See Colosimo and Berardelli (doi:10.1093/brain/awv303) for a scientific commentary on this article.
The influence of clinical features such as age of onset and early autonomic symptoms on survival in multiple system atrophy (MSA) remains unclear. Coon et al. evaluate predictors of survival in the largest patient cohort in MSA published to date. The results clarify discrepancies in the literature and identify prognostic markers.
Keywords: multiple system atrophy, parkinsonism, ataxia, autonomic
See Colosimo and Berardelli (doi:10.1093/brain/awv303) for a scientific commentary on this article.
The influence of clinical features such as age of onset and early autonomic symptoms on survival in multiple system atrophy (MSA) remains unclear. Coon et al. evaluate predictors of survival in the largest patient cohort in MSA published to date. The results clarify discrepancies in the literature and identify prognostic markers.
Abstract
See Colosimo and Berardelli (doi:10.1093/brain/awv303) for a scientific commentary on this article.
Multiple system atrophy is characterized by autonomic failure along with motor symptoms of parkinsonism and/or cerebellar ataxia. There are differing reports on the influence of certain clinical features, including motor subtype (multiple system atrophy–parkinsonism versus multiple system atrophy–cerebellar ataxia), age of onset, gender, and early autonomic symptoms, on the survival in patients with multiple system atrophy. We sought to evaluate overall survival and predictors of survival in a large cohort of patients with multiple system atrophy seen at a single referral centre where objective autonomic testing is routinely performed for this indication. All cases of multiple system atrophy evaluated at Mayo Clinic, Rochester and assessed with an autonomic reflex screen between January 1998 and December 2012 were retrospectively reviewed. A total of 685 patients were identified; 594 met criteria for probable multiple system atrophy, and 91 for possible multiple system atrophy. Multiple system atrophy–parkinsonism was the predominant subtype in 430 patients (63%). Average age of onset was earlier in multiple system atrophy–cerebellar ataxia (58.4 years) compared to multiple system atrophy–parkinsonism (62.3 years; P < 0.001). Median disease duration from symptom onset to death was 7.51 years (95% confidence interval 7.18–7.78) while time from diagnosis to death was 3.33 years (95% confidence interval 2.92–3.59). There was no difference in survival between motor subtypes of multiple system atrophy (P = 0.232). An initial motor symptom was most common (61%) followed by autonomic onset (28%) and combined motor and autonomic symptoms (11%). The initial onset of either motor or autonomic symptoms did not influence length of survival. However, a number of clinical and autonomic laboratory features predicted unfavourable survival in a univariate analysis. A multivariate model retained the following unfavourable predictors of survival: (i) falls within 3 years of onset (hazard ratio 2.31, P < 0.0001); (ii) bladder symptoms (hazard ratio 1.96, P < 0.0001); (iii) urinary catheterization within 3 years of symptom onset (hazard ratio 1.67, P < 0.003); (iv) orthostatic intolerance within 1 year of symptom onset (hazard ratio 1.28, P < 0.014); (v) older age of onset (hazard ratio 1.02, P = 0.001); and (vi) degree of autonomic failure as measured by a validated composite autonomic severity score (hazard ratio 1.07, P < 0.0023). We conclude that carefully selected clinical features can be used to predict survival in patients with multiple system atrophy. Autonomic testing adds an additional, independent predictor of survival, demonstrating its value not only in the diagnosis of multiple system atrophy but also as prognostic marker.
Introduction
Multiple system atrophy (MSA) is a neurodegenerative disorder characterized by autonomic failure with motor involvement of parkinsonism and/or cerebellar ataxia (Fanciulli and Wenning, 2015). MSA is classified based on the predominant motor features into subtypes of either MSA-P (parkinsonism) or MSA-C (cerebellar) (Gilman et al., 2008). Autonomic dysfunction in MSA may include orthostatic hypotension, bowel and bladder disturbances, and sexual dysfunction (Parikh et al., 2002; Fanciulli and Wenning, 2015) with the diagnosis of probable or possible MSA from consensus criteria relying on either orthostatic hypotension or severity of urinary symptoms (Gilman et al., 2008). Objective autonomic testing showing severe adrenergic and sudomotor failure increases the certitude of MSA diagnosis based on autopsy studies (Iodice et al., 2012).
MSA is a fatal disorder with retrospective and prospective studies reporting survival between 6.2 and 10 years (Ben-Shlomo et al., 1997; Kim et al., 2011; Wenning et al., 2013; Low et al., 2015). The reported average age of onset ranges from 53 to 63 years, depending on the study (Watanabe et al., 2002; Gilman et al., 2010; Wenning et al., 2013; Low et al., 2015). There is also great variability in clinical factors that have been reported as predictive of shortened survival. Various reports have indicated that older age of onset (Wenning et al., 1994; Ben-Shlomo et al., 1997; Klockgether et al., 1998; O'Sullivan et al., 2008; Figueroa et al., 2014), gender (male or female) (Wenning et al., 1994; Schrag et al., 2007; O'Sullivan et al., 2008; Kim et al., 2011), subtype of MSA-P (Saito et al., 1994; Schulz et al., 1994; Ben-Shlomo et al., 1997; Kim et al., 2011), and early or initial autonomic symptoms (Watanabe et al., 2002; Tada et al., 2007; O'Sullivan et al., 2008; Figueroa et al., 2014; Low et al., 2015) are associated with worse survival while late development of autonomic failure has been reported as a favourable prognostic factor (Petrovic et al., 2012; Calandra-Buonaura et al., 2013). In early studies, these discrepancies may be due to the absence of uniform diagnostic criteria (Saito et al., 1994; Wenning et al., 1994; Schulz et al., 1994; Testa et al., 1996; Ben-Shlomo et al., 1997) and in more recent reports may be due to low death numbers included for survival analysis (Watanabe et al., 2002; Kim et al., 2011; Wenning et al., 2013; Roncevic et al., 2014). Additionally, few studies have analysed a population of MSA patients with standardized, comprehensive autonomic testing. Standardized autonomic testing is useful in discriminating MSA early in disease (Baschieri et al., 2015); however, the role of autonomic function testing in prognosticating survival is unknown. Therefore, we sought to evaluate overall survival in a large cohort of patients with MSA evaluated at a tertiary referral centre with comprehensive autonomic testing and to determine how MSA phenotype and findings on autonomic testing influence survival.
Materials and methods
Study design
This is a retrospective review of all individuals evaluated at Mayo Clinic in Rochester, MN with the diagnosis of MSA between January 1998 and December 2012. All participants had to have completed an autonomic reflex screen laboratory assessment. Individuals were excluded if there was a secondary cause of parkinsonism or ataxia, or clinical and laboratory features suggested an alternative diagnosis to MSA. This study was approved by the institutional review board of Mayo Clinic, Rochester, MN.
Clinical characterization
All patients were categorized as probable or possible MSA based on consensus criteria (Gilman et al., 2008) and classified by clinical phenotype of MSA-P or MSA-C based on predominant symptom complex and examination findings at initial evaluation. Parkinsonism was defined as bradykinesia plus at least one of the following signs: resting tremor, rigidity, postural instability, gait changes. Cerebellar ataxia was defined as gait ataxia, ataxic dysarthria, limb ataxia, or sustained gaze-evoked nystagmus. When both parkinsonism and cerebellar ataxia were prominent at initial evaluation, the subtype was assigned based on the earliest motor symptom.
Documentation of motor and autonomic symptoms was from the recorded clinical history, neurological examination, and standardized patient-completed symptom questionnaire. This questionnaire assessed for motor symptoms, including the tendency to fall easily and autonomic symptoms including orthostatic intolerance, bowel, and bladder symptoms. Orthostatic intolerance was defined as symptoms of light-headedness, altered vision, nausea, weakness, fatigue, and coat-hangar distribution pain only while standing. Urinary urgency, frequency, and incontinence were considered bladder symptoms unless attributable to a non-neurological cause. In males, erectile dysfunction was only considered the presenting symptom if the onset occurred with motor symptoms or within 1 year of urinary symptoms. Use of levodopa and maximum dose trialled was recorded with response rated as none, modest (including an initial good response but not sustained), or good response. Dream enactment behaviour and stridor were recorded based on documentation in the clinical history.
Chief complaint, previous diagnoses (when given), and autonomic function testing prior to evaluation were obtained from the initial neurologic consultation note. A previous diagnosis of olivopontocerebellar atrophy, striatonigral degeneration or Shy-Drager syndrome was considered as MSA. Initial symptoms were categorized as motor, autonomic, or combined when they occurred simultaneously. Autonomic symptoms were categorized as ‘early’ if they occurred within 1 year of motor symptoms. The onset of falls was analysed as within 1 or 3 years of initial symptom onset.
Autonomic testing
All patients underwent standardized autonomic reflex testing (Autonomic Reflex Screen) which includes quantitative sudomotor axon reflex testing (QSART or QSWEAT), heart rate response to deep breathing, heart rate and blood pressure responses to Valsalva manoeuvre and head-up tilt. If a patient had autonomic testing more than once, the initial test was used for our analysis. A previously validated Composite Autonomic Severity Score (CASS) was assigned which is composed of three subdomains: sudomotor (score range 0–3), cardiovagal (0–3), and adrenergic (0–4). The total CASS score can therefore range from 0 to 10 with 10 indicating the most severe degree of autonomic failure (Low, 1993). Orthostatic hypotension was defined as a drop in systolic blood pressure of ≥30 mmHg or diastolic blood pressure drop of ≥15 mmHg. Supine hypertension was defined as a systolic ≥150 mmHg or diastolic blood pressure ≥90 mmHg. Percentage anhidrosis was obtained from thermoregulatory sweat test results and accounted for in the CASS sudomotor subscore when available.
Imaging
Only MRI scan results reviewed by a neuroradiologist for clinical purposes were included in the study. Findings were deemed consistent with MSA if any of the following signs were included in the report: cerebellar atrophy, hot cross bun sign, hyperintensity of middle cerebellar peduncles, putaminal hypointensity, hyperintense putaminal rim (Brooks and Seppi, 2009).
Survival data
Survival data were obtained from the clinical record. When not available, the Social Security Death Index was queried using patient name and birthdate to obtain date of death. Patients from the USA and Canada without a clinical correspondence within 6 months were contacted by telephone and questioned regarding their clinical course.
Statistical analysis
Statistical analyses were performed using the statistical software SAS, version 9.3 (SAS Inc, N.C.), with statistical significance set at P < 0.05. Summary statistics are presented as mean and standard deviation (SD) for continuous data or frequency and per cent for categorical data. Comparisons between MSA-P and MSA-C for categorical data were performed using χ2 testing or Fisher’s exact test when appropriate. Continuous variables were compared between MSA-P and MSA-C using the Student’s t-test for normally distributed data or Mann-Whitney U-test for skewed data. CASS was analysed as ordinal data. Survival analyses were performed using Kaplan-Meier estimates with survival curves generated from symptom onset to death and from time of diagnosis to death. For patients without a death date, date of last follow-up or last contact was used (censor date). Cox proportionate hazards models were used to calculate univariate and multivariate hazard ratios for survival.
Results
Demographics
A total of 685 patients with MSA were included; 594 patients met consensus criteria for probable MSA and 91 for possible MSA. The predominant subtype was MSA-P in 430 patients (63%) (Table 1). The mean age of onset was 61 years with a range from 34 to 86 years (Table 1). Of the 685 participants, 541 were deceased. Post-mortem examination was performed on 36 patients, which confirmed the diagnosis of MSA (34 of these met criteria for probable MSA at the time of diagnosis at Mayo Clinic). The median time from onset of symptoms to initial evaluation at Mayo was 3.07 years [interquartile range (IQR) 2.03–5.02 years]. A total of 147 patients (21%) had either an initial diagnosis of MSA or suspected diagnosis of MSA. Only 19 patients (3%) had autonomic function testing prior to the initial Mayo evaluation documented in the clinical record. The median duration of clinical follow-up was 22 days (IQR 4–380 days). Patients heralded from 43 of the 50 United States and three Canadian provinces.
Table 1.
Patient demographics
| Overall | MSA-P | MSA-C | P-valuea | |
|---|---|---|---|---|
| Number (%) | 685 (100) | 430 (63) | 255 (37) | - |
| Gender | ||||
| Female | 330 (48) | 203 (47) | 127 (50) | 0.511 |
| Male | 355 (52) | 227 (53) | 128 (50) | - |
| Probable MSA | 594 (87) | 374 (87) | 220 (86) | 0.794 |
| Age of onset | 60.9 (9.6) | 62.3 (9.5) | 58.4 (9.2) | <0.0001 |
aBetween MSA-P and MSA-C.
Values displayed are mean (SD) for continuous variables and frequency (%) for categorical variables.
Clinical phenotype
Motor onset of symptoms was most common (61%), followed by an initial autonomic symptom (28%), while a minority (11%) had simultaneous onset of motor and autonomic symptoms (Table 2). Of autonomic symptoms, bladder symptoms were frequently noted and occurred within a year of symptom onset in 39% of all patients (Table 2). Of the 106 patients with known onset of either intermittent or indwelling urinary catheterization, the mean time from symptom onset to catheterization was 3.3 years [standard deviation (SD) 2.7]. While orthostatic intolerance was common in all patients with MSA, it was more frequently noted in patients with MSA-P (Table 2). Orthostatic intolerance also developed early with over half of all patients with MSA developing orthostasis within the first year of disease (Table 2).
Table 2.
Clinical features
| Feature | Overall | MSA-P | MSA-C | P-valuea |
|---|---|---|---|---|
| n (%) | 685 (100) | 430 (63) | 255 (37) | - |
| Symptom onset | ||||
| Motor onset | 421 (61) | 261 (61) | 160 (63) | 0.595 |
| Autonomic onset | 190 (28) | 127 (30) | 63 (25) | 0.172 |
| Combined motor and autonomic onset | 74 (11) | 42 (10) | 32 (13) | 0.257 |
| Autonomic symptoms | ||||
| Orthostatic intolerance | 460 (67) | 302 (70) | 158 (62) | 0.026 |
| Early orthostatic intoleranceb | 240 (35) | 158 (37) | 82 (32) | 0.224 |
| Bladder symptoms | 572 (84) | 358 (83) | 214 (84) | 0.820 |
| Early bladder symptomsb | 266 (39) | 170 (40) | 96 (38) | 0.624 |
| Urinary catheterization | 111 (16) | 76 (18) | 35 (14) | 0.175 |
| Urinary catheterization within 1 year | 22 (3) | 16 (4) | 6 (2) | 0.377 |
| Urinary catheterization within 3 years | 58 (8) | 38 (9) | 20 (8) | 0.777 |
| Motor symptoms | ||||
| Parkinsonism | 527 (77) | 430 (100) | 97 (38) | <0.0001 |
| Cerebellar ataxia | 347 (51) | 92 (21) | 255 (100) | <0.0001 |
| Falls | 458 (67) | 273 (63) | 185 (73) | 0.015 |
| Falls within 1 year | 189 (28) | 107 (25) | 82 (32) | 0.040 |
| Falls within 3 years | 357 (52) | 205 (48) | 152 (60) | 0.003 |
| Levodopa trial | 354 (52) | 312 (46) | 42 (16) | <0.0001 |
| Levodopa responsec | 150 (43) | 135 (44) | 15 (36) | 0.4063 |
| Sleep symptoms | ||||
| Dream enactment behaviour | 304 (44) | 180 (42) | 124 (49) | 0.085 |
| Stridor | 176 (26) | 116 (27) | 60 (24) | 0.318 |
Values are displayed as frequency (%).
aBetween MSA-P and MSA-C.
bEarly denotes within 1 year of onset.
cPercentage of those trialled on levodopa.
Motor symptoms of parkinsonism and ataxia tended to overlap in our cohort, although parkinsonism was more likely to occur in MSA-C than ataxia in MSA-P (Table 2). Falls were more common in MSA-C patients; however, 67% of all MSA patients experienced falls at some point in the disease course (Table 2). Levodopa was trialled in 52% of all patients with MSA; of the 319 patients with documented dosages, the average maximum levodopa dose was 683 mg per day (SD 472). While 43% of patients had at least an initial response to levodopa (Table 2), only 10% of all MSA patients reported a sustained beneficial response; this did not differ between MSA-P and MSA-C (P = 0.285). Sleep symptoms of dream enactment behaviour and stridor were similar between MSA-P and MSA-C (Table 2). Of the 304 patients with reported dream enactment behaviour, it preceded motor and autonomic onset of symptoms in 34%.
Autonomic testing
The median time from onset of symptoms to autonomic testing was 3.27 years (IQR 2.06 to 5.04 years). On autonomic reflex screen, the mean sudomotor and cardiovagal CASS subscores show moderate impairment with more severe adrenergic failure. The sudomotor and adrenergic CASS subscores did not vary between groups, but mean cardiovagal subscores were mildly worse in patients with MSA-P (1.5 versus 1.2 in MSA-C, P = 0.002). On tilt table testing, the mean drop in blood pressure was 29/8 mmHg at 1 min and 36/11 mmHg at 5 min. Orthostatic hypotension was present in 59% of all patients and was severe with systolic blood pressure falling to less than 90 mmHg in 22% of patients; supine hypertension was noted in 46%. A total of 607 patients underwent thermoregulatory sweat testing; of which 95% had an abnormal result. MSA-P patients were more likely to have an abnormal thermoregulatory sweat test (97%) compared to MSA-C patients (92%) (P = 0.018). The mean percentage anhidrosis was 56 (SD 33) with no difference between MSA-P and MSA-C.
Imaging
Among the 505 patients with an MRI scan reviewed by a neuroradiologist, 34% had findings reported as suggestive of MSA. Patients with MSA-C (n = 143, 56%) were more likely to have findings reported on MRI than MSA-P (n = 91, 21%; P < 0.0001).
Survival
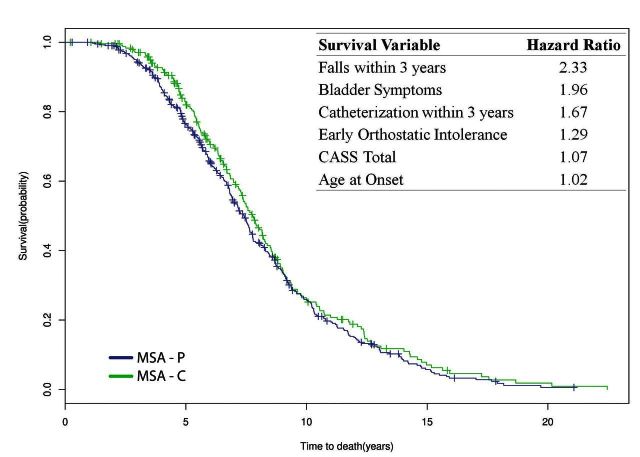
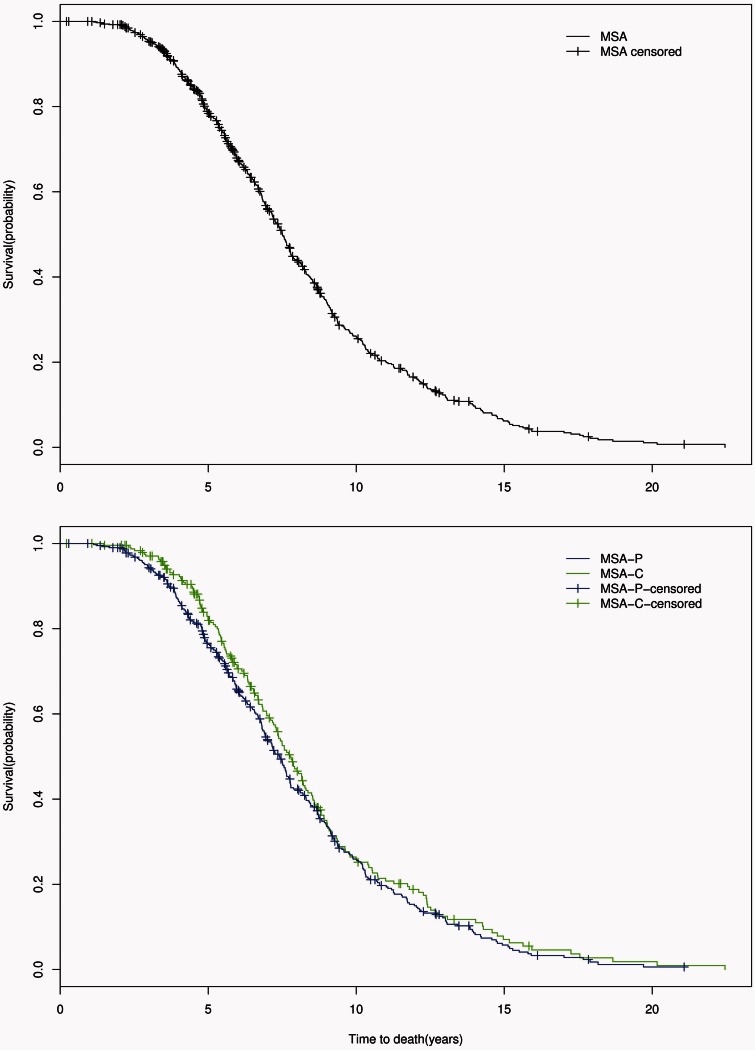
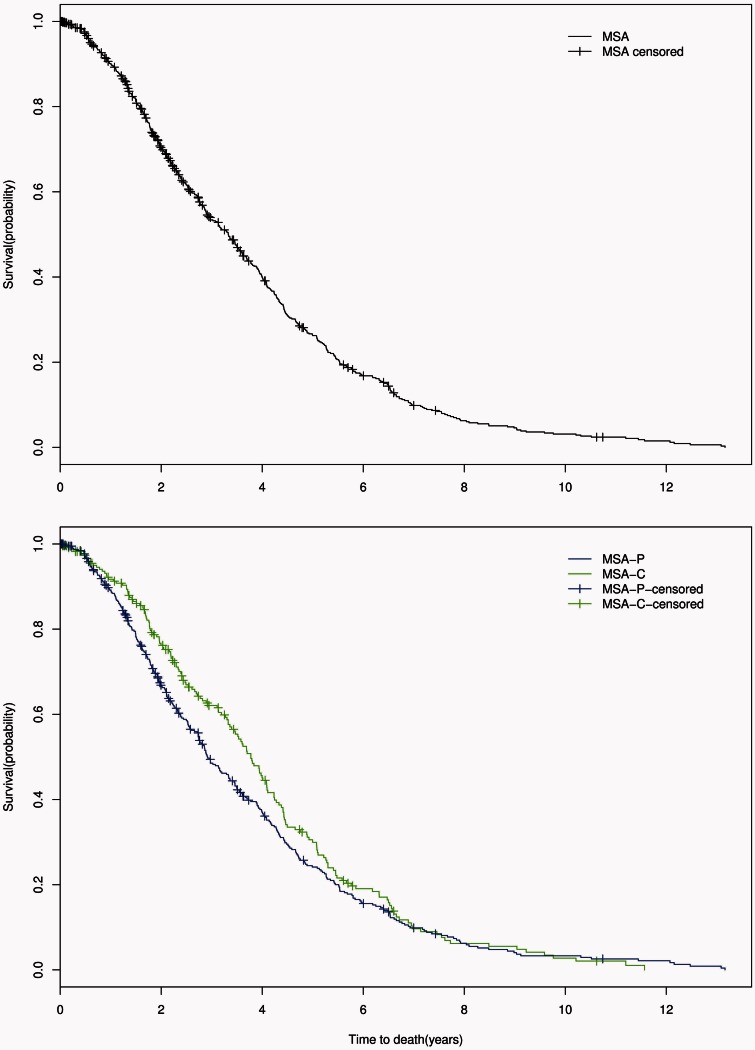
In all MSA patients, the median disease duration from symptom onset to death was 7.51 years [95% confidence interval (CI) 7.18–7.78; IQR 5.42–10.20] while median time from diagnosis to death was 3.33 years (95% CI 2.92–3.59; IQR1.78–5.11). Between MSA-P and MSA-C, there was no difference in overall survival (P = 0.232) with symptom onset to death survival curves shown in Fig. 1. From the time of diagnosis, there was also no significant difference in survival between MSA subtypes (P = 0.132); diagnosis to death survival curves are shown in Fig. 2. However, there was a trend for shorter median survival time from diagnosis to death in MSA-P (2.92 years, 95% CI 2.74–3.36) compared to MSA-C (3.78 years, 95% CI 3.36–4.09).
Figure 1.
Symptom onset to death. Censored Kaplan-Meier survival curves for probability of death from symptom onset in all patients with MSA (top); in patients with MSA-P (blue) compared with those with MSA-C (green) (bottom).
Figure 2.
Diagnosis to death. Censored Kaplan-Meier survival curves for probability of death from time of diagnosis in all patients with MSA (top); in patients with MSA-P (blue) compared with those with MSA-C (green) (bottom).
Survival predictors
Univariate analysis of the influence of clinical factors and findings on autonomic testing on survival from symptom onset to death and time of diagnosis to death are shown in Supplementary Table 1. Neither MSA subtype, classification as probable or possible MSA, or gender were significantly associated with survival. Factors associated with shortened survival included later age at onset, early autonomic symptoms, the presence of bladder symptoms, early urinary catheterization need, early falls, stridor, and severity of autonomic dysfunction. For every one point increase in CASS, the hazard ratio is 1.05 (P = 0.015). This is further illustrated by comparing the median survival times in those with a low CASS of 2 which is 7.8 years (IQR 6.2–8.9) versus a high score of 10, which is 4.3 years (IQR 3.6–5.9). Therefore, there is a 3.5 year difference in median survival between patients with mild autonomic dysfunction compared with severe autonomic failure shown on autonomic function testing at the time of diagnosis. Severity of autonomic dysfunction at the time of diagnosis was furthermore a predictor of shortened survival from the time of diagnosis. In comparison, the presence of falls within 3 years led to a shortened median survival of 2.1 years (median survival 6.7 years, IQR 6.0–6.9 versus 8.8 years, IQR 8.2–9.4). The median survival difference for the presence of bladder symptoms was 2.4 years (6.1 years, IQR 5.6–6.7 versus 8.5 years, IQR 7.8–9.0), urinary catheterization within 3 years was 2.4 years (5.3 years, IQR 4.8–6.1 versus 7.7 years, IQR 7.4–8.1) and early orthostatic intolerance was 1.0 years (6.8 years, IQR 6.3–7.5 versus 7.8 years, IQR 7.4–8.3).
Predictors of shortened overall survival based on multivariate analysis are shown in Table 3. Six variables were maintained in the final model as independent predictors of shortened survival. The strongest predictor was falls occurring within 3 years of symptom onset. Four of the other five independent predictors relate to autonomic symptoms or the degree of autonomic impairment. As age of onset differed between MSA subtype with older age of onset in MSA-P, overall survival between subtype was analysed adjusted for age of onset and showed no difference (P = 0.607). Surprisingly, stridor was not maintained as an independent predictor in the final model, possibly related to the fact that we found significant associations between stridor and several other predictors in that model (falls within 3 years, bladder symptoms, CASS, and age at onset).
Table 3.
Multivariate analysis of survival factors in MSA
| Variable | From symptom onset |
|||
|---|---|---|---|---|
| Hazard ratio | Lower 95% CI | Upper 95% CI | P-value | |
| Falls within 3 years | 2.33 | 1.92 | 2.83 | <0.0001 |
| Bladder symptoms | 1.96 | 1.61 | 2.38 | <0.0001 |
| Urinary catheterization within 3 years | 1.67 | 1.19 | 2.35 | 0.003 |
| Early orthostatic intolerancea | 1.29 | 1.06 | 1.58 | 0.011 |
| CASS total | 1.07 | 1.02 | 1.11 | 0.002 |
| Age at onset | 1.02 | 1.01 | 1.03 | 0.001 |
aWithin 1 year of onset.
Discussion
This study on survival and predictors of survival in MSA is to our knowledge the largest reported cohort of MSA patients. Also unique to this study is that all patients were seen and diagnosed at a single North American referral centre, and that all patients underwent comprehensive standardized autonomic testing. In this group of patients, we found a median survival of 7.51 years with no difference between MSA subtype or gender. However, older age of onset and both motor and autonomic clinical features predicted shorter survival. In particular, motor symptoms of falls within 3 years and autonomic manifestations of bladder symptoms, urinary catheterization within 3 years, and orthostatic intolerance developing within a year from onset were the strongest predictors of shorter survival. The severity of autonomic failure on autonomic reflex screen and thermoregulatory sweat test also portended significantly worse overall survival in MSA and predicted shorter survival from the time of diagnosis.
Our findings reinforce those of the North American Multiple System Atrophy Natural History Study showing no difference of survival between MSA subtypes of MSA-P and MSA-C (Low et al., 2015). This differs from the findings of the European Natural History study, which found worse survival in MSA-P (Gilman et al., 2008; Wenning et al., 2013). Patients included in our study were solely from the USA and Canada and the observed differences may reflect a true population difference. Another possibility is that a younger age of onset observed in MSA-C patients is what influences the longer survival in MSA-C reported in smaller studies (Schulz et al., 1994; Ben-Shlomo et al., 1997; Wenning et al., 2013) as we found that older age of onset predicted shorter survival. However, we found that MSA subtype is not a significant predictor of survival even when accounting for age of onset. We also observed a trend for shorter duration from diagnosis to death in MSA-P compared to MSA-C. This may be due to greater difficulty differentiating MSA-P from other forms of parkinsonism leading to a diagnosis later in the disease course. While we found no influence on gender for overall survival, females had a longer duration from diagnosis to death. This raises the question of gender differences in MSA; females may be diagnosed earlier than males or this could be an artefact of a large study population.
While the initial onset with autonomic symptoms was not associated with shortened overall survival, early autonomic symptoms in disease course, particularly bladder symptoms and severe urinary symptoms (requiring urinary catheterization), negatively affected survival. These findings are similar to other studies showing early autonomic involvement predicting worse prognosis (Tada et al., 2007; Wenning et al., 2013; Figueroa et al., 2014; Low et al., 2015) but also underscore previous findings that the initial presenting symptom is of less prognostic importance (Roncevic et al., 2014). Additionally, the findings from this cohort confirm the presence of stridor as an important prognostic feature when assessed in isolation (Silber and Levine, 2000). However, the presence of stridor as predictor of survival is not retained in the final multivariate model as it loses its predictive value in the presence of the other variables in the model. Recognition of stridor during evaluation prompting treatment may have also influenced the prognostic effect of stridor.
Considering the importance of autonomic dysfunction for the clinical phenotype of MSA, having results of objective autonomic testing upon diagnosis available for our entire patient cohort is a strength of this study. The finding of several variables derived from autonomic testing, including the finding that generalized autonomic failure is associated with shorter survival, underscores the importance of standardized autonomic testing not only for the diagnosis of MSA but also as an important prognostic indicator. The severity of autonomic failure as assessed by severity score, presence and degree of orthostatic hypotension, and degree of anhidrosis was highly predictive of overall survival and naturally, even more predictive of survival from the time of diagnosis.
The severity of autonomic dysfunction at the time of diagnosis seen in this study was similar to our previous autopsy-confirmed studies’ cases (Iodice et al., 2012; Figueroa et al., 2014). The large percentages of patients with orthostatic hypotension and supine hypertension support the clinical recommendation that patients should have blood pressure monitoring both supine and upright, particularly when on blood pressure augmenting medications. Thermoregulatory sweat test frequently showed widespread anhidrosis and the severity of anhidrosis was associated with survival. This further supports the diagnostic and prognostic utility of thermoregulatory sweat test in complementing other autonomic tests in MSA.
Weaknesses of this study include its retrospective nature; patients were seen by different providers over a long time period, which may account for a difference in recording of symptoms. For instance, the presence of dream enactment behaviour was lower than expected, which also may be accounted for by decreased awareness of sleep findings in the early part of the study (Palma et al., 2015). However, this drawback is limited due to the homogeneity of the diagnostic approach within a single neurological department, the availability of detailed electronic records for the entire timeframe of this study, and the use of patient-completed questionnaires, which allowed for standardization of some of the recorded symptoms (including falls and urinary symptoms). While all patients had to meet consensus criteria for probable or possible MSA, and we were sensitive to exclude those with competing diagnoses, there is a potential for misdiagnosis. As the vast majority of our patients met criteria for probable MSA, we suspect, however, a low misdiagnosis rate based on agreement between current consensus criteria and autopsy-confirmed cases (Iodice et al., 2012; Figueroa et al., 2014). While only 36 patients underwent post-mortem evaluation, MSA was confirmed in all of these cases.
Besides its single centre nature and the availability of standardized autonomic testing in all cases, additional strengths of this study over previously reported cohorts include the uniquely large size of this cohort and the large number of confirmed deaths. These factors support high confidence in the calculated median survival of 7.5 years for patients with MSA in North America. The identified prognostic predictors may prove useful in guiding an individualized response to patients’ questions about prognosis.
Funding
This publication was supported by NIH (P01NS44233, U54NS065736, K23NS075141, UL1RR24150), Mayo Funds, Cure MSA Foundation, and Mayo CTSA (UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- CASS
composite autonomic severity score
- MSA-C/P
multiple system atrophy-cerebellar/parkinsonism
References
- Baschieri F, Calandra-Buonaura G, Doria A, Mastrolilli F, Palareti A, Barletta G, et al. Cardiovascular autonomic testing performed with a new integrated instrumental approach is useful in differentiating MSA-P from PD at an early stage. Parkinsonism Relat Disord 2015; 21: 477–82. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Wenning GK, Tison F, Quinn NP. Survival of patients with pathologically proven multiple system atrophy: a meta-analysis. Neurology 1997; 48: 384–93. [DOI] [PubMed] [Google Scholar]
- Brooks DJ, Seppi K. Proposed neuroimaging criteria for the diagnosis of multiple system atrophy. Mov Disord 2009; 24: 949–64. [DOI] [PubMed] [Google Scholar]
- Calandra-Buonaura G, Guaraldi P, Sambati L, Lopane G, Cecere A, Barletta G, et al. Multiple system atrophy with prolonged survival: is late onset of dysautonomia the clue? Neurol Sci 2013; 34:1875–8. [DOI] [PubMed] [Google Scholar]
- Fanciulli A, Wenning GK. Multiple-system atrophy. N Engl J Med 2015; 372: 1375–6. [DOI] [PubMed] [Google Scholar]
- Figueroa JJ, Singer W, Parsaik A, Benarroch EE, Ahlskog JE, Fealey RD, et al. Multiple system atrophy: prognostic indicators of survival. Mov Disord 2014; 29: 1151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Low PA, May S, Tanner CM, Stern MB, Sandroni P, et al. Prospective 5-year natural history study of probable multiple system atrophy (MSA) in 175 North American subjects. Clin Auton Res 2010; 20: 327. [Google Scholar]
- Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus conference on the diagnosis of multiple system atrophy. Neurology 2008; 71: 670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iodice V, Lipp A, Ahlskog JE, Sandroni P, Fealey RD, Parisi JE, et al. Autopsy confirmed multiple system atrophy cases: Mayo experience and role of autonomic function tests. J Neurol Neurosurg Psychiatry 2012; 83: 453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Jeon BS, Lee JY, Yun JY. Survival of Korean patients with multiple system atrophy. Mov Disord 2011; 26: 909–12. [DOI] [PubMed] [Google Scholar]
- Klockgether T, Ludtke R, Kramer B, Abele M, Burk K, Schols L, et al. The natural history of degenerative ataxia: a retrospective study in 466 patients. Brain 1998; 121: 589–600. [DOI] [PubMed] [Google Scholar]
- Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc 1993; 68: 748–52. [DOI] [PubMed] [Google Scholar]
- Low PA, Reich SG, Jankovic J, Shults CW, Stern MB, Novak P, et al. Natural history of multiple system atrophy in North America: a prospective cohort study. Lancet Neurol 2015; 14: 710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain 2008; 131: 1362–72. [DOI] [PubMed] [Google Scholar]
- Palma JA, Fernandez-Cordon C, Coon EA, Low PA, Miglis MG, Jaradeh S, et al. Prevalence of REM sleep behavior disorder in multiple system atrophy: a multicenter study and meta-analysis. Clin Auton Res 2015; 25: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh SM, Diedrich A, Biaggioni I, Robertson D. The nature of the autonomic dysfunction in multiple system atrophy. J Neurol Sci 2002; 200: 1–10. [DOI] [PubMed] [Google Scholar]
- Petrovic IN, Ling H, Asi Y, Ahmed Z, Kukkle PL, Hazrati LN, et al. Multiple system atrophy-parkinsonism with slow progression and prolonged survival: a diagnostic catch. Mov Disord 2012; 27: 1186–90. [DOI] [PubMed] [Google Scholar]
- Roncevic D, Palma JA, Martinez J, Goulding N, Norcliffe-Kaufmann L, Kaufmann H. Cerebellar and parkinsonian phenotypes in multiple system atrophy: similarities, differences and survival. J Neural Transm 2014; 121: 507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Matsuoka Y, Takahashi A, Ohno Y. Survival of patients with multiple system atrophy. Intern Med 1994; 33: 321–5. [DOI] [PubMed] [Google Scholar]
- Schrag A, Selai C, Mathias C, Low P, Hobart J, Brady N, et al. Measuring health-related quality of life in MSA: the MSA-QoL. Mov Disord 2007; 22: 2332–8. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Klockgether T, Petersen D, Jauch M, Muller-Schauenburg W, Spieker S, et al. Multiple system atrophy: natural history, MRI morphology, and dopamine receptor imaging with 123IBZM-SPECT. J Neurol Neurosurg Psychiatry 1994; 57: 1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber MH, Levine S. Stridor and death in multiple system atrophy. Mov Disord 2000; 15: 699–704. [DOI] [PubMed] [Google Scholar]
- Tada M, Onodera O, Tada M, Ozawa T, Piao YS, Kakita A, et al. Early development of autonomic dysfunction may predict poor prognosis in patients with multiple system atrophy. Arch Neurol 2007; 64: 256–60. [DOI] [PubMed] [Google Scholar]
- Testa D, Filippini G, Farinotti M, Palazzini E, Caraceni T. Survival in multiple system atrophy: a study of prognostic factors in 59 cases. J Neurol 1996; 243: 401–4. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E, et al. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain 2002; 125: 1070–83. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Ben Shlomo Y, Magalhaes M, Daniel SE, Quinn NP. Clinical features and natural history of multiple system atrophy. An analysis of 100 cases. Brain 1994; 117: 835–45. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S, et al. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol 2013; 12: 264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.