Abstract
Although primary central nervous system (CNS) germ cell tumors (GCTs) are one of the most treatable types of malignant brain tumor, a subset of patients remain resistant to standard chemotherapy. Gain-of-function mutations of the c-Kit gene, and KIT protein expression, have been observed in a number of GCTs, including testicular seminoma, ovarian dysgerminoma and mediastinal seminoma in various ethnic groups. Although a small number of studies have reported the role of c-Kit in CNS GCTs, few have focused on Chinese patients exhibiting CNS GCTs. In the present study, the frequency and location of c-Kit mutations and KIT protein expression levels in CNS GCTs were investigated in 30 patients, between January 1994 and October 2014. Immunohistochemical assays suggested that KIT protein expression was present in 59.1% patients (66.7% in males and 42.9% in females); however, no statistically significant correlation was identified between KIT protein expression and patient clinicopathological features. By performing PCR amplification and direct sequencing, 4 mutational hot spots of the c-Kit gene (exons 9, 11, 13 and 17) were examined, and c-Kit gene mutation was identified in 1/17 (5.9%) CNS germinoma cases. This mutation was located in exon 11 at codon 557–558 WK (Tryptophan-Lysine). No c-Kit gene mutations were detected in non-germinomatous GCTs. Imatinib, a tyrosine kinase inhibitor, may be an effective treatment against standard chemotherapy-resistant CNS germinoma patients exhibiting c-Kit mutations.
Keywords: intracranial germinoma, c-kit mutation, imatinib
Introduction
Primary central nervous system (CNS) germ cell tumors (GCTs) are a rare heterogeneous group of lesions located in the CNS (1). CNS GCTs occur primarily in children and adolescents, with ~90% of cases arising before the age of 20 years (2). Pathological classification of CNS GCTs, according to the World Health Organization criteria, comprises germinoma, teratoma, yolk sac tumor, embryonal carcinoma, choriocarcinoma and mixed GCT (3). Although CNS GCTs are considered to be one of the most treatable types of malignant brain tumor, and may be treated using neoadjuvant therapy in combination with pre/post-chemoradiotherapy, a subset of patients remain resistant to standard chemotherapy agents, primarily due to the clinical and histological heterogeneity of CNS GCTs (4). Therefore, the development of an understanding of the detailed molecular mechanisms underlying CNS GCTs is imperative, in order to discover novel potential treatments for tumors that demonstrate resistance to traditional therapeutic strategies.
The proto-oncogene c-Kit encodes a transmembrane tyrosine kinase (TK) receptor, containing an extracellular domain with five immunoglobulin-like repeats (D1 distal-D5 juxtamembrane), a transmembrane domain, a juxtamembrane domain, and TK 1 and 2 domains (5). The natural c-Kit ligand, stem cell factor (SCF), binds distal D1, D2 and D3 domains and activates downstream signaling, including Src family kinase, phosphoinositide 3-kinase and mitogen-activated protein kinase signaling pathways, promoting cell migration, proliferation and apoptosis resistance (6). SCF/KIT signaling has a significant role in a number of normal tissues, including germ cells, melanocytes, mast cells and interstitial cells of Cajal (7–10). The absence of KIT or SCF expression in mice results in death, suggesting an irreplaceable role of SCF/KIT signaling during embryonic or perinatal death (11). In addition, human c-Kit mutations in exons 8, 9, 11, 13 and 17 have been identified in 75–80% of gastrointestinal stromal tumors (GISTs) (12). c-Kit mutations have additionally been frequently identified in 30% of GCTs, including testicular seminoma, ovarian dysgerminoma and mediastinal seminoma (13–15).
It has been observed that there is significant genetic variation for the same disease among certain ethnic groups (16). Considering the fact that mutation of c-Kit in CNS GCTs has been reported in a number of studies (17,18), and no study to the best of our knowledge has focused on Chinese populations, the present study investigated 4 mutant hot spots of the c-Kit gene (exons 9, 11, 13 and 17) using polymerase chain reaction (PCR) amplification and direct sequencing, to identify the presence, frequency and location of c-Kit mutations. In addition, KIT protein expression was detected using immunohistochemistry, and its correlation with patient clinicopathological data was analyzed.
Materials and methods
Patients and specimens
Between January 1994 and October 2014, 21 male and 9 female Chinese patients (male:female ratio, 2.3:1), diagnosed with primary intracranial GCTs at Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University (Shanghai, China) were enrolled in the present study. None of the patients had received chemotherapy or radiotherapy prior to surgery. A total of 39 formalin-fixed paraffin-embedded tumor tissue samples, and one fresh tumor tissue, were collected from primary intracranial GCT patients. All tumor samples were reviewed by two pathologists in order to verify the diagnosis. The basic clinical characteristics of patients are presented in Table I. The present study was approved by the Ethics Committee of Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University (Shanghai, China). Written informed consent for the use of patient tissue specimens in the present study was obtained from all patients.
Table I.
Clinicopathological data, c-Kit gene mutations and c-Kit IHC staining in 23 intracranial germ cell tumors.
| Case number | Age, years | Gender | Location | Histological type | Size, cm | c-Kit IHC | c-Kit mutation |
|---|---|---|---|---|---|---|---|
| 1 | 12 | F | Sellar | G | 3.5 | 2+ | Wild-type |
| 2 | 14 | M | Pineal | G | 1.5 | 4+ | Wild-type |
| 3 | 9 | M | Pineal | G | 5.0 | 3+ | Wild-type |
| 4 | 17 | M | Third ventricle | G | 2.5 | 3+ | Wild-type |
| 5 | 7 | M | Third ventricle | IT | 2.5 | – | Wild-type |
| 6 | 15 | M | Pineal | G | 3.0 | 4+ | 557,558 |
| 7 | 16 | M | Hypothalamus | G | 5.0 | 3+ | Wild-type |
| 8 | 30 | M | Sellar | G | 2.0 | 2+ | Wild-type |
| 9 | 17 | F | Hypothalamus | G | 3.0 | 4+ | Wild-type |
| 10 | 8 | M | Basal ganglia | G | 5.5 | 2+ | Wild-type |
| 11 | 13 | F | Sellar | G | 3.0 | 2+ | Wild-type |
| 12 | 11 | F | Suprasellar | G | 3.0 | 1+ | Wild-type |
| 13 | 11 | F | Sellar | G | 4.5 | 3+ | Wild-type |
| 14 | 16 | M | Sellar | G | 2.0 | – | Wild-type |
| 15 | 18 | M | Hypothalamus | G | 3.0 | 4+ | Wild-type |
| 16 | 30 | M | Hypothalamus | G | 2.5 | 3+ | Wild-type |
| 17 | 25 | M | Third ventricle | G | 5.0 | 3+ | Wild-type |
| 18 | 31 | M | Pineal | MT | 3.0 | – | Wild-type |
| 19 | 18 | M | Hypothalamus | MT | 5.0 | – | Wild-type |
| 20 | 24 | M | Pineal | MGCT | 3.0 | 2+ | Wild-type |
| 21 | 11 | M | Pineal | G | 3.0 | 4+ | Wild-type |
| 22 | 9 | F | Pineal | MGCT | 3.5 | 1+ | Wild-type |
| 23 | 12 | F | Sellar | EC | 4.0 | – | Wild-type |
IHC, immunohistochemistry; G, germinoma; MT, mature teratoma; IT, immature teratoma; EC, embryonal carcinoma; MGCTs, mixed germ cell tumors; F, female; M, male.
Immunohistochemistry and staining evaluation
Surgical specimens were fixed in 10% formalin (Guanghua Sci-Tech Co., Ltd., Guangdong, China) and embedded in paraffin. Sections (4-µm) were cut and stained by hematoxylin and eosin (Sangon Biotech Co., Ltd., Shanghai, China). Additional 4-µm sections were deparaffinized using xylene and rehydrated in a graded series of ethanol. The deparaffinized sections were subsequently incubated with 3% H2O2 to inhibit any endogenous peroxidase activity, followed by microwave treatment for antigen retrieval prior to incubation with primary antibody, using a two-step polymer method (EnVision™; Dako, Glostrup, Denmark). The sections were incubated in a humidified chamber at 4°C overnight, following addition of polyclonal rabbit anti-human c-Kit (A4502; Dako) at working dilutions of 1:100. Subsequently, secondary antibody (anti-rabbit IgG; cat no. A042301; Dako) was added following rinsing with phosphate-buffered saline. The sections were incubated at room temperature for 30 min, subsequently immunoreactivity was detected using 3,3-diaminobenzidine (Sangon Biotech Co., Ltd.) for 15 min, and finally counterstained using hematoxylin. GIST tissues were obtained from Ren Ji Hospital (School of Medicine, Shanghai Jiao Tong University) and utilized as a positive control for staining. Negative controls were prepared using blocking serum instead of primary antibody. c-Kit expression was assessed based on the intensity and extent of membranous staining. The semi-quantitative scoring system was based on the immunoreactive score (IRS), which was defined as the product of percentage of positive cells (PP) and staining intensity (SI). PP was scored as follows: 0, no staining; 1+, 1–25% stained; 2+; 26–50% stained; 3+, 51–75%, stained; and 4+, >75% stained. An IRS of 0, 1+ and 2+ were defined as weak expression and an IRS of ≥3+ as strong expression. SI was scored from 0–3 according to staining colour: 0, no staining; 1, faint yellow; 2, yellow-brown; 3, sepia. The cells were veiwed under a microscope (CX31; Olympus Corporation, Tokyo, Japan).
DNA extraction
Genomic DNA was extracted from paraffin-embedded tumor specimens using a QIAamp DNA mini kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's protocols. The quality and concentration of DNA was assessed using a spectrophotometer (NanoDrop™ 2000; Thermo Fisher Scientific, Waltham, MA, USA) and resolved on a 0.8% TBE gel (Thermo Fisher Scientific).
The amplified DNA fragments were purified using a GFX PCR DNA and Gel Band Purification kit (GE Healthcare Life Sciences, Chalfont, UK) DNA fragments that aligned with exons 9, 11, 13 and 17 were amplified by PCR, using various primers from Invitrogen (Thermo Fisher Scientific; Table II). Each PCR reaction consisted of 5 µl 10X PCR buffer, 5 µl magnesium chloride (25 mmol/l), 1 µl 10 mmol/l deoxynucleotide triphosphates, 0.5 units Taq DNA polymerase (Bio-Rad Laboratories, Inc., Hercules, CA, USA), 2 µl genomic DNA and 1 µl of each primer (10 µmol/l), in a final volume of 50 µl. The cycling conditions were as follows: 94°C for 5 min, 35 cycles at 94°C for 30 sec, at 60°C for 30 sec and 72°C for 30 sec, with a final extension step at 72°C for 10 min. The amplified fragments were purified using a EZ-10 Spin Column PCR Products Purification kit (Bio Basic Canada, Inc., Markham, ON, Canada) and direct sequencing was performed using an ABI Prism® 3100 genetic analyzer (Applied Biosystems; Thermo Fisher Scientific). The sequencing results were analyzed using Chromas Lite software (version 2.1.1; Technelysium Pty Ltd., Brisbane, Australia), with a signal to noise ratio of >98%. Each sample was sequenced at ≥2 times.
Table II.
Summary of primer sequences utilized for amplification and sequencing of c-Kit exons 9, 11, 13 and 17.
| Exon | Primer | Sequence 5→3 | Annealing temperature, °C | Fragment size, bp |
|---|---|---|---|---|
| c-Kit 9 | F | TCCTAGAGTAAGCCAGGGCTT | 54 | 284 |
| R | TGGTAGACAGAGCCTAAACATCC | |||
| c-Kit 11 | F | CTGAGACAATAATTATTAAAAGGTGA | 55 | 227 |
| R | TTATGTGTACCCAAAAAGGTGACA | |||
| c-Kit 13 | F | GCTTGACATCAGTTTGCCAG | 54 | 193 |
| R | AAAGGCAGCTTGGACACGGCTTTA | |||
| c-Kit 17 | F | TACAAGTTAAAATGAATTTAAATGGT | 53 | 228 |
| R | AAGTTGAAACTAAAAATCCTTTGC |
F, forward; R, reverse.
Statistical analysis
Statistical analyses were performed using SPSS for Windows (version 17.0; SPSS, Inc., Chicago, IL, USA). The χ2 or Fishers exact tests were used for categorical and ordinal variables. P<0.05 was considered to indicate a statistically significant difference, and all reported P-values were two-sided.
Results
Variable clinical characteristics of the patients
The mean age of diagnosis of intracranial GCTs was 17.2 years (range, 7–31 years). The duration of symptoms prior to diagnosis ranged from 5 days to 5 years, with a mean duration of 9.7 months. A total of 23 patients (76.7%), including all of the female patients investigated in the present study, were younger than 20 years at diagnosis. Intracranial GCT sites included the sellar region, pineal gland, hypothalamus, third ventricle, basal ganglia and others. The sellar region and pineal gland were the most common sites for intracranial GCT occurrence. Clinical presentations were dependent on the location and size of the tumor in the intracranial region. Symptoms varied at diagnosis, and included headaches, visual disturbances, signs of increased intracranial pressure and endocrine abnormalities (Table I).
KIT protein is expressed in intracranial GCT
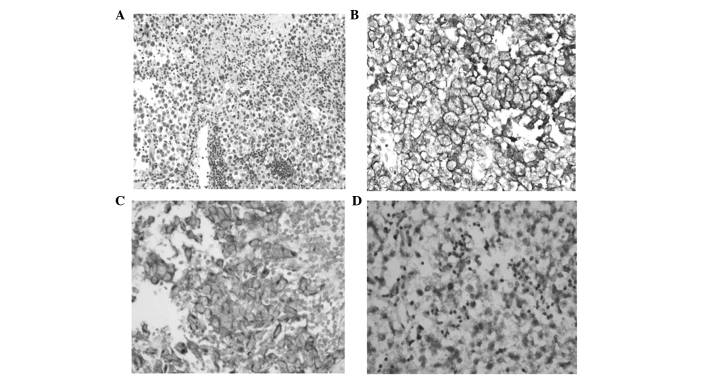
KIT protein, which was primarily expressed in the cytoplasmic membrane, was detected in 21/22 germinoma (95.5%) and 2/2 mixed GCT cases. However, expression of KIT protein was not observed in any of the teratoma (4/4), embryonal carcinoma (1/1) or choriocarcinoma (1/1) cases. High levels of staining were observed in 13/22 (59.1%; 66.7% in males and 42.9% in females) germinoma cases (Fig. 1). As presented in Table III KIT protein expression did not correlate with mutation of the c-Kit gene or any patient clinicopathological parameters, including age, gender, tumor size, tumor location and prognosis.
Figure 1.
Representative results of c-Kit immunohistochemical staining of tumor cells in intracranial germinomas (magnification, ×200). (A) H&E staining, (B) and (C) strong c-Kit expression and (D) weak c-Kit expression. H&E, hematoxylin and eosin.
Table III.
Comparison of clinicopathological data between germinoma patients with high and low KIT protein expression.
| KIT expression | |||
|---|---|---|---|
| Variable | High, n=13 | Low, n=9 | P-value |
| Median age (range) | 17 (9–30) | 15 (8–30) | 1 |
| Gender (male:female) | 10:3 | 5:4 | 0.376 |
| Maximum size, cm | 3.45 ± 1.21 | 3.17 ± 1.29 | 0.611 |
| Location | |||
| Sellar | 1 | 4 | |
| Pineal | 4 | 1 | |
| Third ventricle | 2 | 1 | |
| Hypothalamus | 4 | 1 | 0.395 |
| Basal ganglia | 1 | 1 | |
| Suprasella | 1 | 1 | |
| Alive and well | 13 | 9 | |
| c-Kit mutation | 1 | 0 | 1 |
Statistics were obtained by χ2 or Fisher's exact test.
c-Kit gene mutation was observed in a single germinoma patient
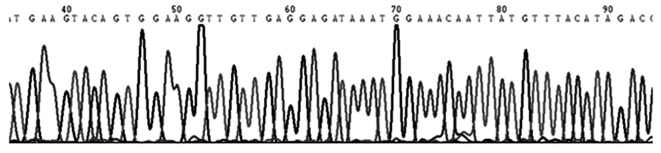
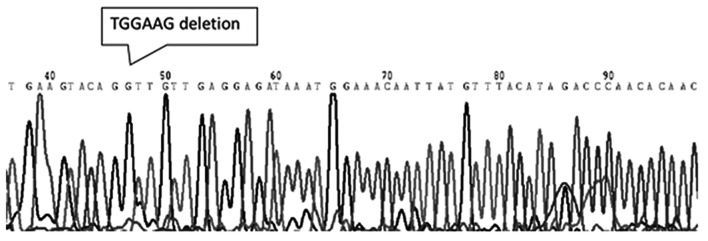
A total of 23 specimens (17 germinoma and 5 non-germinomatous cases) were screened for mutations in the c-Kit gene. Among 17 intracranial germinoma cases, no gene mutations was identified in 16 patients (Fig. 2) and only one c-Kit gene mutation was identified in one patient (5.9%). The observed mutation was located in exon 11, which encodes the juxtamembrane, and was classified as an in-frame deletion at codon 557–558 WK [Tryptophan (Trp)-Lysine (Lys)]. The mutation was considered to a be gain-of-function type (Fig. 3). None of the investigated germinoma cases exhibited mutations in exons 9, 13 or 17 of the c-Kit gene. No mutations were detected in exons 9, 11, 13 or 17 of the c-Kit gene in any of the non-germinomatous GCT cases, including 3 teratomas and 2 mixed GCTs, in the present study.
Figure 2.
Genomic sequencing of exon 11 of the c-Kit gene, demonstrating a wild-type sequence.
Figure 3.
Genomic sequencing of exon 11 of the c-Kit gene. This case exhibited an in-frame deletion mutation (deletion 6 bp, TGGAAG) at codon 557–558 WK (Tryptophan-Lysine). The mutation was considered to be a gain-of-function type.
Discussion
Due to significant advances in treatment options for CNS germinoma patients, it has become essential to develop novel therapeutic strategies for patients that achieve no response to standard chemotherapeutic agents. Due to its expression and mutation in CNS GCTs, c-Kit is a significant potential therapeutic target (18). KIT protein expression in intracranial germinoma cases has been reported in a number of previous studies (17,18). Sakuma et al (17) identified that 100% of intracranial germinoma cases investigated demonstrated membranous KIT protein expression. Similar to these previous findings, the results of the present study revealed that KIT protein expression was detectable in 95.5% of intracranial germinoma cases. However, KIT protein expression was not observed to significantly correlate with c-Kit gene mutation or patient clinicopathological parameters, including age, gender, tumor size, tumor location and prognosis, which was in accordance with multiple types of tumors with the exceptions of the GISTs (19).
In the present study, no mutations were detected in exons 9, 11, 13, and 17 of the c-Kit gene in non-germinomatous GCT cases, including 3 teratomas and 2 mixed GCTs, which was a similar finding to the results of a number of previous studies (5,20–22). The present study identified that 1/17 (5.88%) CNS germinoma cases exhibited a c-Kit gene mutation in exon 11, which encodes the juxtamembrane, and this mutation was classified as an in-frame deletion at codon 557–558 WK (Trp-Lys). The results of the present study differed from previous studies, which reported that c-Kit mutations were identified in 4/16 (25%) and 3/13 (23%) Japanese patients exhibiting germinomas (17,18). In addition, these previous studies demonstrated that 75% of germinoma cases exhibiting mutations possessed a point mutation at exon 17 (D816V, D820V and N822Y), and only 25% of gene mutations were observed to occur at exon 11 (17,18). There may be several reasons for the discrepancy observed between the results of the present and previous studies. The present study was performed on a cohort of Chinese patients, while the previous studies were performed on Japanese patient cohorts (17,18). Therefore, the differences between these populations may be one of the most plausible explanations underlying the discrepancy observed between the results of the present study and previous studies. Furthermore, as CNS GCTs are rare tumors, it is often only possible to study small sample sizes, which may also be responsible for the inconsistencies in results. Therefore, studies aiming to enroll increased numbers of CNS GCT patients are required, and the association between population factors and c-Kit gene mutations in CNS GCTs requires further investigation.
Gain-of-function mutations of c-Kit have been identified in a number of neoplasms, including GIST (23), mastocytosis (24) and hematologic malignancies (25). In addition, GIST patients exhibiting c-Kit mutations in exon 11, which encodes the juxtamembrane domain, have been reported to exhibit unfavorable prognosis (26). In agreement with the results of a number of previous studies, in the present study, the patient exhibiting a c-Kit mutation encountered recurrence 8 months subsequent to primary surgical excision, which suggested that c-Kit mutation may be a gain-of-function type mutation responsible for refractory intracranial germinomas. However, there was no statistically significant correlation observed between c-Kit protein expression and gene mutation in the present study.
Imatinib, a TK inhibitor, has been utilized in order to block the activated c-Kit receptor TK (27). A previous study has demonstrated that imatinib is able to exert a significant suppressive effect on the activation of the mutational c-Kit gene (28). CNS GCT cases exhibiting a mutant c-Kit gene within exon 11 have been reported to demonstrate sensitivity to imatinib treatment (29). However, other studies identified that a mutant c-Kit gene, which exhibited a codon 816 mutation at exon 17, resulted in resistance to imatinib treatment in GISTs (27,30). Therefore, it is possible that sensitivity to imatinib may depend on the type and site of the c-Kit mutation, implying that imatinib may not be a suitable treatment for certain patients exhibiting intracranial GCT. In the present study, a missense mutation in exon 11 of the c-Kit gene was detected in a CNS GCT patient. This patient was diagnosed with germinoma exhibiting no notable clinicopathological features, and experienced recurrence 8 months subsequent to primary surgical excision. As the patient was harboring an activated mutation, they may have demonstrated resistance to traditional anticancer therapy and sensitivity to treatment with imatinib. Germinoma patients exhibiting high levels of KIT protein expression may benefit from gene mutation analysis, which may assist in determination of whether imatinib treatment is appropriate.
In conclusion, KIT protein expression was detected in 95.5% of germinoma cases and was not observed to correlate with c-Kit gene mutation or clinicopathological parameters. In addition, the present study identified that 1/17 (5.9%) patients possessed mutations at exon 11 of the c-Kit gene, which differed from the results of a number of previous studies based on Japanese patient cohorts (17,18). No mutations were detected in non-germinomatous GCT cases. To the best of our knowledge, the present study was the first investigation into the expression and mutation of c-Kit in Chinese patients exhibiting CNS GCTs. Additional studies investigating increased numbers of intracranial GCT patients are required, and whole exome sequencing of c-Kit may additionally be conducted in order to identify additional mutant locations.
Acknowledgements
The authors would like to thank the Science and Technology Commission of Shanghai Municipality (grant no. 134119a9502) for providing the funding for the present study.
References
- 1.Thakkar JP, Chew L, Villano JL. Primary CNS germ cell tumors: Current epidemiology and update on treatment. Med Oncol. 2013;30:496. doi: 10.1007/s12032-013-0496-9. [DOI] [PubMed] [Google Scholar]
- 2.Dufour C, Guerrini-Rousseau L, Grill J. Central nervous system germ cell tumors: An update. Curr Opin Oncol. 2014;26:622–626. doi: 10.1097/CCO.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subbiah V, Meric-Bernstam F, Mills GB, Shaw KR, Bailey AM, Rao P, Ward JF, Pagliaro LC. Next generation sequencing analysis of platinum refractory advanced germ cell tumor sensitive to Sunitinib (Sutent®) a VEGFR2/PDGFRβ/c-kit/FLT3/RET/CSF1R inhibitor in a phase II trial. J Hematol Oncol. 2014;7:52. doi: 10.1186/s13045-014-0052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakuma Y, Sakurai S, Oguni S, Hironaka M, Saito K. Alterations of the c-kit gene in testicular germ cell tumors. Cancer Sci. 2003;94:486–491. doi: 10.1111/j.1349-7006.2003.tb01470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruse G, Metcalfe DD, Olivera A. Functional deregulation of KIT: Link to mast cell proliferative diseases and other neoplasms. Immunol Allergy Clin North Am. 2014;34:219–237. doi: 10.1016/j.iac.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi P. Transcriptional control of KIT gene expression during germ cell development. Int J Dev Biol. 2013;57:179–184. doi: 10.1387/ijdb.130014pr. [DOI] [PubMed] [Google Scholar]
- 8.Yamada T, Hasegawa S, Inoue Y, Date Y, Yamamoto N, Mizutani H, Nakata S, Matsunaga K, Akamatsu H. Wnt/β-catenin and kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J Invest Dermatol. 2013;133:2753–2762. doi: 10.1038/jid.2013.235. [DOI] [PubMed] [Google Scholar]
- 9.Reber LL, Marichal T, Galli SJ. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012;33:613–625. doi: 10.1016/j.it.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 11.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 12.Rammohan A, Sathyanesan J, Rajendran K, Pitchaimuthu A, Perumal SK, Srinivasan U, Ramasamy R, Palaniappan R, Govindan M. A gist of gastrointestinal stromal tumors: A review. World J Gastrointest Oncol. 2013;5:102–112. doi: 10.4251/wjgo.v5.i6.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coffey J, Linger R, Pugh J, Dudakia D, Sokal M, Easton DF, Bishop Timothy D, Stratton M, Huddart R, Rapley EA. Somatic KIT mutations occur predominantly in seminoma germ cell tumors and are not predictive of bilateral disease: Report of 220 tumors and review of literature. Genes Chromosomes Cancer. 2008;47:34–42. doi: 10.1002/gcc.20503. [DOI] [PubMed] [Google Scholar]
- 14.Hoei-Hansen CE, Kraggerud SM, Abeler VM, Kaern J, Rajpert-De Meyts E, Lothe RA. Ovarian dysgerminomas are characterised by frequent KIT mutations and abundant expression of pluripotency markers. Mol Cancer. 2007;6:12. doi: 10.1186/1476-4598-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terada T. Mediastinal seminoma with multiple KIT gene mutations. Pathology. 2009;41:695–697. doi: 10.3109/00313020903305852. [DOI] [PubMed] [Google Scholar]
- 16.Yuan F, Shi M, Ji J, Shi H, Zhou C, Yu Y, Liu B, Zhu Z, Zhang J. KRAS and DAXX/ATRX gene mutations are correlated with the clinicopathological features, advanced diseases, and poor prognosis in Chinese patients with pancreatic neuroendocrine tumors. Int J Biol Sci. 2014;10:957–965. doi: 10.7150/ijbs.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakuma Y, Sakurai S, Oguni S, Satoh M, Hironaka M, Saito K. c-kit gene mutations in intracranial germinomas. Cancer Sci. 2004;95:716–720. doi: 10.1111/j.1349-7006.2004.tb03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamakura Y, Hasegawa M, Minamoto T, Yamashita J, Fujisawa H. C-kit gene mutation: Common and widely distributed in intracranial germinomas. J Neurosurg. 2006;104(Suppl):173–180. doi: 10.3171/ped.2006.104.3.173. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen M, Lasota J. KIT (CD117): A review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205–220. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 20.McIntyre A, Summersgill B, Grygalewicz B, Gillis AJ, Stoop J, van Gurp RJ, Dennis N, Fisher C, Huddart R, Cooper C, et al. Amplification and overexpression of the KIT gene is associated with progression in the seminoma subtype of testicular germ cell tumors of adolescents and adults. Cancer Res. 2005;65:8085–8089. doi: 10.1158/0008-5472.CAN-05-0471. [DOI] [PubMed] [Google Scholar]
- 21.Willmore-Payne C, Holden JA, Chadwick BE, Layfield LJ. Detection of c-kit exons 11- and 17-activating mutations in testicular seminomas by high-resolution melting amplicon analysis. Mod Pathol. 2006;19:1164–1169. doi: 10.1038/modpathol.3800623. [DOI] [PubMed] [Google Scholar]
- 22.Cheng L, Roth LM, Zhang S, Wang M, Morton MJ, Zheng W, Karim Abdul FW, Montironi R, Lopez-Beltran A. KIT gene mutation and amplification in dysgerminoma of the ovary. Cancer. 2011;117:2096–2103. doi: 10.1002/cncr.25794. [DOI] [PubMed] [Google Scholar]
- 23.Lennartsson J, Rönnstrand L. Stem cell factor receptor/c-Kit: From basic science to clinical implications. Physiol Rev. 2012;92:1619–1649. doi: 10.1152/physrev.00046.2011. [DOI] [PubMed] [Google Scholar]
- 24.Bodemer C, Hermine O, Palmérini F, Yang Y, Grandpeix-Guyodo C, Leventhal PS, Hadj-Rabia S, Nasca L, Georgin-Lavialle S, Cohen-Akenine A, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804–815. doi: 10.1038/jid.2009.281. [DOI] [PubMed] [Google Scholar]
- 25.Corbin AS, O'Hare T, Gu Z, Kraft IL, Eiring AM, Khorashad JS, Pomicter AD, Zhang TY, Eide CA, Manley PW, et al. KIT signaling governs differential sensitivity of mature and primitive CML progenitors to tyrosine kinase inhibitors. Cancer Res. 2013;73:5775–5786. doi: 10.1158/0008-5472.CAN-13-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calibasi G, Baskin Y, Alyuruk H, Cavas L, Oztop I, Sagol O, Atila K, Ellidokuz H, Yilmaz U. Molecular analysis of the KIT gene in gastrointestinal stromal tumors with novel mutations. Appl Immunohistochem Mol Morphol. 2014;22:37–45. doi: 10.1097/PAI.0b013e318284a074. [DOI] [PubMed] [Google Scholar]
- 27.Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 28.Corless CL, Ballman KV, Antonescu CR, Kolesnikova V, Maki RG, Pisters PW, Blackstein ME, Blanke CD, Demetri GD, Heinrich MC, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32:1563–1570. doi: 10.1200/JCO.2013.51.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou YY, Tan YS, Sun MH, Wei YK, Xu JF, Lu SH, A-Ke-Su SJ, Zhou YN, Gao F, Zheng AH, et al. C-kit gene mutation in human gastrointestinal stromal tumors. World J Gastroenterol. 2004;10:1310–1314. doi: 10.3748/wjg.v10.i9.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tetzlaff ED, Davey MP. Optimizing adherence to adjuvant imatinib in gastrointestinal stromal tumor. J Adv Pract Oncol. 2013;4:238–250. [PMC free article] [PubMed] [Google Scholar]