Abstract
Environmental pollution is a current area of focus worldwide, particularly heavy metal pollution. Feasible prevention or therapeutic strategies are required. Exploration of the correlation between ω-3 polyunsaturated fatty acids (ω-3 PUFAs) and intestinal epithelial cells injured by heavy metals may be of significance for intestinal health. In the present study, the effects of ω-3 PUFAs on the rat intestinal crypt cell line (IEC-6) injured by heavy metals and its mechanisms were determined according to the evaluation of cell viability and expression levels of reactive oxygen species (ROS), epidermal growth factor (EGF) and interleukin-6 (IL-6). The results demonstrated that ω-3 PUFAs can improve the viability of IEC-6 cells injured by heavy metals and the expression level of ROS was correlated with oxidative damage; the increased expression level of inflammatory factors is associated with cell apoptosis. In the present study, ω-3 PUFAs significantly decreased the expression levels of ROS, EGF and IL-6. This indicates that the protective action of ω-3 PUFAs was associated with a decrease of oxidative damage and pro-inflammatory cytokine expression against the damage of heavy metals.
Keywords: ω-3 PUFAs, heavy metals, intestinal crypt cell line, oxidative damage
Introduction
Environmental pollution and food safety problems are becoming increasingly serious and are directly threatening human health. In particular, the effect and mechanism of heavy metal pollution on health has received increasing attention from investigators worldwide. Heavy metals have toxic effects on the whole body system, particularly on the nerve, blood, digestive tract, heart, kidney and immune systems; they enter the body through ingestion (1–3). Intestinal epithelial cells are a major medium between the internal and external environment; and are also the frontline of the defensive system to resist the invasion of exotic pathogens.
Previous studies have shown that ω-3 polyunsaturated fatty acids (ω-3 PUFAs), such as eicosapentaenoic acid (EPA; C20:5, ω3) and docosahexaenoic acid (DHA; C22:6, ω3), contributed to preventing or reducing the incidence of peptic ulcer disease and obesity (4–6), and improving the prognosis of several chronic inflammatory diseases, including atherosclerosis, systemic lupus erythematosus, psoriasis, inflammatory bowel disease and rheumatoid arthritis (7–10). The anti-inflammatory characteristics of ω-3 PUFAs, including decreasing cyclooxygenase-2 expression and cytokines, are consistent in numerous studies (11–13) and evidence the hypothesis that inhibition of inflammatory responses may be a viable protective strategy against heavy metal damage.
In order to understand the effects of ω-3 PUFAs on intestinal crypt (IEC-6) cells injured by heavy metals, the present study investigated the effect of EPA and DHA on the viability of heavy metal-injured IEC-6 cells and the expression levels of reactive oxygen species (ROS), epidermal growth factor (EGF) and interleukin-6 (IL-6).
Materials and methods
Materials
High-glucose Dulbecco's modified Eagle's medium (DMEM) and trypsin were obtained from Gino Biomedical Technology Co., Ltd. (Hangzhou, China). Fetal bovine serum was purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Rat intestinal crypt cells (IEC-6) were from the American Type Culture Collection (Manassas, VA, USA). EPA, DHA and dimethylsulphoxide (DMSO) were from Sigma-Aldrich (St. Louis, MO, USA), and all other chemicals were of analytical grade.
Cell culture
IEC-6 cells were cultured in high-glucose DMEM supplemented with 10% heat-inactivated fetal bovine serum (at pH 7.2), and placed in a 5% CO2-humidified incubator at 37°C. The culture medium was replaced every 48 h. CuSO4, CrCl3 and Pb(CH3COO)2·3H2O were dissolved in high-purity water at a concentration of 100 mmol/l as a stock solution, and was filtered prior to use. Stock solutions (20 mmol/l) of EPA and DHA were prepared in anhydrous ethanol, respectively, and diluted with sterile water prior to use.
MTT assay
The IEC-6 cells in the logarithmic growth period were seeded in 96-well plates at a density of 104 cells/well. After 4-h culture, the cells were treated with 10 µl of different concentrations of DHA, EPA and metal ions (Cu2+, Cd3+ and Pb2+) and maintained for 48 h. Following this, 20 µl of MTT solution (5 mg/ml) was supplemented at the end of the incubation period. Cells were incubated for another 3 h at 37°C before being removed from the cultured medium. Subsequently, 150 µl of DMSO was added to each well, and the formazan crystals were dissolved on a horizontal oscillator. The optical density was detected at the 570 nm wavelength by an ELISA microplate reader and cell viability was calculated as follows.
By contrast, the effect of preincubation with 10 µl of different concentrations of metal ions for 1 h followed by further exposure to various doses of DHA and EPA was investigated.
ROS, EGF and IL-6 measurement
IEC-6 cells were seeded in 96-well plates at a density of 2×105 cells/well. Subsequently, heavy metal-exposed IEC-6 cells were treated with ω-3 PUFAs. Subsequently, IEC-6 cells and culture medium were collected for the measurement of ROS, EGF and IL-6 using the LXA4 ELISA kit, according to the manufacturer's protocol (Shanghai Lianshuo Biological Technology Co., Ltd., Shanghai, China). The results were corrected by the protein level based on the reference manual of the bicinchoninic acid protein assay kit (Shanghai Beyotime Biotechnology, Shanghai, China).
Statistical analysis
All the data are expressed as mean ± standard deviation, and was analyzed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). Significant differences of analyses between varying groups were analyzed by analysis of variance.
Results
Effect of heavy metal (Cu2+, Cd3+ and Pb2+) and ω-3 PUFA treatments on cell viability
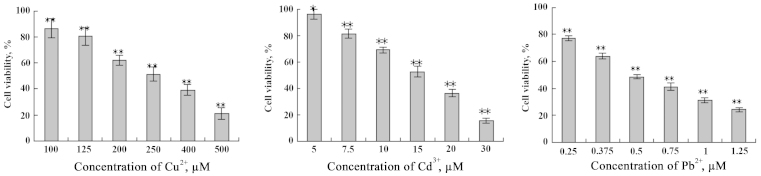
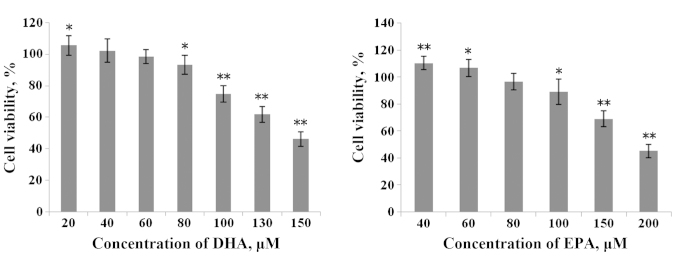
In the present study, to evaluate the damage effect of heavy metals (Cu2+, Cd3+ and Pb2+) on IEC-6 cells, cell viability was detected by the MTT assay. Supplementation of Cu2+ (100–500 µM), Cd3+ (5–30 µM) and Pb2+ (0.25–1.5 mM) to the IEC-6 cells resulted in a significant dose-dependent decrease in cell viability when compared with the control group (Fig. 1). According to the demonstrated results, concentrations of 500, 250 and 125 µM of Cu2+ with 30 and 15 µM of Cd3+, and 7.5 µM of Cd3+ with 1, 0.5 and 0.25 mM of Pb2+ were chosen for the subsequent experiments. Similarly, IEC-6 cells were incubated with DHA, ranging from 20 to 150 µM and EPA from 40 to 200 µM for 48 h, respectively. Simultaneously with the increase of concentration, cell viability decreased gradually (Fig. 2). According to these results, 60 µM DHA and 80 µM EPA were chosen for the subsequent experiments, as they exhibited no significant effect on IEC-6 cell viability.
Figure 1.
Effect of heavy metal ions (Cu2+, Cd3+ and Pb2+) at different concentrations on the viability of IEC-6 cells. *P<0.05; **P<0.01.
Figure 2.
Effect of ω-3 polyunsaturated fatty acids (DHA and EPA) at different concentrations on the viability of IEC-6 cells. *P<0.05; **P<0.01. DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
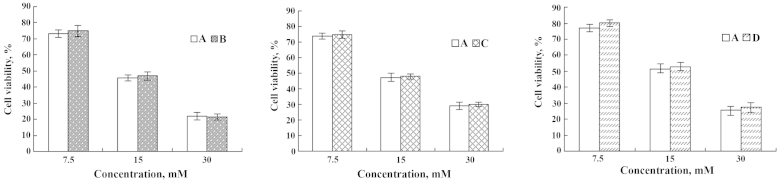
Effects of DHA and EPA on viability of IEC-6 cells injured by Cu2+
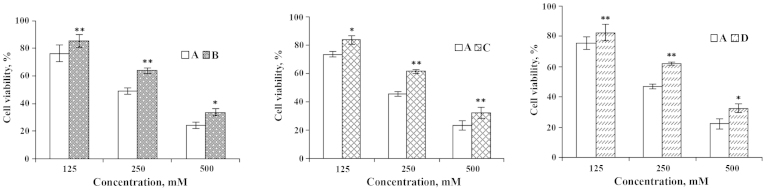
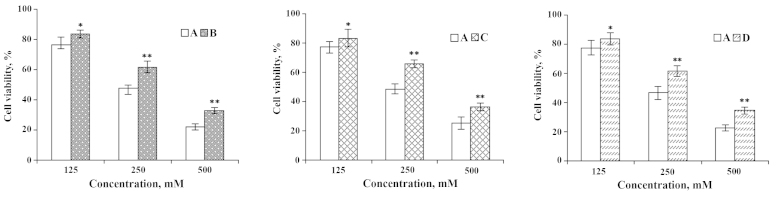
In order to study the effect of ω-3 PUFAs on IEC-6 cells injured by heavy metals, the IEC-6 cell culture was supplemented with the two ω-3 PUFAs and a metal ion. Following pre-incubation of cells with 250 µM of Cu2+ for 1 h followed by further exposure to various doses of DHA and EPA, cell viability increased 14.78 and 14.29%, respectively (Figs. 3 and 4). By contrast, pre-incubation of cells with 10 µl DHA and EPA for 1 h prior to an addition of 250 µM Cu2+, the cell viability was enhanced 15.83 and 16.94%, respectively (Figs. 3 and 4). Furthermore, while DHA or EPA with 250 µM of Cu2+ were treated simultaneously, IEC-6 viable cells were increased by 15.05 and 14.74% (Figs. 3 and 4). These results enable us to conclude that DHA and EPA could significantly improve the cell viability of IEC-6 cells despite being inflicted by Cu2+.
Figure 3.
Effects of DHA on IEC-6 cells injured by Cu2+. (A) Cu2+-injured; (B) Cu2+-injured and subsequently repaired by DHA; (C) DHA treatment followed by Cu2+-injury; and (D) simultaneous addition of Cu2+ and DHA. *P<0.05; **P<0.01. DHA, docosahexaenoic acid.
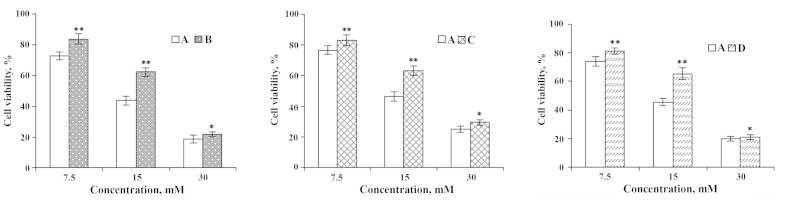
Figure 4.
Effects of EPA on IEC-6 cells injured by Cu2+. (A) Cu2+-injured; (B) Cu2+ injured and subsequently repaired by EPA; (C) EPA treatment followed by Cu2+-injury; and (D) simultaneous addition of Cu2+ and EPA. *P<0.05; **P<0.01. EPA, eicosapentaenoic acid.
Effects of DHA and EPA on the viability of IEC-6 cells injured by Cd3+
The protective effect on the IEC-6 cells following DHA, EPA and Cd3+ exposure was also investigated. DHA was shown to have no significant effect on the 15 µM Cd3+-injured IEC-6 cells (Fig. 5). By contrast, EPA had a significant effect on the cell viability, with increases of 18.59, 16.88 and 19.71%, respectively (Fig. 6). Therefore EPA, but not DHA, has a protective effect on the IEC-6 cells injured by Cd3+.
Figure 5.
Effects of DHA on IEC-6 cells injured by Cd3+. (A) Cd3+-injured; (B) Cd3+ injured and subsequently repaired by DHA; (C) DHA treatment followed by Cd3+-injury; and (D) simultaneous addition of Cd3+ and DHA. DHA, docosahexaenoic acid.
Figure 6.
Effects of EPA on IEC-6 cells injured by Cd3+. (A) Cd3+-injured; (B) Cd3+ injured and subsequently repaired by EPA; (C) EPA treatment followed by Cd3+-injury; and (D) simultaneous addition of Cd3+ and EPA. *P<0.05; **P<0.01. EPA, eicosapentaenoic acid.
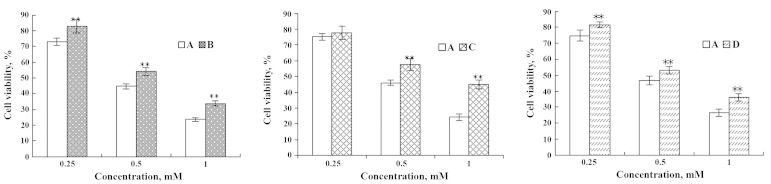
Effects of DHA and EPA on viability of IEC-6 cells injured by Pb2+
Cell viabilities of 1 mM Pb2+-inflicted IEC-6 cells increased to 10.16 and 7.65% following treatment with DHA and EPA (Figs. 7 and 8). Additionally, the cell viabilities were enhanced by 20.73 and 9.76%, respectively, as cells were pre-incubated with 10 µl DHA and EPA for 1 h prior to supplementation with 1 mM of Pb2+ (Figs. 7 and 8). When the cells were simultaneously treated with DHA or EPA and 1 mM of Pb2+, the cell viabilities were observed to increase by 9.83 and 7.08%, respectively (Figs. 7 and 8).
Figure 7.
Effects of DHA on IEC-6 cells injured by Pb2+. (A) Pb2+-injured; (B) Pb2+ injured and subsequently repaired by DHA; (C) DHA treatment followed by Pb2+-injury; and (D) simultaneous addition of Pb2+ and DHA. **P<0.01. DHA, docosahexaenoic acid.
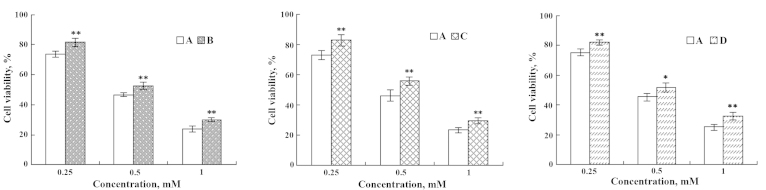
Figure 8.
Effects of EPA on IEC-6 cells injured by Pb2+. (A) Pb2+-injured; (B) Pb2+ injured and subsequently repaired by EPA; (C) EPA treatment followed by Pb2+-injury; and (D) simultaneous addition of Pb2+ and EPA. **P<0.01. EPA, eicosapentaenoic acid.
Effects of DHA and EPA on the ROS, EGF and IL-6 levels of IEC-6 cells injured by Cu2+
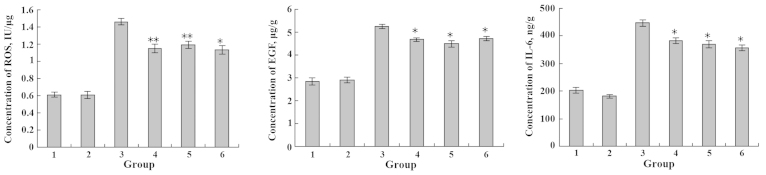
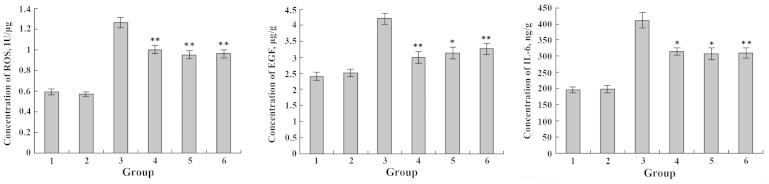
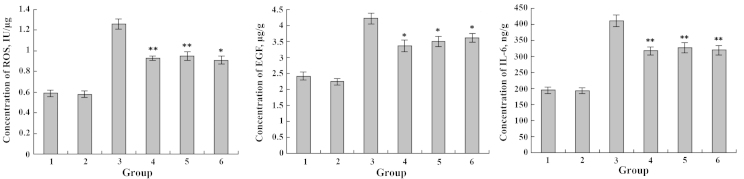
The IEC-6 cell lines were exposed to various concentrations of DHA or EPA and Cu2+. After 48 h, the levels of ROS, EGF and IL-6 secreted by IEC-6 cells were detected. The amounts of ROS, EGF and IL-6 generated by the IEC-6 cells incubated with Cu2+ were significantly higher in comparison with the untreated control (**P<0.01). By contrast, incubation of the IEC-6 cells with DHA and EPA resulted in a substantial decrease of the IL-6 level when compared with the control group (P>0.05) (Figs. 9 and 10). Regardless of the additional sequence of DHA or EPA and Cu2+, DHA and EPA significantly reduced the expression levels of ROS, EGF and IL-6 in the IEC-6 cells (**P<0.01; *P<0.05) (Figs. 9 and 10). Therefore, DHA and EPA had a protective effect on the IEC-6 cells damaged by Cu2+ by reducing the expression levels of ROS, EGF and IL-6.
Figure 9.
Effects of Cu2+ and DHA on the expression levels of ROS, EGF and IL-6. 1, blank group; 2, only DHA; 3, only Cu2+; 4, Cu2+ and DHA added simultaneously; 5, Cu2+ injured followed by DHA repair; 6, DHA treatment followed by Cu2+ injury. *P<0.05; **P<0.01. DHA, docosahexaenoic acid; ROS, reactive oxygen species; EGF, epidermal growth factor; IL-6, interleukin-6.
Figure 10.
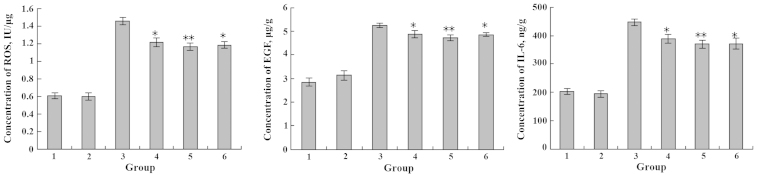
Effects of Cu2+ and EPA on the expression levels of ROS, EGF and IL-6. 1, blank group; 2, only EPA; 3, only Cu2+; 4, Cu2+ and EPA added simultaneously; 5, Cu2+-injured followed by EPA repair; 6, EPA treatment followed by Cu2+-injury. *P<0.05; **P<0.01. EPA, eicosapentaenoic acid; ROS, reactive oxygen species; EGF, epidermal growth factor; IL-6, interleukin-6.
Effects of DHA and EPA on ROS, EGF and IL-6 levels of IEC-6 cells injured by Cd3+
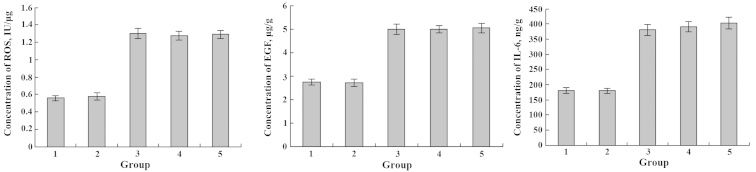
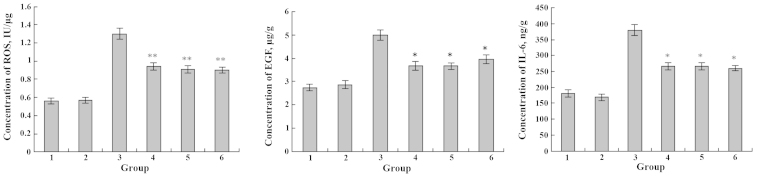
Similarly, incubation of IEC-6 cells with DHA and EPA for 48 h induced no significant variation in the secretion of ROS, EGF and IL-6; however, the level of ROS, EGF and IL-6 substantially increased after treatment of Cd3+ (Figs. 11 and 12). DHA treatment exhibited changes in the secretion level of ROS, EGF and IL-6 in the Cd3+-injured IEC-6 cells (Fig. 11). Notably, when IEC-6 cells were supplemented with EPA, as shown in Fig. 12, there was an evident reduction in the secretion of ROS, EGF and IL-6 levels.
Figure 11.
Effects of Cd3+ and DHA on the expression levels of ROS, EGF and IL-6. 1, blank group; 2, only DHA; 3, only Cd3+; 4, Cd3+-injured followed by DHA repair; 5, DHA treatment followed by Cd3+-injury. *P<0.05; **P<0.01. DHA, docosahexaenoic acid; ROS, reactive oxygen species; EGF, epidermal growth factor; IL-6, interleukin-6.
Figure 12.
Effects of Cd3+ and EPA on the expression levels of ROS, EGF and IL-6. 1, blank group; 2, only EPA; 3, only Cd3+; 4, Cd3+ and EPA added simultaneously; 5, Cd3+-injured followed by EPA repair; 6, EPA treatment followed by Cd3+-injury. *P<0.05; **P<0.01. EPA, eicosapentaenoic acid; ROS, reactive oxygen species; EGF, epidermal growth factor; IL-6, interleukin-6.
Effects of DHA and EPA on the ROS, EGF and IL-6 levels of IEC-6 cells injured by Pb2+
Supplementation of DHA and EPA to IEC-6 cells did not significantly affect the expression levels of ROS, EGF and IL-6 (P>0.05). Conversely, a substantial increase was observed as Pb2+ was added (**P<0.01) (Figs. 13 and 14). This result indicates that treatment with DHA and EPA induced a similar decrease in the content of ROS, EGF and IL-6 in Pb2+-injured IEC-6 cells.
Figure 13.
Effects of Pb2+ and DHA on the expression levels of ROS, EGF and IL-6. 1, blank group; 2, only DHA; 3, only Pb2+; 4, Pb2+ and DHA added simultaneously; 5, Pb2+-injured followed by DHA repair; 6, DHA treatment followed by Pb2+-injury. *P<0.05; **P<0.01. DHA, docosahexaenoic acid; ROS, reactive oxygen species; EGF, epidermal growth factor; IL-6, interleukin-6.
Figure 14.
Effects of Pb2+ and EPA on the expression levels of ROS, EGF and IL-6. 1, blank group; 2, only EPA; 3, only Pb2+; 4, Pb2+ and EPA added simultaneously; 5, Pb2+-injured followed by EPA repair; 6, EPA treatment followed by Pb2+-injury. *P<0.05; **P<0.01. EPA, eicosapentaenoic acid; ROS, reactive oxygen species; EGF, epidermal growth factor; IL-6, interleukin-6.
Discussion
Heavy metal pollution is becoming increasingly serious and can cause a direct risk of harm to human health. Currently, exhaust fumes from automobile vehicles, industrial discharge, consumer waste and soil pollution are major sources of heavy metal pollution (14). Plants are heavy metal-contaminated through polluted soils; once animals have been fed with these contaminated plants, the animal carcasses and milk will be contaminated. The ingestion of those foods poses a severe health risks for humans (15). The heavy metals can accumulate in the human body through the food chain. As long as heavy metals have accumulated in living organisms, the body will be hard to degrade (16). Long-term exposure to heavy metals increases the risk of developing cancer, kidney failure, mental growth retardation and central and peripheral nervous system injury. There is substantial evidence supporting that the consumption of the ω-3 PUFAs, EPA and DHA, are associated with health benefits. Despite increasing evidence of the beneficial effect of ω-3 PUFAs, little is known concerning the effects of EPA and DHA on intestinal epithelial cells injured by heavy metals. The present study aimed to elucidate the effects of ω-3 PUFAs (EPA and DHA) on the viability of intestinal epithelial IEC-6 cells exposed to heavy metals and the levels of ROS, EGF and IL-6.
The present results demonstrated that ω-3 PUFAs could increase the percentage of IEC-6 viable cells. The levels of ROS, EGF and IL-6 of IEC-6 cells injured by heavy metals increased, but decreased when EPA and DHA were administered. In addition, the ω-3 PUFAs significantly reduced the expression level of ROS. The current studies generally support that ROS is inseparably associated with oxidative damage (17–19). The level of ROS increased when the IEC-6 cells were treated with the heavy metals; heavy metals may cause oxidative damage to cells, thus inducing the cells to secrete a large amount of ROS, which eventually triggers cell apoptosis and also increases the level of the inflammatory factors EGF and IL-6. Overall, the beneficial effects of DHA and EPA on damage caused by the heavy metals were observed in the present study.
The present data support the assertion that ω-3 PUFAs could alleviate the adverse effects caused by heavy metal poisoning through improving the survival rates of intestinal crypt cell line (IEC-6). The results also suggest that ω-3 PUFAs protected the IEC-6 cells from the damage of heavy metals by reducing the expression levels of ROS, EGF and IL-6. In conclusion, ω-3 PUFA supplementation is a promising direction to alleviate the damages caused by heavy metals.
Acknowledgements
The present study was supported by the Health Bureau of Zhejiang Province (grant no. 2013KYA108) and Health and Family Planning Commission of Zhejiang Province (grant nos. 2013KYA108 and 2014KYA260).
References
- 1.Oszlánczi G, Papp A, Szabó A, Nagymajtényi L, Sápi A, Kónya Z, Paulik E, Vezér T. Nervous system effects in rats on subacute exposure by lead-containing nanoparticles via the airways. Inhal Toxicol. 2011;23:173–181. doi: 10.3109/08958378.2011.553248. [DOI] [PubMed] [Google Scholar]
- 2.Coen N, Mothersill C, Kadhim M, Wright EG. Heavy metals of relevance to human health induce genomic instability. J Pathol. 2001;195:293–299. doi: 10.1002/path.950. [DOI] [PubMed] [Google Scholar]
- 3.Raghunath R, Tripathi RM, Kumar AV, Sathe AP, Khandekar RN, Nambi KS. Assessment of Pb, Cd, Cu, and Zn exposures of 6- to 10-year-old children in Mumbai. Environ Res. 1999;80:215–221. doi: 10.1006/enrs.1998.3919. [DOI] [PubMed] [Google Scholar]
- 4.Itariu BK, Zeyda M, Leitner L, Marculescu R, Stulnig TM. Treatment with n-3 polyunsaturated fatty acids overcomes the inverse association of vitamin D deficiency with inflammation in severely obese patients: A randomized controlled trial. PLoS One. 2013;8:e54634. doi: 10.1371/journal.pone.0054634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itariu BK, Zeyda M, Hochbrugger EE, Neuhofer A, Prager G, Schindler K, Bohdjalian A, Mascher D, Vangala S, Schranz M, et al. Long-chain n-3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: A randomized controlled trial. Am J Clin Nutr. 2012;96:1137–1149. doi: 10.3945/ajcn.112.037432. [DOI] [PubMed] [Google Scholar]
- 6.Spencer M, Finlin BS, Unal R, Zhu B, Morris AJ, Shipp LR, Lee J, Walton RG, Adu A, Erfani R, et al. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes. 2013;62:1709–1717. doi: 10.2337/db12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 8.Belluzzi A. N-3 fatty acids for the treatment of inflammatory bowel diseases. Proc Nutr Soc. 2002;61:391–395. doi: 10.1079/PNS2002171. [DOI] [PubMed] [Google Scholar]
- 9.Clark WF, Parbtani A, Naylor CD, Levinton CM, Muirhead N, Spanner E, Huff MW, Philbrick DJ, Holub BJ. Fish oil in lupus nephritis: Clinical findings and methodological implications. Kidney Int. 1993;44:75–86. doi: 10.1038/ki.1993.215. [DOI] [PubMed] [Google Scholar]
- 10.De Caterina R, Caprioli R, Giannessi D, Sicari R, Galli C, Lazzerini G, Bernini W, Carr L, Rindi P. n-3 fatty acids reduce proteinuria in patients with chronic glomerular disease. Kidney Int. 1993;44:843–850. doi: 10.1038/ki.1993.320. [DOI] [PubMed] [Google Scholar]
- 11.Hall JC, Priestley JV, Perry VH, Michael-Titus AT. Docosahexaenoic acid, but not eicosapentaenoic acid, reduces the early inflammatory response following compression spinal cord injury in the rat. J Neurochem. 2012;121:738–750. doi: 10.1111/j.1471-4159.2012.07726.x. [DOI] [PubMed] [Google Scholar]
- 12.Luchtman DW, Meng Q, Song C. Ethyl-eicosapentaenoate (E-EPA) attenuates motor impairments and inflammation in the MPTP-probenecid mouse model of Parkinson's disease. Behav Brain Res. 2012;226:386–396. doi: 10.1016/j.bbr.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 13.Okabe N, Nakamura T, Toyoshima T, Miyamoto O, Lu F, Itano T. Eicosapentaenoic acid prevents memory impairment after ischemia by inhibiting inflammatory response and oxidative damage. J Stroke Cerebrovasc Dis. 2011;20:188–195. doi: 10.1016/j.jstrokecerebrovasdis.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Jin X, Balasubramanian VV, Selvan ST, Sawant DP, Chari MA, Lu GQ, Vinu A. Highly ordered mesoporous carbon nitride nanoparticles with high nitrogen content: A metal-free basic catalyst. Angew Chem Int Ed Engl. 2009;48:7884–7887. doi: 10.1002/anie.200903674. [DOI] [PubMed] [Google Scholar]
- 15.He D, Qiu B, Peng JH, Peng L, Hu LX, Hu Y. Heavy metal contents and enrichment characteristics of dominant plants in a lead-zinc tailings in Xiashuiwan of Hunan Province. Huan Jing Ke Xue. 2013;34:3595–3600. (In Chinese) [PubMed] [Google Scholar]
- 16.Quan SX, Yan B, Lei C, Yang F, Li N, Xiao XM, Fu JM. Distribution of heavy metal pollution in sediments from an acid leaching site of e-waste. Sci Total Environ. 2014;499:349–355. doi: 10.1016/j.scitotenv.2014.08.084. [DOI] [PubMed] [Google Scholar]
- 17.Polimeno L, Rossi R, Mastrodonato M, Montagnani M, Piscitelli D, Pesetti B, De Benedictis L, Girardi B, Resta L, Napoli A, et al. Augmenter of liver regeneration, a protective factor against ROS-induced oxidative damage in muscle tissue of mitochondrial myopathy affected patients. Int J Biochem Cell Biol. 2013;45:2410–2419. doi: 10.1016/j.biocel.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Labuschagne CF, Brenkman AB. Current methods in quantifying ROS and oxidative damage in Caenorhabditis elegans and other model organism of aging. Ageing Res Rev. 2013;12:918–930. doi: 10.1016/j.arr.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Nzengue Y, Steiman R, Rachidi W, Favier A, Guiraud P. Oxidative stress induced by cadmium in the C6 cell line: Role of copper and zinc. Biol Trace Elem Res. 2012;146:410–419. doi: 10.1007/s12011-011-9265-9. [DOI] [PubMed] [Google Scholar]