Abstract
Anti-glomerular basement membrane (GBM) disease is characterized by circulating anti-GBM antibodies and deposition of these antibodies in the renal GBM. Renal involvement in anti-GBM is more severe when compared with other types of immune-mediated glomerulonephritis, and the majority of patients manifest progressive renal failure, leading to end-stage renal disease. In a limited number of cases, anti-GBM disease has been shown to be accompanied with other immune-mediated glomerulonephritis. The present study reported the case of a 50-year-old female patient presenting with rapidly progressive glomerulonephritis, who was diagnosed with anti-GBM disease with IgA nephropathy. The patient achieved a relatively good therapeutic outcome with administration of corticosteroids plus mycophenolate mofetil (MMF), which may prove to be a novel treatment option for this rare disease; however, the exact underlying mechanism requires further in-depth investigation.
Keywords: anti-glomerular basement membrane disease, immunoglobulin A nephropathy, mycophenolate mofetil
Introduction
Anti-glomerular basement membrane (anti-GBM) disease is a classic organ-specific autoimmune disease, mainly involving the lungs and kidneys. It is characterized by circulating anti-GBM antibodies and deposition of these antibodies in the renal GBM (1,2). The incidence of anti-GBM disease is estimated to be 1 case per million per year, but it accounts for ~20% of all cases of rapidly progressive or crescentic glomerulonephritis, and the age distribution is bimodal, 20–30 years old and 60–70 years old (3,4). Renal pathological analysis in anti-GBM patients has previously demonstrated that immunoglobulin (Ig) G with or without C3 was linearly deposited in the basement membrane, while light microscopy mainly identified crescentic nephritis (5). Renal involvement in anti-GBM is more severe when compared with other types of immune-mediated glomerulonephritis. The majority of patients present progressive renal failure, resulting in end-stage renal disease, and require long-term treatment to improve the prognosis (3). According to the Kidney Disease - Improving Global Outcomes Clinical Practice Guideline for glomerulonephritis, corticosteroids and cyclophosphamide in combination with plasmapheresis are recommended as the initial immunosuppressive treatment for anti-GBM disease (6). The association between anti-GBM disease and antineutrophil cytoplasmic antibody-associated vasculitis has been well established in an increased number of studies (7–9). However, only a limited number of studies have reported the association between anti-GBM disease and IgA nephropathy or other immune complex glomerulonephritis, such as Schönlein-Henoch purpura and membranous glomerulonephritis (10–13).
The present study reports the case of a patient who was diagnosed with anti-GBM disease with IgA nephropathy, and was successfully treated with corticosteroids in combination with mycophenolate mofetil (MMF).
Case report
A 50-year-old female was admitted to the Kidney Institute of PLA (Changzheng Hospital, Shanghai, China) in June 2014 with complaints of gross hematuria and rapidly decreasing kidney function for the past 2 weeks. The patient did not experience any fever, skin rash and hemoptysis. Gross hematuria was presented 2 weeks prior to admission, and laboratory tests performed in a local hospital reported hematuria with a red blood cell (RBC) count of 336 cells/µl (the normal level is <25 cells/µl), as well as decreased kidney function with a serum creatinine (Scr) level of 157 µmol/l (the normal level is 61–116 μmol/l) and blood urea nitrogen level of 11.29 mmol/l (the normal level is 2.9–7.2 mmol/l). Ultrasonography reveal that the kidneys were normal in size and shape, with the exception of several small kidney stones. The diagnosis of renal lithiasis was established at the local hospital, without administration of any specific treatment. However, the patient was advised to drink more water and was prescribed a traditional Chinese medicine called Loosestrife (Guangxi Wantong Pharmaceutical Co., Ltd., Nanning, China). However, gross hematuria did not show any improvement 10 days later, and further laboratory tests suggested a rapidly decreasing kidney function with an Scr level of 220 µmol/l.
Subsequently, the patient was transferred to Changzheng Hospital for further diagnosis and treatment. Physical examination was not remarkable. Laboratory examination upon admission included counting the number of RBCs per HP by observing the urine under a DM2500 HP microscope (Leica, Heidelberg, Germany). The results showed heavy hematuria [10–15 RBCs per high-power field (HP); the normal level is <3 RBCs per HP], with 90% dysmorphic RBCs, mild urinary protein excretion (0.41 g/24 h; the normal level is <0.15 g/24 h), and decreased kidney function with an Scr level of 232 µmol/l. In addition, the hemoglobin (Hb) level was 99 g/l (the normal level is 110–150 g/l) and the erythrocyte sedimentation rate (ESR) was 141 mm/h (the normal level is 0–20 mm/h), while a positive concentration serum monoclonal anti-GBM antibody (isolated from bovine kidney as an antigen) was reported (258.3 EU/ml; cat no. EA 1251–9601 G, EUROIMMUN Medical Laboratory Diagnostics Co., Ltd, Luebeck, Germany). Serologic tests for antinuclear, anti-double-strand DNA and anti-neutrophil cytoplasmic antibodies were all negative. In addition, the serum levels of IgA, IgG, IgM, complement C3 and C4, and total hemolytic complement (CH50) were within the normal ranges. Chest X-ray radiography (Ysio 50019, Siemens, Munich, Germany) was also normal, while ultrasonography (ACUSON S2000 Ultrasound system, Siemens, Chicago, IL, USA) showed normal shape of kidneys without kidney stones, and the sizes of the right and left kidneys were 102×51 and 101×51 mm (the normal level is 90–110×40–60 mm), respectively. This can be due to the previous diagnosis being wrong or by the removal of the kidney stones with the previous treatment. Previous medical history of the patient revealed that the Scr was 57 µmol/l at 6 months prior to admission, while the patient had a 20-year history of recurrent tonsillitis. Based on the aforementioned findings, rapidly progressive glomerulonephritis was diagnosed and renal biopsy was immediately performed.
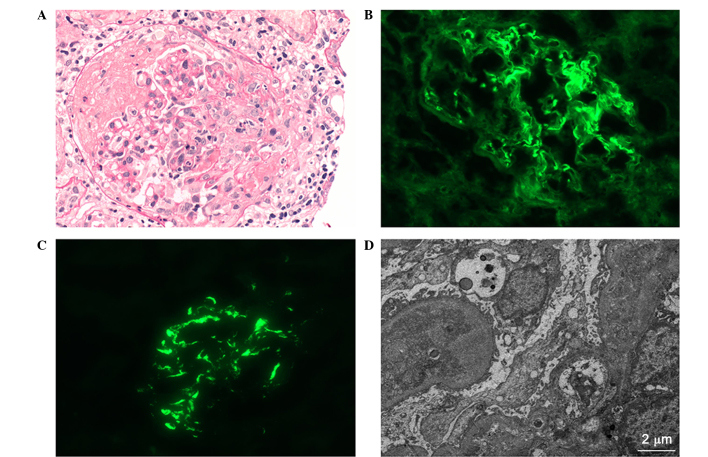
Light microscopy (DM2500, Leica) identified 18 glomeruli with 12 cellular crescents and 4 cellulofibrous crescents, and the glomeruli presented capillary occlusion with segmental fibrinoid necrosis (Fig. 1A). Numerous necrotic tubular epithelial cells and diffused infiltration of inflammatory cells indicated severe injury to the tubulointerstitium. The small arterial walls were thickened without significant evidence of necrosis or hyaline degeneration. Immunofluorescent examination (DM2500, Leica) revealed that strong IgG (3+) linear deposition in the capillary loop (Fig. 1B), along with mesangial staining for IgA (4+; Fig. 1C), IgM (1+) and C3 (2+). The staining intensity was scored as follows: 0+, negative; 1+, mild; 2+, moderate or 3+, strong (8). Furthermore, electron microscopy (Hitachi 7700, Tokyo, Japan) demonstrated electron-dense mass deposition in the mesangium (Fig. 1D). Based on the aforementioned findings, the diagnosis of anti-GBM disease with IgA nephropathy was confirmed.
Figure 1.
Pathology of renal biopsy. (A) Light microscopy showing hypercellularity with cellularofibrous crescent (PAS staining). (B) Immunofluorescence showing linear deposition of IgG along the GBM. (C) Immunofluorescence showing lumpy deposition of IgA in the mesangium. (D) Electron microscopy revealing electrondense deposition in the mesangium. Ig, immunoglobulin; GBM, glomerular basement membrane.
The patient was immediately administered 500 mg/day intravenous methylprednisolone (Pfizer Ltd., Kent, UK) for 3 days, which was then changed to 60 mg/day oral prednisone (Shanghai Xinyi Pharmaceutical Co., Ltd, Shanghai, China); however, the dosage was quickly decreased to 20 mg/day two weeks later due to the side effect of hyperglycemia. Treatment with cyclophosphamide and plasmapheresis were then recommended, however this treatment was not accepted by the patient due to the possibility of side effects. Therefore, MMF (0.5 g, twice a day; Roche Pharmaceutical Co., Ltd, Shanghai, China) in combination with prednisone (20 mg/day) were administered to the patient and the dose of prednisone was decreased by 5 mg every month. Four months later the prednisone treatment stopped. One month later gross hematuria was gradually relieved following MMF treatment. Further laboratory examinations after 3 months of MMF treatment showed the following results: Hematuria with 5–6 RBCs/HP; Scr level, 102 µmol/l; Hb, 112 g/l; ESR, 22 mm/h; positivity for anti-GBM antibody (176.0 EU/ml). After 6 months, the Scr level was found to be 100 µmol/l and the ESR was 23 mm/h, showing no significant difference as compared with these levels 3 months before, whereas the Hb level was increased to 129 g/l and the test for anti-GBM antibody was negative. The total MMF treatment was 15 months in total. Patient informed consent was obtained prior to the study.
In the last observation in February 2016 the MMF treatment had ceased and the laboratory examinations demonstrated the following results: Urine test showed 4 5 RBCs/HP; Scr level, 74 μmol/l; Hb, 132 g/l; ESR, 4 mm/h and the test for anti GBM antibody was negative.
Discussion
In the present study, a rare case of anti-GBM disease with IgA nephropathy was reported. Previous animal studies have demonstrated that complement activation through the classical complement pathway is one of the major mechanisms underlying glomerular injury in anti-GBM disease (14,15). According to this proposed theory, complement C1q may serve an important role in the underlying mechanism of anti-GBM disease. However, a number of studies have reported that complement C1q deposition was seldom present along the GBM in the renal tissue of patients, and circulating and urinary levels of C1q were not significantly correlated with the severity of kidney injury (8,16). Hu et al (9) further found that, even in patients with C1q deposition, no association of C1q with the disease activity and severity was identified, suggesting that the classical complement pathway may not serve a pathogenic role in the development of kidney injury in human anti-GBM disease. In the present case, immunofluorescence examination did not identify any C1q deposition, which is consistent with previous findings (9). However, the diagnosis of anti-GBM disease remains appropriate in the present case.
As the progression of anti-GBM disease can be rapid and the outcome is greatly associated with the severity at presentation, treatment must be initiated immediately. It is widely recognized that corticosteroids and cyclophosphamide in combination with plasmapheresis is the preferred standard treatment administered as the initial immunosuppressive therapy for anti-GBM disease (6). Notably, in the present study, the patient declined the standard treatment for anti-GBM disease, however a relatively improved therapeutic outcome with less side-effects was achieved compared with treatment with corticosteroids in combination with MMF. To date, there is little clinical evidence to recommend MMF for the anti-GBM disease therapy, although previous animal experiments in a rat model of anti-GBM disease have suggested the preventive effect of MMF against glomerular crescent formation (17). The case reported in the present study may provide useful evidence concerning the effect of MMF for the clinical treatment of anti-GBM disease.
As mentioned earlier, strong IgA deposition in the renal tissue may have a profound impact on the pathogenesis of this rare disease (11,18). The findings from randomized controlled trials (RCTs) investigating MMF administration in IgA nephropathy are conflicting, since favorable outcomes of MMF have only been demonstrated in studies conducted in China (19–22). This is due to the fact that Asian IgG nephropathy patients may be more sensitive to MMF treatment, and the variation of ethnicity may be partly the reason for this observation. Based on the updated treatment options, the use of MMF is recommended for selected patients in whom steroid therapy has failed or who are intolerant to steroid therapy in IgA nephropathy (23). Nevertheless, MMF may prove to be a novel treatment for this rare disease, although the exact underlying mechanism requires further in-depth investigation.
In conclusion, the present study reported a case with anti-GBM disease and IgA nephropathy, in which the patient responded well to corticosteroids plus MMF treatment. The underlying mechanism renders further in-depth examination.
References
- 1.Lahmer T, Heemann U. Anti-glomerular basement membrane antibody disease: a rare autoimmune disorder affecting the kidney and the lung. Autoimmun Rev. 2012;12:169–173. doi: 10.1016/j.autrev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR, Wieslander J, Kashtan C, Borza DB, Neilson EG, et al. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med. 2010;363:343–354. doi: 10.1056/NEJMoa0910500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang W, McDonald SP, Hawley CM, Badve SV, Boudville NC, Brown FG, Clayton PA, Campbell SB, de Zoysa JR, Johnson DW. Anti-glomerular basement membrane antibody disease is an uncommon cause of end-stage renal disease. Kidney Int. 2013;83:503–510. doi: 10.1038/ki.2012.375. [DOI] [PubMed] [Google Scholar]
- 4.Cui Z, Zhao MH. Advances in human antiglomerular basement membrane disease. Nat Rev Nephrol. 2011;7:697–705. doi: 10.1038/nrneph.2011.89. [DOI] [PubMed] [Google Scholar]
- 5.Jennette JC, Nickeleit V. Anti-glomerular basement membrane glomerulonephritis and Goodpasture's syndrome. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall's Pathology of the Kidney. 6th. Lippincott Williams and Wilkins; Philadelphia, PA: 2007. pp. 615–641. [Google Scholar]
- 6.Kidney Disease - Improving Global Outcomes Glomerulonephritis Work Group: KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Inter Suppl 2. 2012:139–274. [Google Scholar]
- 7.Hellmark T, Niles JL, Collins AB, McCluskey RT, Brunmark C. Comparison of anti-GBM antibodies in sera with or without ANCA. J Am Soc Nephrol. 1997;8:376–385. doi: 10.1681/ASN.V83376. [DOI] [PubMed] [Google Scholar]
- 8.Fischer EG, Lager DJ. Anti-glomerular basement membrane glomerulonephritis: A morphologic study of 80 cases. Am J Clin Pathol. 2006;125:445–450. doi: 10.1309/NPTP4UKV7JU3ELMQ. [DOI] [PubMed] [Google Scholar]
- 9.Hu SY, Jia XY, Yang XW, Yu F, Cui Z, Zhao MH. Glomerular C1q deposition and serum anti-C1q antibodies in anti-glomerular basement membrane disease. BMC Immunol. 2013;14:42. doi: 10.1186/1471-2172-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao B, Li M, Xia W, Wen Y, Qu Z. Rapidly progressive glomerulonephritis due to anti-glomerular basement membrane disease accompanied by IgA nephropathy: A case report. Clin Nephrol. 2014;81:138–141. doi: 10.5414/CN107213. [DOI] [PubMed] [Google Scholar]
- 11.Wang A, Wang Y, Wang G, Zhou Z, Xun Z, Tan X. Mesangial IgA deposits indicate pathogenesis of anti-glomerular basement membrane disease. Mol Med Rep. 2012;5:1212–1214. doi: 10.3892/mmr.2012.809. [DOI] [PubMed] [Google Scholar]
- 12.Carreras L, Poveda R, Bas J, Mestre M, Rama I, Carrera M. Goodpasture syndrome during the course of a Schönlein-Henoch purpura. Am J Kidney Dis. 2002;39:E21. doi: 10.1053/ajkd.2002.32799. [DOI] [PubMed] [Google Scholar]
- 13.Kielstein JT, Helmchen U, Netzer KO, Weber M, Haller H, Floege J. Conversion of Goodpasture's syndrome into membranous glomerulonephritis. Nephrol Dial Transplant. 2001;16:2082–2085. doi: 10.1093/ndt/16.10.2082. [DOI] [PubMed] [Google Scholar]
- 14.Otten MA, Groeneveld TW, Flierman R, Rastaldi MP, Trouw LA, Faber-Krol MC, Visser A, Essers MC, Claassens J, Verbeek JS, et al. Both complement and IgG fc receptors are required for development of attenuated antiglomerular basement membrane nephritis in mice. J Immunol. 2009;183:3980–3988. doi: 10.4049/jimmunol.0901301. [DOI] [PubMed] [Google Scholar]
- 15.Sheerin NS, Springall T, Carroll MC, Hartley B, Sacks SH. Protection against anti-glomerular basement membrane (GBM)-mediated nephritis in C3-and C4-deficient mice. Clin Exp Immunol. 1997;110:403–409. doi: 10.1046/j.1365-2249.1997.4261438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma R, Cui Z, Liao YH, Zhao MH. Complement activation contributes to the injury and outcome of kidney in human anti-glomerular basement membrane disease. J Clin Immunol. 2013;33:172–178. doi: 10.1007/s10875-012-9772-2. [DOI] [PubMed] [Google Scholar]
- 17.Takeda S, Takahashi M, Sado Y, Takeuchi K, Hakamata Y, Shimizu H, Kaneko T, Yamamoto H, Ito C, Ookawara S, et al. Prevention of glomerular crescent formation in glomerulonephritis by mycophenolate mofetil in rats. Nephrol Dial Transplant. 2004;19:2228–2236. doi: 10.1093/ndt/gfh302. [DOI] [PubMed] [Google Scholar]
- 18.Trpkov K, Abdulkareem F, Jim K, Solez K. Recurrence of anti-GBM antibody disease twelve years after transplantation associated with de novo IgA nephropathy. Clin Nephrol. 1998;49:124–128. [PubMed] [Google Scholar]
- 19.Maes BD, Oyen R, Claes K, Evenepoel P, Kuypers D, Vanwalleghem J, Van Damme B, Vanrenterghem YF. Mycophenolate mofetil in IgA nephropathy: Results of a 3-year prospective placebo-controlled randomized study. Kidney Int. 2004;65:1842–1849. doi: 10.1111/j.1523-1755.2004.00588.x. [DOI] [PubMed] [Google Scholar]
- 20.Frisch G, Lin J, Rosenstock J, Markowitz G, D'Agati V, Radhakrishnan J, Preddie D, Crew J, Valeri A, Appel G. Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: A double-blind randomized controlled trial. Nephrol Dial Transplant. 2005;20:2139–2145. doi: 10.1093/ndt/gfh974. [DOI] [PubMed] [Google Scholar]
- 21.Tang S, Leung JC, Chan LY, Lui YH, Tang CS, Kan CH, Ho YW, Lai KN. Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int. 2005;68:802–812. doi: 10.1111/j.1523-1755.2005.00460.x. [DOI] [PubMed] [Google Scholar]
- 22.Tang SC, Tang AW, Wong SS, Leung JC, Ho YW, Lai KN. Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int. 2010;77:543–549. doi: 10.1038/ki.2009.499. [DOI] [PubMed] [Google Scholar]
- 23.Hogan J, Mohan P, Appel GB. Diagnostic tests and treatment options in glomerular disease: 2014 update. Am J Kidney Dis. 2014;63:656–666. doi: 10.1053/j.ajkd.2013.09.019. [DOI] [PubMed] [Google Scholar]