:813–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.