Abstract
The ability of non-steroidal anti-inflammatory drugs (NSAIDs) to modulate γ-aminobutyrate (GABA)-activated currents via Ca2+-activated Cl− channels in rat dorsal root ganglion neurons (DRG), was examined in the present study. During the preparation of DRG neurons harvested from Sprague-Dawley rats, the whole-cell recording technique was used to record the effect of NSAIDs on GABA-activated inward currents, and the expression levels of the TMEM16A and TMEM16B subunits were revealed. In the event that DRG neurons were pre-incubated for 20 sec with niflumic acid (NFA) and 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) prior to the administration of GABA, the GABA-induced inward currents were diminished markedly in the majority of neurons examined (96.3%). The inward currents induced by 100 µmol/l GABA were attenuated by (0±0.09%; neurons = 4), (5.32±3.51%; neurons = 6), (21.3±4.00%; neurons = 5), (33.8±5.20%; neurons = 17), (52.2±5.10%; neurons = 4) and (61.1±4.12%; neurons = 12) by 0.1, 1, 3, 10, 30 and 100 µmol/l NFA, respectively. The inward currents induced by 100 µmol/l GABA were attenuated by (13.8±6%; neurons = 6), (23.2±14.7%; neurons = 6) and (29.7±9.1%; neurons = 9) by 3, 10 and 30 µmol/l NPPB, respectively. NFA and NPPB dose-dependently inhibited GABA-activated currents with half maximal inhibitory concentration (IC50) values of 6.7 and 11 µmol/l, respectively. The inhibitory effect of 100 µmol/l NFA on the GABA-evoked inward current were also strongly inhibited by nitrendipine (NTDP; an L-type calcium channel blocker), 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis (a highly selective calcium chelating reagent), caffeine (a widely available Ca2+ consuming drug) and calcium-free extracellular fluid, in a concentration-dependent manner. Immunofluorescent staining indicated that TMEM16A and TMEM16B expression was widely distributed in DRG neurons. The results suggest that NSAIDs may be able to regulate Ca2+-activated chloride channels to reduce GABAA receptor-mediated inward currents in DRGs.
Keywords: non-steroidal anti-inflammatory drugs, niflumic acid, γ-aminobutyrate-induced inward current, modulatory, Ca2+-activated Cl− channels, dorsal root ganglion
Introduction
γ-aminobutyrate (GABA) is a crucial inhibitory neurotransmitter in the mammalian peripheral nervous system (PNS) and central nervous system (CNS) (1). GABAA receptor is a pentamer comprised of multiple subunits (α−6, β1–3, γ1–3, π, ε, δ and θ) with an absolute chloride ion channel and diversiform allosteric binding sites through which rapid inhibitory synaptic neurotransmission may be modulated (1). The GABAA receptor is a favorable target for therapeutic agents including steroids, barbiturates, benzodiazepines, anesthetics and convulsants (2). Recently, it has also been proposed that the β-subunit has an important role in determining the chloride ion selectivity of GABAA receptors (3,4).
GABAA receptor antagonists niflumic acid (NFA) and 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), are the only chloride ion channel blockers able to protect cells from excitotoxicity (5). Accumulating evidence suggests that non-steroidal anti-inflammatory drugs (NSAIDs) modulate GABAA receptor function in heterologous expression systems (6). NSAIDS at clinically relevant concentrations (low micromolar) are sufficient to potentiate β2/3-containing GABAA receptors (7). The NSAID-sensitive α1β2γ2 receptor subtype is the predominant and the largest GABAA receptor population in mammalian PNS and CNS (7). In addition to their effect on GABAA receptors, fenamate NSAIDs also affect a variety of other ion channels (8,9). Several drugs that have an effect on GABAA receptor function have been revealed to depend on subtypes (subunits combinations), and on specific amino acids situs of specific subunits (10).
Halliwell et al (11) revealed that the regulation of GABAA receptors by one particular anti-inflammatory agent, mefenamic acid, was dependent on the β-subunit. Conversely, Sinkkonen et al (12) reported that the potentiation of α1β2γ2 receptors by NFA was dependent on the presence of a γ2 subunit, which also effects mefenamic acid modulation (11). Antagonism of the α6β2γ2 receptor subtype by NFA has also been reported (12), and the substitution of an α4 subunit reduced the mefenamic potentiation of α1β2γ2 receptors by 50% (13). Similar observations have been observed in electrophysiological studies regarding the actions of mefenamic acid, pentobarbital and etomidate (11,14,15).
The aim of the present study is to use conventional whole-cell patch-clamp recordings, immunofluorescence and NSAIDs, including NFA and NPPB, to investigate the effect of Ca2+-activated Cl− channels (CaCCs) on GABA-induced currents in the dorsal root ganglion (DRG) of rats. The present study intended to elucidate the diversity of the modulatory effect route of NSAIDs on GABA-activated currents via CaCCs.
Materials and methods
Isolation of DRG neurons
A total of 120 Sprague-Dawley rats (SDRs) were provided by the Experimental Animal Center of Xinjiang Medical University, Urumqi, China (certificate no. SCXK 2003-0001; age, 8–10 weeks; weight, 250–280 g) irrespective of gender. All protocols were approved by the Institutional Animal Care and Use Committee at the Medical College of Shihezi University (Shihezi, China) and were consistent with the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health (16). SDRs were bred in separate specific pathogen-free cages at a relative humidity of 40–70%, (24±3°C), 100–120 lx/12-h dark:light illumination and free access to food and water. The DRG neuron selection and the separation process are described in our previous studies (17,18). Rats were sacrificed by decapitation.
Electrophysiological recordings
A gap-free recording with a sampling interval of 50 msec (17,18) was performed in the present study. Briefly, with the aid of a whole-cell patch clamp amplifier, perforated patch-clamp recordings in the whole-cell mode were performed. Using an Axon 700B amplifier (Axon, San Jose, CA, USA) and pCLAMP version 0.2 hardware and software (Axon), currents were recorded from the DRG neurons in vitro. The room temperature was set at 22–24°C. The resistance of the recording pipette ranged from 3 to 5 MΩ. The experimental procedures were performed according to the Regulations for the Administration of Affairs. Concerning Experimental Animals, formulated by the Ministry of Science and Technology of the People's Republic of China (The Ministry of Science and TechnoIogy of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2011).
Immunofluorescent staining of TMEM16A and TMEM16B to determine expression in the DRG
Rats were anesthetized with 0.3% (w/v) sodium pentobarbital [Sangon Biotech (Shanghai,) Co., Ltd., Shanghai, China], and perfused through the aorta with 0.9% (w/v) normal saline, followed by fresh 4% (w/v) paraformaldehyde in phosphate-buffered saline [both purchased from Sangon Biotech (Shanghai,) Co., Ltd.] for 10 min for tissue fixation. The lumbar DRG at level L4–6 to the nerve injury was removed rapidly and placed in 4% (w/v) paraformaldehyde in PBS for 24 h. The L4–6 DRG were cut into 5-µm sections with a freezing microtome (CM1510S; Leica Biosystems, Wetzlar, Germany). Immunofluorescent staining was performed using rabbit anti-TMEM16A polyclonal antibody (1:20; sc-135235) and goat anti-TMEM16B polyclonal antibody (1:20; sc-169622) (both purchased from Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C. The sections were incubated for 1 h in a solution containing donkey anti-rabbit IgG-fluorescein isothiocyanate (FITC; 1:50; 711-095-152) and donkey anti-goat IgG-tetramethylrhodamine (TRITC; 1:50; 705-025-003) (both purchased from Jackson ImmunoResearch, West Grove, PA, USA) at 37°C. Several tissue sections were selected for double-labeling of TNEM16A and TMEM16B and were incubated in a mixture of primary antibodies against TNEM16A and TMEM16B, followed by donkey anti-rabbit IgG conjugated with FITC and donkey anti-goat IgG conjugated with TRITC. Slides were then examined by confocal microscopy (LSM710; Carl Zeiss AG, Oberkochen, Germany). Quantitative analysis of TMEM16A and TMEM16B expression in the DRG was performed by measuring the mean absorbance at 488 and 550 nm (Zeiss LSM 510 System; Carl Zeiss, Jena, Germany) following laser confocal microscopy and using analysis software (ZEN 2009 Light Edition; Carl Zeiss).
Drug application
GABA, muscimol, bicuculline, NFA, NPPB, caffeine and NTDP were purchased from Sigma-Aldrich, (St. Louis, MO, USA). 1,2-Bis(2-aminophenoxy)ethane-N, N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl ester; BAPTA-AM) was from Merck Millipore (Darmstadt, Germany). Rabbit anti-TMEM16A polyclonal antibody and donkey anti-rabbit IgG-FITC were purchased from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA). All drugs used in electrophysiological recordings were dissolved in extracellular fluid and applied by gravity flow from a home-made perfusion system consisting a row of tubules connected with a series of individual reservoirs (17). This rapid solution exchange system was manipulated by shifting the tubules horizontally with a micromanipulator (17,18). The time of pre-perfusion of antagonists was 0.5–5 min, and the time of pre-perfusion of GABA was 5–10 sec.
Statistical analysis
Statistical analysis of the data was performed using SPSS (version 13.0; SPSS, Inc., Chicago, IL, USA) and the values of GABA-activated currents are presented as mean ± standard error of the mean and a Student's t test was used to assess the significance. P<0.05 was considered to indicate a statistically significant difference.
Results
GABA-induced inward currents
Treatment with different concentrations of GABA (1–1,000 µmol/l) activated an inward current in the majority of cells (94.32%, 150/159) examined. The GABA-induced response was concentration-dependent, and displayed evidence of desensitization at high concentrations (Fig. 1). The activation threshold was ~1 µmol/l and the maximal response was achieved at 300 µmol/l GABA. The value of the dissociation constant was ~30 µmol/l, derived from a concentration-response curve (17,18). The averaged amplitude of 100 µmol/l GABA-evoked inward current was (1.29±0.72 nA; neurons = 52). The selective GABAA receptor agonist, muscimol (100 µmol/l) mimicked the GABA-evoked response (neurons = 8). A selective GABAA receptor antagonist, bicuculline (100 µmol/l), suppressed GABA (neurons = 9) and muscimol-evoked (neurons = 8) inward currents (Fig. 1).
Figure 1.
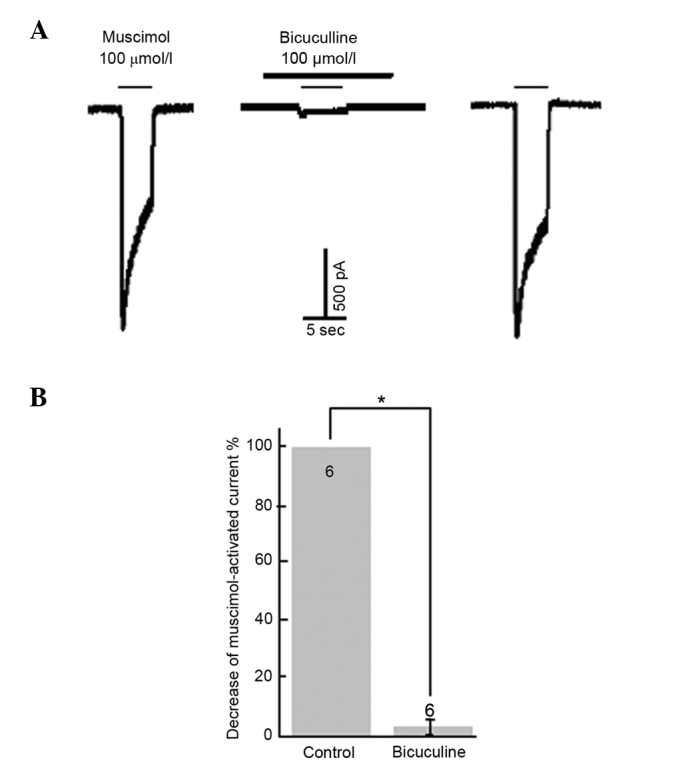
Inhibition of GABAA receptor antagonist on muscimol-induced depolarization. (A) Blockade of 100 µmol/l muscimol-induced inward current by GABAA receptor antagonist bicuculline (100 µmol/l). (B) Statistical results indicating the ability of bicuculline to block muscimol-activated currents. *P<0.05 vs. control. Numbers on bars=number of neurons.
Inhibition of GABA-induced inward currents by NFA and NPPB
NFA and NPPB were pre-incubated for 20 sec prior to application of GABA, resulting in the marked attenuation of the GABA-induced inward current in the majority of the neurons examined (96.3%, 52/54). Inhibition of GABA-induced responses by NFA and NPPB were concentration-dependent. The inward currents induced by 100 µmol/l GABA were suppressed by (0±0.09%; neurons=4), (5.32±3.51%; neurons=6), (21.3±4.00%; neurons=5), (33.8±5.20%; neurons=17), (52.2±5.10%; neurons=4), (61.1±4.12%; neurons=12) and (57.6±4.20%; neurons=4) by 0.1, 1, 3, 10, 30, 100 and 300 µmol/l NFA, respectively (Fig. 2A). The inhibition threshold was ~0.1 µmol/l and the maximal inhibition was achieved at 300 µmol/l NFA. The IC50 value was ~6.7 µmol/l derived from the concentration-inhibition curve (Fig. 2B). NFA did not alter the half maximal effective concentration EC50 value for GABA (~30 µmol/l) (17,19), but reduced the maximal GABA currents by ~60%. The inward current induced by 100 µmol/l GABA was suppressed by 13.8±6.7%, neurons=6, P<0.05; 23.2±14.7%, neurons=6, P<0.01 and (29.7±9.1%, neurons=9, P<0.01, by 3, 10 and 30 µmol/l NPPB, respectively. The inhibition threshold was ~1 µmol/l and the maximal inhibition was achieved by 30 µmol/l NPPB. The IC50 value was ~11 µmol/l (Fig. 3).
Figure 2.
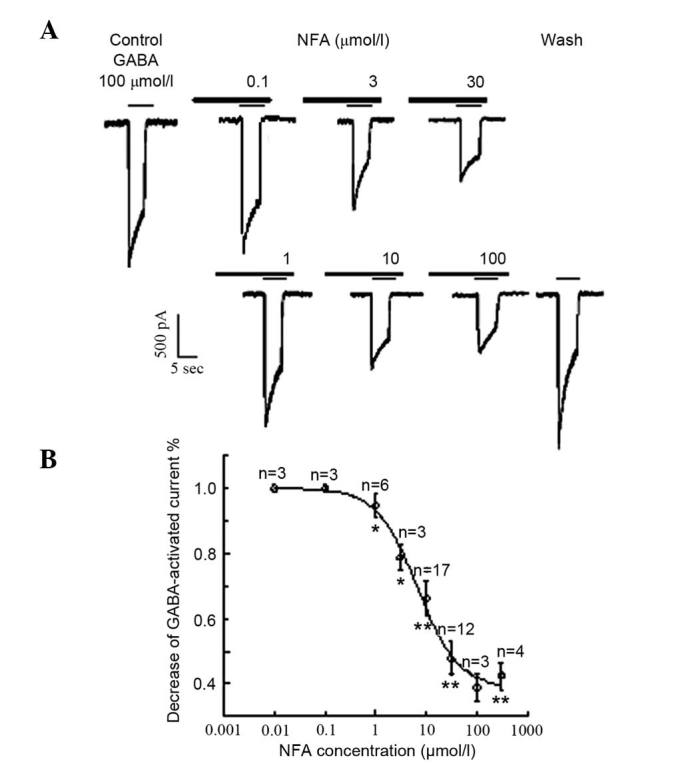
Effects of different concentrations of NFA on GABA-activated inward currents. (A) Results of 100 µmol/l GABA-activated inward currents in the different-concentration of NFA (0.1–100 µmol/l). (B) Inward current induced by 100 µmol/l GABA was suppressed by 0±0.09% (neurons=4), 5.32±3.51% (neurons=6), 21.3±4.00% (neurons=5), 33.8±5.20% (neurons=17), 52.2±5.10% (neurons=4), 61.1±4.12% (neurons=12) and 57.6±4.20% (neurons=4) by 0.1, 1, 3, 10, 30, 100 and 300 µmol/l NFA (neurons=3–17), respectively. *P<0.05 and **P<0.01 vs. control. Data are results of a paired t test.
Figure 3.
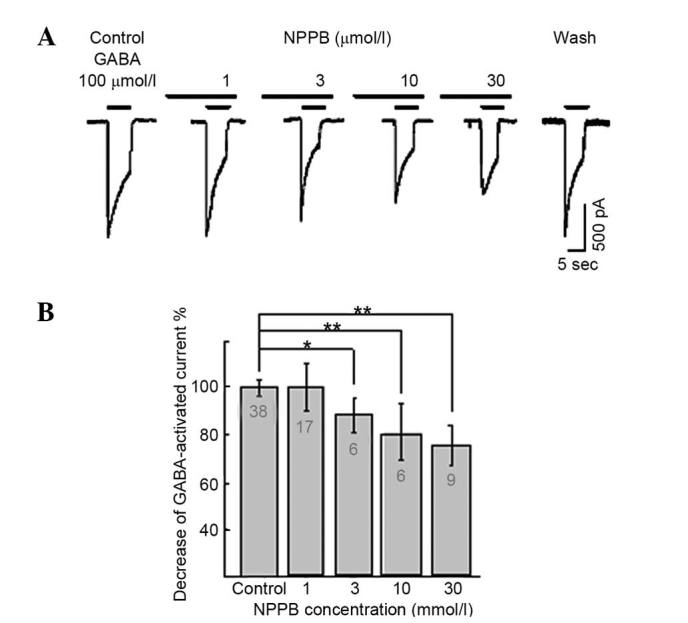
Effects of different-concentration of NPPB on GABA-activated inward currents. (A) Results of 100 µmol/l GABA-activated inward currents with different concentrations of NPPB (1–30 µmol/l). (B) Inward current induced by GABA (100 µmol/l) was suppressed by 13.8±6.7, 23.2±14.7 and 29.7±9.1% by 3, 10 and 30 µmol/l NPPB, respectively. *P<0.05, **P<0.01 vs. control. Results as determined by paired t test. Numbers on bars = number of neurons. NPPB, 5-nitro-2-(3-phenylpropylamino) benzoic acid; GABA, γ-aminobutyrate.
Effects of NTDP and extracellular calcium on GABA-induced inward currents
The L-type calcium channel blocker, NTDP (0.1–30 µmol/l), inverted the inhibitory effect of 100 µmol/l NFA on 100 µmol/l GABA-induced inward current (Fig. 4). The inhibitory ratio of NFA on inward current induced by GABA were (59.6±8.70%, neurons=4, P>0.05; 43.6±5.10%, neurons=3, P<0.05; 32.3±6.62%, neurons=8, P<0.01; 8.7±7.6%, neurons=6, P<0.01 and 8.6±7.4%, neurons=4, P<0.01, in the presence of 0.1, 1, 3, 10 and 30 µmol/l NTDP, respectively. To investigate the effect of extracellular free calcium on NFA, calcium-free extracellular fluid was utilized. The inhibitory effect of NFA on GABA-evoked inward currents was strongly suppressed by calcium-free extracellular fluid (P<0.01; Fig. 4). The 100 µmol/l GABA-activated current with 100 µmol/l NFA were 1,298.8±124.4 pA; neurons = 22 and 775.9±104.9 pA; neurons = 6; P<0.01, in the presence and absence of calcium-free extracellular solution, respectively. The inhibition ratio of NFA on the GABA-evoked inward current was 8.4±7.2% (neurons = 6, P<0.01) in the calcium-free extracellular solution.
Figure 4.
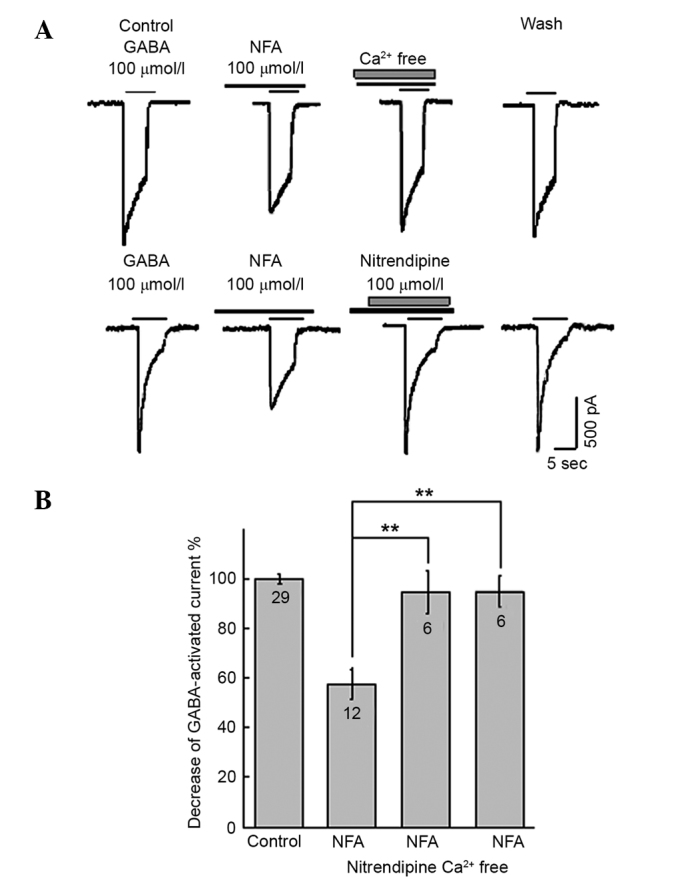
Effects of nitrendipine (NTDP) and exreacellular Ca2+ on GABA-activated inward currents. (A) Results of 100 µmol/l GABA-activated inward currents in the presence of 100 µmol/l NFA, 100 µmol/l NFA + calcium-free extracellular solution and 100 µmol/l NFA + 10 µmol/l NTDP. (B) Inward current induced by 100 µmol/l GABA was suppressed by 62.2±12.6, 8.7±7.6 and 8.4±7.2% by NFA, NFA + NTDP and NFA + calcium-free extracellular solution, respectively. **P<0.01. Paired t test. Numbers on bars=number of neurons. NFA, niflumic acid; GABA, γ-aminobutyrate.
Effect of intracellular calcium on the GABA-induced inward current by intracellular calcium
To investigate the effect of intracellular free calcium on the GABA-induced inward current, BAPTA-AM was utilized and caffeine. The inhibitory effect of NFA on GABA-evoked inward current was strongly suppressed by BAPTA-AM and caffeine. The inhibition ratio of NFA on the GABA-evoked inward current was decreased by 48.2±15.7% (neurons=9, P<0.01) and 38.7±13.2% (neurons=7, P<0.05) with BAPTA-AM (100 µmol/l) and caffeine (30 µmol/l), respectively (Fig. 5).
Figure 5.
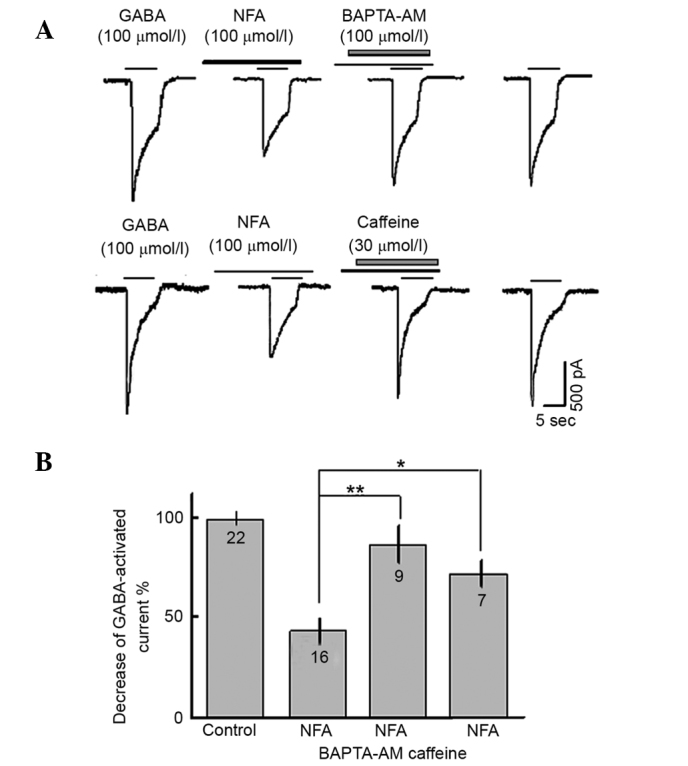
Effects of intracellular Ca2+ on GABA-activated inward currents. (A) Results of 100 µmol/l GABA-activated inward currents in the presence of 100 µmol/l NFA, 100 µmol/l NFA + 100 µmol/l BAPTA-AM and 100 µmol/l NFA + 30 µmol/l caffeine. (B) Inward current induced by GABA was suppressed by 61.8±13.4, 18.9±17.6 and 28.4±13.2% NFA, NFA + BAPTA-AM and NFA + caffeine, respectively. *P<0.05, **P<0.01. Results as determined by paired t test. Numbers on bars = number of neurons. NFA, niflumic acid; GABA, γ-aminobutyrate; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N,N-tetraacetic acid.
Distribution of TMEM16A and TMEM16B subunits expressed in DRG neurons
Immunofluorescence staining revealed that TMEM16A and TMEM16B expression was widely distributed in DRG neurons, and was predominately located in the cell membranes of various diameters (Fig. 6A and B). Thus, TMEM16A and TMEM16B were co-expressed in the membranes of DRG neurons (Fig. 6C).
Figure 6.
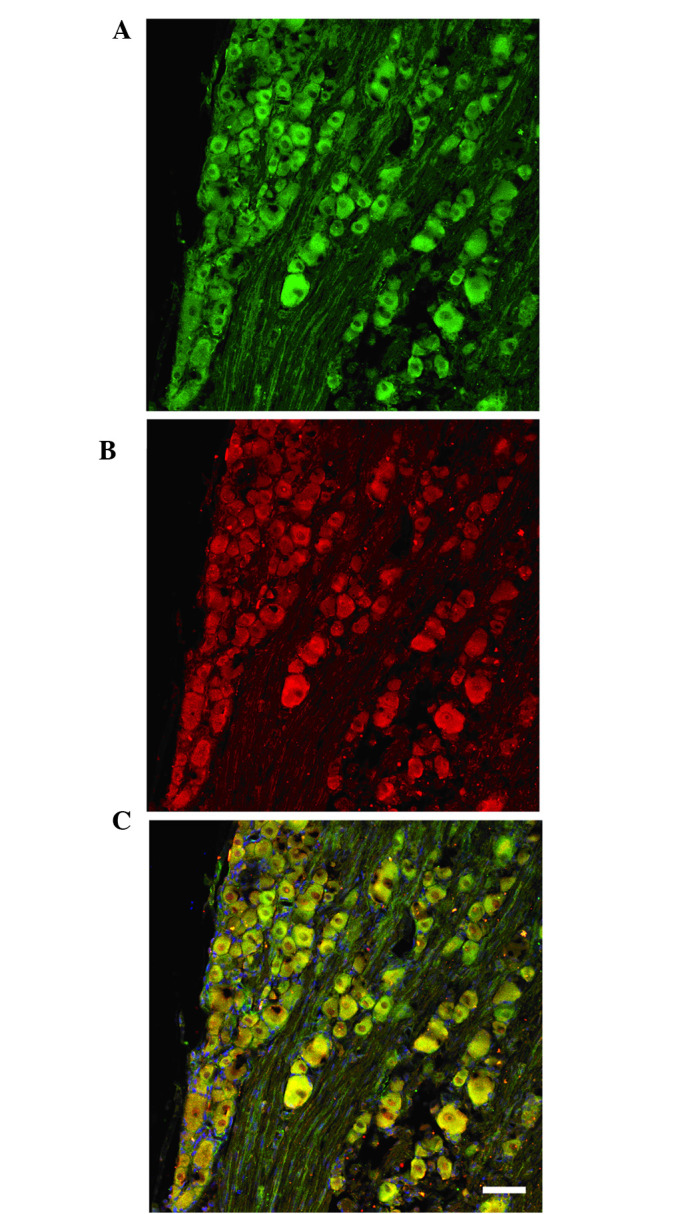
Confocal images of TMEM16A and TMEM16B subunits expressed in the L4–6 dorsal root ganglion (DRG). Immunofluorescent staining indicated that (A) TMEM16A and (B) TMEM16B expression were widely distributed in DRG neurons, and were mainly located in cell membranes of various diameters. (C) A and B overlaid (scale bars, 100 µm).
Discussion
GABAA receptor mediates GABA-evoked membrane depolarization responses, or inward current, since the selective GABAA receptor agonist muscimol mimicked GABA-evoked responses, and the selective GABAA receptor antagonist bicuculline blocked GABA-activated membrane responses in rat DRG neurons (17,19). The results of the present study indicated that NFA and NPPB, non-steroid anti-inflammatory agents, reduced GABA-activated inward currents. The present study also revealed that NTDP, an L-type calcium channel blocker, a calcium-free extracellular solution, BAPTA-AM, which is a membrane permeable Ca2+ chelator and caffeine, a Ca2+ consuming drug, also reduced the inhibitory effect of NFA on the GABA-activated inward current. Furthermore, the current study demonstrated that the TMEM16A and TMEM16B subunits were expressed in rat DRG neurons.
A number of cells express CaCCs, which have several physiological functions, including their being developmentally adjusted with maximum peak expression in the period of peripheral synaptogenesis in DRG neurons (20). An association was observed between the expression of CaCCs and the growth competence of sensory neurons (21). CaCCs activation augments after depolarization following spike firing of action potential in neonatal rat DRG neurons (22,23). Axotomy upregulates the expression of CaCCs in adult sensory, nodose and sympathetic ganglion neurons (21,24,25). Additionally, with regard to electrical activity, CaCCs have an important role in other basic cellular functions such as cell adhesion, apoptosis and potentially in volume regulation (26,27). In 2008, the announcements that three labs had cloned genes that encoded classical CaCCs generated considerable interest (28–30); the 2 genes that have been definitively shown to encode CaCCs termed TMEM16A and TMEM16B. CaCCs are activated by an increase in intracellular free calcium concentration following either internal calcium release from Ca2+ stores, or external calcium entry through Ca2+ channel (31). NFA and NPPB have been indicated to suppress the activity of CaCCs, and NPPB inhibits chloride ion flux through other anionic channels (32). The results of the present study show that NFA and NPPB are able to significantly inhibit the GABA-activated inward current, and NTDP, calcium-free extracellular fluid and BAPTA-AM may significantly reduce the inhibitory effect of NFA on GABA-activated inward current. The present results also suggest that NSAIDs have an important role in the GABA-activated inward current via CaCCs in DRG neurons in rats, and support the hypothesis proposed by the present study. GABA activates the GABAA receptor to open chloride ion channels, the chloride ion efflux induces the depolarization response of the membrane of DRG neurons (17). Conversely, voltage dependent L-type Ca2+ channels may be activated by depolarization, and lead to increased intracellular Ca2+. Furthermore, the NFA-induced increase in intracellular Ca2+ is likely due to Ca2+ release from an intracellular store (33–35). CaCCs are activated by an increase in intracellular calcium concentration, which in turn increases the driving force for chloride ion efflux (28). Finally, the synergistic action of chloride ion efflux via GABAA receptors and NFA-sensitive CaCCs results in GABA-activated currents or depolarization responses in rat DRG neurons. The depolarization arising from Cl− efflux through CaCCs indicates a mechanism of electrical amplification of the GABA-activated currents (Fig. 7).
Figure 7.
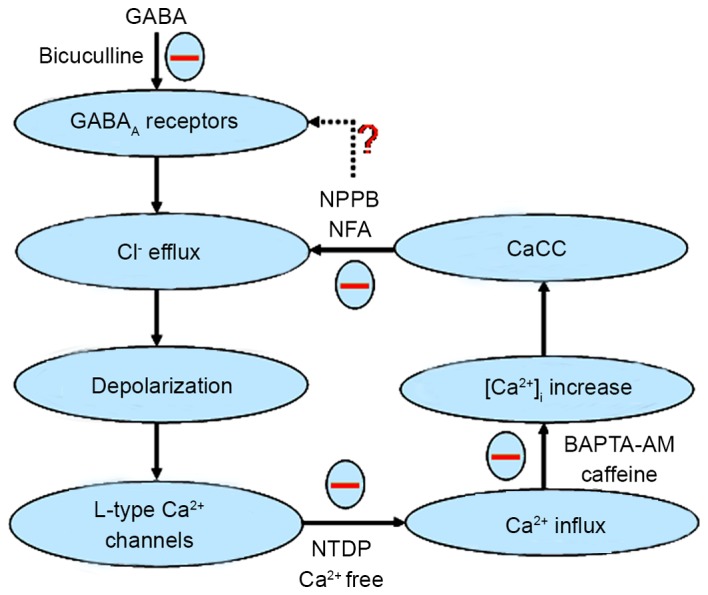
Schematic displaying the effects of CaCCs on GABA-activated inward currents and depolarization. GABA activates the GABAA receptor to open the Cl− channel and the Cl− efflux induces the depolarization response (inward current) of the membrane of dorsal root ganglion (DRG) neurons. Then, voltage dependent L-type Ca2+ channels are activated by the depolarization, and give rise to an increase in intracellular Ca2+. CaCCs are activated by an increase in intracellular Ca2+ concentration which, in turn, increases the driving force for Cl− efflux. Finally, the synergistic action of the chloride ion efflux through GABAA receptors and NFA-sensitive CaCCs causes GABA-activated currents or depolarization response in rat DRG neurons. ‘−’ expressed function was inhibitory, ‘?’ expressed function was not clear. GABA, γ-aminobutyrate; NPPB, 5-nitro-2-(3-phenylpropylamino) benzoic acid; NFA, niflumic acid; CaCC, Ca2+-activated Cl− channels; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N,N-tetraacetic acid; NTDP, nitrendipine.
The binding of intracellular signaling molecules or extracellular ligands activates a conformational change that opens or closes the pores of ligand-gated ion channels such as GABA, glutamate, serotonin and acetylcholine, as well as the cyclic nucleotide-gated ion channels that have important roles in sensory biology (17). However, the structure and function of ligand-gated ion channels and their integral receptors have yet to be elucidated. Therefore, it is important to develop novel tools to investigate the interactions between various receptor subunits, which may benefit the future designing of receptor subtype-selective therapies.
Ion channel gating is influenced by several factors, including the binding of another ligand and intracellular Ca2+ (36). GABAA integrates the actions of a wide range of therapeutic agents, including steroids, benzodiazepines, barbiturates, convulsants and anesthetics (2). Previous studies have indicated that NFA is able to directly act on GABAA and NMDA receptors (5,12,37). Notably, another chloride blocker, mefenamic acid, is also able to directly activate GABAA receptors (38). NFA functions as a positive allosteric modulator of α1β2γ2, and a negative modulator of α6β2 and α6β2γ2 (and α1β2) GABAA receptors. Despite the knowledge that NFA shares the same site as furosemide to mediate its inhibitory effect, the site for the positive regulation remains elusive, and is dependant on the presence of the γ2 subunit, yet separable from the benzodiazepine binding site (11,12).
It has been suggested that the niflumate potentiation of GABAA function is through a pure direct allosteric mechanism (12). Conversely, it has been reported that the activation of GABAA opens NFA-sensitive anion channels (39,40). Furthermore, GABAA-mediated chloride ion influx lowers the magnitudes of NFA- and NPPB-sensitive chloride currents in motorneurons. NFA and other fenamate blockers of CaCCs (41,42), and recombinant GABAA (43). The expression levels of rat homopentamer GABAA receptors display GABA-independent activation (44). In addition, NFA activates single chloride ion channels, likely to be an isoform of the GABAA receptors in mouse sperm (45). Therefore, it is possible that alternative mechanisms exists for NFA action on the GABAA receptor.
In the present study, NFA did not alter the EC50 value but reduced the maximal response of GABA currents, which is consistent with noncompetitive antagonism. Similar results have been reported for furosemide (46) and NFA (12) previously. Changes in the expression and function of α2, but not α6 subunits of GABAA, were observed in L4-L6 DRG neurons by whole-cell patch-clamp and immunofluorescence.
Further experiments are needed to determine whether NFA and NPPB may directly act upon the GABAA α2 subunit in DRG neurons, and the physiological activator of CaCCs may also provide further elucidation regarding the contribution of CaCCs to electrical activity. As NSAIDS are highly subtype-selective, further studies to investigate its behavioral and cognitive effects are warranted (11). We propose that expression of CaCCs in DRG should increase in response to peripheral nerve injury, and enhance the responses of GABAA receptors. Therefore, the upregulation of CaCCs may prominently enhance ‘the presynaptic inhibition’ of GABA in the primary afferent endings, and have involvement in pain modulation.
Acknowledgements
The authors thank Dr Hong-Zhen Hu for reading the manuscript and for valuable suggestions. The present study was supported by the National Natural Science Foundation of China (grant no. 30160026 to Dr Jun-Qiang Si) and the Youth Science and Technology Innovation Special Foundation of Xinjiang Production and Construction Corps (China; grant no. 2010JC33 to Dr Li Li).
Glossary
Abbreviations
- GABA
γ-aminobutyrate
- PNS
periphery nervous system
- CNS
central nervous system
- NFA
niflumic acid
- NPPB
5-nitro-2-(3-phenylpropylamino) benzoic acid
- NSAIDs
non-steroidal anti-inflammatory drugs
- CaCCs
Ca2+-activated Cl− channels
- DRG
dorsal root ganglion
- SDRs
Sprague-Dawley rats
- NTDP
nitrendipine
References
- 1.Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdellès B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, et al. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann N Y Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- 2.Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Brain Res Rev. 1999;29:196–217. doi: 10.1016/S0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- 3.Smith AJ, Oxley B, Malpas S, Pillai GV, Simpson PB. Compounds exhibiting selective efficacy for different beta subunits of human recombinant gamma-aminobutyric acid A receptors. J Pharmacol Exp Ther. 2004;311:601–609. doi: 10.1124/jpet.104.070342. [DOI] [PubMed] [Google Scholar]
- 4.Jensen ML, Timmermann DB, Johansen TH, Schousboe A, Varming T, Ahring PK. The beta subunit determines the ion selectivity of the GABAA receptor. J Biol Chem. 2002;277:41438–41447. doi: 10.1074/jbc.M205645200. [DOI] [PubMed] [Google Scholar]
- 5.Babot Z, Cristòfol R, Suñol C. Excitotoxic death induced by released glutamate in depolarized primary cultures of mouse cerebellar granule cells is dependent on GABAA receptors and niflumic acid-sensitive chloride channels. Eur J Neurosci. 2005;21:103–112. doi: 10.1111/j.1460-9568.2004.03848.x. [DOI] [PubMed] [Google Scholar]
- 6.Wallenstein MC. Attenuation of epileptogenesis by nonsteroidal anti-inflammatory drugs in the rat. Neuropharmacology. 1991;30:657–663. doi: 10.1016/0028-3908(91)90087-R. [DOI] [PubMed] [Google Scholar]
- 7.McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/S0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood IA, Large WA. Comparison of the effects of fenamates on Ca-activated chloride and potassium currents in rabbit portal vein smooth muscle cells. Br J Pharmacol. 1995;116:2939–2948. doi: 10.1111/j.1476-5381.1995.tb15948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malykhina AP, Shoeb F, Akbarali HI. Fenamate-induced enhancement of heterologously expressed HERG currents in Xenopus oocytes. Eur J Pharmacol. 2002;452:269–277. doi: 10.1016/S0014-2999(02)02330-0. [DOI] [PubMed] [Google Scholar]
- 10.Korpi ER, Gründer G, Lüddens H. Drug interactions at GABA (A) receptors. Prog Neurobiol. 2002;67:113–159. doi: 10.1016/S0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell RF, Thomas P, Patten D, James CH, Martinez-Torres A, Miledi R, Smart TG. Subunit-selective modulation of GABAA receptors by the non-steroidal anti-inflammatory agent, mefenamic acid. Eur J Neurosci. 1999;11:2897–2905. doi: 10.1046/j.1460-9568.1999.00709.x. [DOI] [PubMed] [Google Scholar]
- 12.Sinkkonen ST, Mansikkamäki S, Möykkynen T, Lüddens H, Uusi-Oukari M, Korpi ER. Receptor subtype-dependent positive and negative modulation of GABA (A) receptor function by niflumic acid, a nonsteroidal anti-inflammatory drug. Mol Pharmacol. 2003;64:753–763. doi: 10.1124/mol.64.3.753. [DOI] [PubMed] [Google Scholar]
- 13.Whittemore ER, Yang W, Drewe JA, Woodward RM. Pharmacology of the human gamma-aminobutyric acid A receptor alpha 4 subunit expressed in Xenopus laevis oocytes. Mol Pharmacol. 1996;50:1364–1375. [PubMed] [Google Scholar]
- 14.Thompson SA, Whiting PJ, Wafford KA. Barbiturate interactions at the human GABAA receptor: Dependence on receptor subunit combination. Br J Pharmacol. 1996;117:521–527. doi: 10.1111/j.1476-5381.1996.tb15221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci USA. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Division of Research Resources: Guide for the care and use of laboratory animals. 8th. National Academies Press; Washington, DC: 2011. Institute of Laboratory Animal Resources (US). Committee on Care, Use of Laboratory Animals, and National Institutes of Health (US) [Google Scholar]
- 17.Si JQ, Zhang ZQ, Li CX, Wang LF, Yang YL, Li ZW. Modulatory effect of substance P on GABA-activated currents from rat dorsal root ganglion. Acta Pharmacol Sin. 2004;25:623–629. [PubMed] [Google Scholar]
- 18.Cheng HJ, Ma KT, Li L, Zhao L, Wang Y, Si JQ. Differential expression of alpha-adrenoceptor subtypes in rat dorsal root ganglion after chronic constriction injury. Hua Zhong Ke Ji Da Xue Xue Bao. 2014;34:322–329. doi: 10.1007/s11596-014-1277-1. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 19.Ma KT, Si JQ, Zhang ZQ, Zhao L, Fan P, Jin JL, Li XZ, Zhu L. Modulatory effect of CCK-8S on GABA-induced depolarization from rat dorsal root ganglion. Brain Res. 2006;1121:66–75. doi: 10.1016/j.brainres.2006.08.094. [DOI] [PubMed] [Google Scholar]
- 20.Bernheim L, Bader CR, Bertrand D, Schlichter R. Transient expression of a Ca2+-activated Cl− current during development of quail sensory neurons. Dev Biol. 1989;136:129–39. doi: 10.1016/0012-1606(89)90136-X. [DOI] [PubMed] [Google Scholar]
- 21.André S, Boukhaddaoui H, Campo B, Al-Jumaily M, Mayeux V, Greuet D, Valmier J, Scamps F. Axotomy-induced expression of calcium-activated chloride current in subpopulations of mouse dorsal root ganglion neurons. J Neurophysiol. 2003;90:3764–3773. doi: 10.1152/jn.00449.2003. [DOI] [PubMed] [Google Scholar]
- 22.De Castro F, Geijo-Barrientos E, Gallego R. Calcium-activated chloride current in normal mouse sympathetic ganglion cells. J Physiol. 1997;498:397–408. doi: 10.1113/jphysiol.1997.sp021866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer ML. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Vives MV, Gallego R. Calcium-dependent chloride current induced by axotomy in rat sympathetic neurons. J Physiol. 1994;475:391–400. doi: 10.1113/jphysiol.1994.sp020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lancaster E, Oh EJ, Gover T, Weinreich D. Calcium and calcium-activated currents in vagotomized rat primary vagal afferent neurons. J Physiol. 2002;540:543–556. doi: 10.1113/jphysiol.2001.013121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdel-Ghany M, Cheng HC, Elble RC, Pauli BU. Focal adhesion kinase activated by beta (4) integrin ligation to mCLCA1 mediates early metastatic growth. J Biol Chem. 2002;277:34391–34400. doi: 10.1074/jbc.M205307200. [DOI] [PubMed] [Google Scholar]
- 27.Elble RC, Pauli BU. Tumor suppression by a proapoptotic calcium-activated chloride channel in mammary epithelium. J Biol Chem. 2001;276:40510–40517. doi: 10.1074/jbc.M104821200. [DOI] [PubMed] [Google Scholar]
- 28.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 29.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 30.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott RH, Sutton KG, Griffin A, Stapleton SR, Currie KP. Aspects of calcium-activated chloride currents: A neuronal perspective. Pharmacol Ther. 1995;66:535–565. doi: 10.1016/0163-7258(95)00018-C. [DOI] [PubMed] [Google Scholar]
- 32.Frings S, Reuter D, Kleene SJ. Neuronal Ca2+-activated Cl-channels-homing in on an elusive channel species. Prog Neurobiol. 2000;60:247–289. doi: 10.1016/S0301-0082(99)00027-1. [DOI] [PubMed] [Google Scholar]
- 33.Cruickshank SF, Baxter LM, Drummond RM. The Cl(−) channel blocker niflumic acid releases Ca(2+) from an intracellular store in rat pulmonary artery smooth muscle cells. Br J Pharmacol. 2003;140:1442–1450. doi: 10.1038/sj.bjp.0705571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L, Ma KT, Zhao L, Si JQ. Niflumic acid hyperpolarizes the smooth muscle cells by opening BK(Ca) channels through ryanodine-sensitive Ca(2+) release in spiral modiolar artery. Sheng Li Xue Bao. 2008;60:743–750. [PubMed] [Google Scholar]
- 35.Li L, Ma KT, Zhao L, Si JQ, Zhang ZS, Zhu H, Li J. Niflumic acid hyperpolarizes smooth muscle cells via calcium-activated potassium channel in spiral modiolar artery of guinea pigs. Acta Pharmacol Sin. 2008;29:789–799. doi: 10.1111/j.1745-7254.2008.00803.x. [DOI] [PubMed] [Google Scholar]
- 36.Levitan IB. Signaling protein complexes associated with neuronal ion channels. Nat Neurosci. 2006;9:305–310. doi: 10.1038/nn1647. [DOI] [PubMed] [Google Scholar]
- 37.Lerma J, del Rio Martin R. Chloride transport blockers prevent N-methyl-D-aspartate receptor-channel complex activation. Mol Pharmacol. 1992;41:217–222. [PubMed] [Google Scholar]
- 38.Coyne L, Su J, Patten D, Halliwell RF. Characterization of the interaction between fenamates and hippocampal neuron GABA(A) receptors. Neurochem Int. 2007;51:440–446. doi: 10.1016/j.neuint.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raiteri L, Schmid G, Prestipino S, Raiteri M, Bonanno G. Activation of alpha 6 GABAA receptors on depolarized cerebellar parallel fibers elicits glutamate release through anion channels. Neuropharmacology. 2001;41:943–951. doi: 10.1016/S0028-3908(01)00138-1. [DOI] [PubMed] [Google Scholar]
- 40.Van Damme P, Callewaert G, Eggermont J, Robberecht W, Van Den Bosch L. Chloride influx aggravates Ca2+-dependent AMPA receptor-mediated motoneuron death. J Neurosci. 2003;23:4942–4950. doi: 10.1523/JNEUROSCI.23-12-04942.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White MM, Aylwin M. Niflumic and flufenamic acids are potent reversible blockers of Ca2 (+)-activated Cl-channels in Xenopus oocytes. Mol Pharmacol. 1990;37:720–724. [PubMed] [Google Scholar]
- 42.Korn SJ, Bolden A, Horn R. Control of action potentials and Ca2+ influx by the Ca (2+)-dependent chloride current in mouse pituitary cells. J Physiol. 1991;439:423–437. doi: 10.1113/jphysiol.1991.sp018674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodward RM, Polenzani L, Miledi R. Effects of fenamates and other nonsteroidal anti-inflammatory drugs on rat brain GABAA receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1994;268:806–817. [PubMed] [Google Scholar]
- 44.Sigel E, Baur R, Malherbe P, Möhler H. The rat beta 1-subunit of the GABAA receptor forms a picrotoxin-sensitive anion channel open in the absence of GABA. FEBS Lett. 1989;257:377–379. doi: 10.1016/0014-5793(89)81576-5. [DOI] [PubMed] [Google Scholar]
- 45.Espinosa F, de la Vega-Beltrán JL, López-González I, Delgado R, Labarca P, Darszon A. Mouse sperm patch-clamp recordings reveal single Cl-channels sensitive to niflumic acid, a blocker of the sperm acrosome reaction. FEBS Lett. 1998;426:47–51. doi: 10.1016/S0014-5793(98)00305-6. [DOI] [PubMed] [Google Scholar]
- 46.Korpi ER, Kuner T, Seeburg PH, Lüddens H. Selective antagonist for the cerebellar granule cell-specific gamma-aminobutyric acid type A receptor. Mol Pharmacol. 1995;47:283–289. [PubMed] [Google Scholar]


