Abstract
Acute acalculous cholecystitis (AAC) constitutes 5–10% of all cases of cholecystitis in adults, and is even less common in children. The recent literature has described an association between primary Epstein-Barr virus (EBV) infection and AAC, however, it still remains an uncommon presentation of the infection. Most authors advise that the management of AAC in patients with primary EBV infection should be supportive, since the use of antibiotics does not seem to alter the severity or prognosis of the illness. Furthermore, surgical intervention has not been described as necessary or indicated in the management of uncomplicated AAC associated with EBV infection. We report a case of a 16-year-old Lebanese girl with AAC associated with primary EBV infection. She presented to the emergency department, with high-grade fever, fatigue, vomiting and abdominal pain. Liver enzymes were elevated with a cholestatic pattern, and imaging confirmed the diagnosis of AAC. She was admitted to the regular floor, and initial management was conservative. Owing to persistence of fever, antibiotics were initiated on day 3 of admission. She had a smooth clinical course and was discharged home after a total of 9 days, with no complications.
Background
Acute acalculous cholecystisis (AAC) compromises 5–10% of all cases of overall cholecystitis, and usually occurs in patients with a history of trauma, recent surgery, shock, burns, sepsis, critical illness, total parenteral nutrition or prolonged fasting.1–5 The recent literature has described an association between AAC and primary Epstein-Barr virus infection (EBV), however, it still remains an infrequent and uncommon complication of primary EBV. In a recent review of the literature published by Agergaard and Larsen3 in April 2015, the authors found a total of only 27 cases, the last being their own, of AAC associated with primary EBV infection. The exact pathogenesis of the condition remains unclear, and the possibility of a genetic or gender predisposition has been previously discussed in the literature but is still unproven.3
We chose to report this case for several reasons. As mentioned above, AAC is an atypical manifestation of primary EBV infection, and is not commonly reported in the paediatric/adolescent population. Our presentation of the case will add to the available literature and, possibly, in the future, help contribute to determining the pathogenesis of the disease. Furthermore, this case represents the first report of the condition in a Lebanese adolescent girl.
Case presentation
A 16-year-old girl, previously healthy, presented to our emergency department with a 10-day history of high-grade fever reaching 40°C. The fever was associated with a sore throat, and moderate-to-severe periumbilical and epigastric abdominal pain that was non-radiating and not associated with food intake. She also reported fatigue, vomiting, decreased oral intake and a headache.
She had been admitted to an outside hospital for a total of 4 days, where she had received clarithromycin for 3 days. The fever and abdominal pain persisted, however, so she was transferred to the American University in Beirut Medical Center (AUBMC) for further management. EBV Immunoglobulin M (IgM) serology taken at the outside hospital returned positive (specific serological investigation unavailable).
In our emergency department, the patient's vitals were stable (blood pressure was 100/60mm Hg, temperature 37°C, heart rate 97 bpm, respiratory rate 20 breaths per minute and oxygen saturation of 97% on room air). On physical examination, she was noted to have periorbital swelling. There was neither cervical lymphadenopathy, tonsillar exudates, nor pharyngitis. On abdominal examination, she had periumbilical and epigastric tenderness on palpation and hepatosplenomegaly (liver felt 2–3 cm below costal margin, spleen felt 2 cm below costal margin). McBurney's sign and Murphy's sign were both negative.
Investigations
Initial work up included: complete blood count (CBC), electrolytes, liver function tests, albumin, prothrombin time (PT) and activated partial thromboplastin time (aPTT), and C reactive protein (CRP). Urine culture, blood culture and cytomegalovirus (CMV) and EBV serologies were also taken (reference ranges provided parenthetically).
CBC revealed no major abnormality except for lymphocytosis. White cell count (WCC) count was 11 500/mm3 (4000–11 000/mm3) with 75% (25–40%) lymphocytes. Platelet count was 194 000/mm3 (150 000–400 000/mm3). Liver enzyme tests showed a mildly elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) with a cholestatic pattern and direct hyperbilirubinaemia. Total bilirubin 2.3 mg/dL (0–1.2 mg/dL), direct bilirubin 1.9 mg/dL (0–0.3 mg/dL), AST 163 IU/L (0–50 IU/L), ALT 136 IU/L (0–50 IU/L) and γ-glutamyl transferase (g-GT) 158 IU/L (10–50 IU/L). PT and aPTT were both normal.
Chemistries revealed hypoalbuminaemia of 27 g/L (36–53 g/L), low carbon dioxide of 18 mmol/L (24–30 mmol/L) and normal lipase of 18 U/L (13–60 U/L). All other labs values were normal.
CMV serologies were negative, however, EBV viral capsid antigens (VCA) IgM and IgG were both positive. CRP was mildly elevated at 14.5 mg/L (0–2.5 mg/L).
Owing to the history of moderate to severe abdominal pain, a CT of the abdomen was initially performed in the emergency department and showed thickening of the gallbladder wall, hepatosplenomegaly, periportal tracking, minimal ascites and infradiaphragmatic lymphadenopathy (retroperitoneal, mesenteric and perisplenic lymph nodes) (figure 1). In order to better visualise the gallbladder, ultrasound was performed, which revealed significant wall thickening reaching 1.56 cm with stratification and oedema, no gallstones, no biliary ductal dilation and a positive sonographic Murphy's sign (figure 2). The findings were in keeping with AAC.
Figure 1.
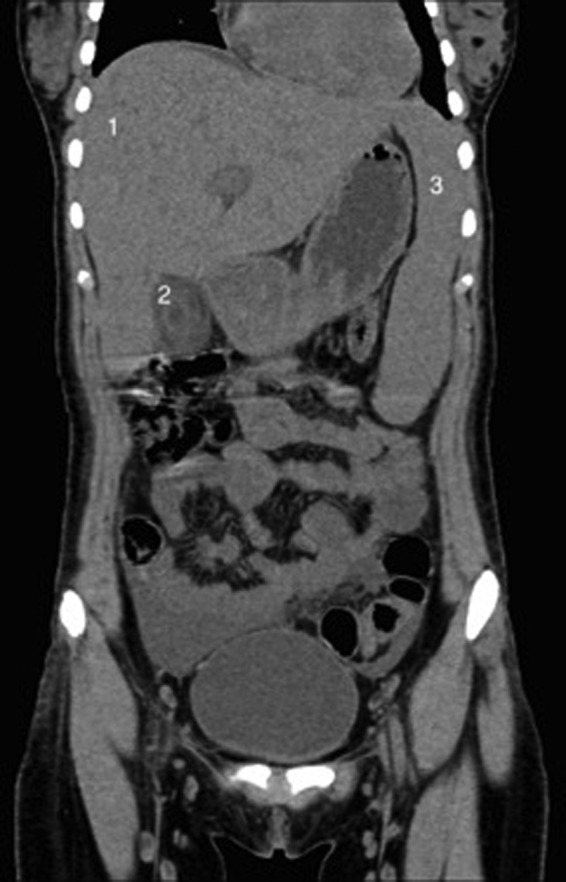
Abdominal CT scan. Coronal views showing a thickened gallbladder wall, hepatosplenomegaly, periportal tracking, minimal ascites and infradiaphragmatic lymphadenopathy; (*) gallbladder (1) liver (2) gallbladder.
Figure 2.
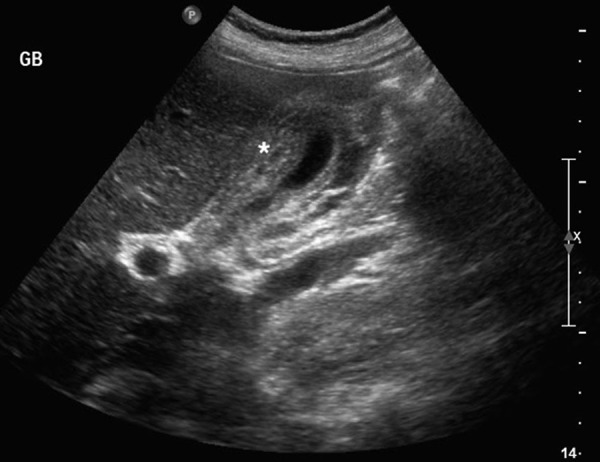
Ultrasound of gallbladder. Significant thickening of gallbladder wall (1.56 cm) without evidence of cholelithiasis; (*) gallbladder.
Differential diagnosis
The main differential diagnoses included other aetiologies of abdominal pain, such as appendicitis or hepatitis; hepatitis being more likely in light of the history of EBV infection. AAC secondary to other infectious aetiologies (viral or bacterial) could also be considered in the differential diagnosis (figure 3).
Figure 3.
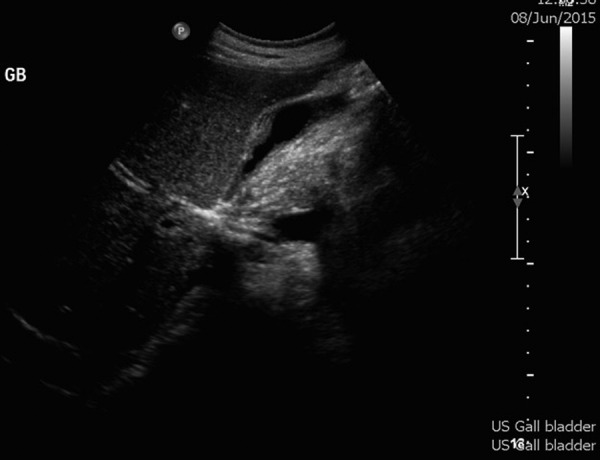
Repeat ultrasound of gallbladder. Thickened gallbladder wall (1.4 cm) and no evidence of gallstones.
Treatment
Initially, treatment was supportive, and included intravenous hydration, pain management and anti-emetics. Antibiotics were not initiated on admission since the AAC was most likely secondary to primary EBV infection.
However, later, due to persistence of fever and possibility of superimposed infection, the patient was started on ciprofloxacin on day 3 of admission and on Flagyl on day 4 of admission.
She had an uncomplicated hospital stay. The fever defervesced on day 4, approximately 8 h after the initial dose of ciprofloxacin, and her abdominal pain gradually improved. Repeat ultrasound on day 3 of admission revealed the same findings, with a thickened gallbladder wall (1.4 cm) and no evidence of gallstones.
A repeat CBC carried out on day 5 of admission showed mild leucocytosis, lymphocytosis with atypical lymphocytes, mild neutropenia and a normal platelet count; and a WCC count of 12 800/mm3 (normal range 4000–11 000/mm3) with 84% lymphocytes (normal range 25–40%), an absolute neutrophil count of 1280/mm3 (normal range 1600–7200/mm3) and platelets of 221 000/mm3 (150 000–400 000/mm3). No repeat assessment of CRP level was performed.
Most of the patient's liver function tests normalised over the course of her hospital stay, except for alkaline phosphatase (ALP) and g-GT, which decreased over time but remained elevated above normal values (table 1).
Table 1.
Liver function tests over time*
| Day of admission |
||||
|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 8 | |
| AST | 163 | 150 | 114 | 48 |
| ALT | 136 | 132 | 118 | 68 |
| Total bilirubin | 2.3 | 2.5 | 1.6 | 1 |
| Direct bilirubin | 1.9 | 2.1 | 1.3 | 0.8 |
| g-GT | 158 | 218 | 209 | 149 |
| ALP | 579 | 625 | 505 | |
*Abbreviations and reference ranges indicated parenthetically. Aspartate aminotransferase (AST, 0–50 IU/L), alanine aminotransferase (ALT, 0–50 IU/L), total bilirubin (0–1.2 mg/dL), direct bilirubin (0–0.3 mg/dL), γ-glutamyl transferase (g-GT, 10–50 IU/L) and alkaline phosphatase (ALP, 26–235 IU/L).
Blood culture taken in the emergency department remained negative after 5 days of incubation.
The patient was discharged home 8 days after admission to continue Flagyl and ciprofloxacin for a total course of 10 days.
Outcome and follow-up
The patient was discharged after a total of 9 days, with no complications. She followed up and was doing well in the Outpatient Clinic. Liver function tests repeated at that time were all normal.
Discussion
EBV is a herpes virus that resides in the host's memory B cells and is transmitted through saliva.2 3 5 In young children, the infection is often asymptomatic. In older adolescents, the most common manifestation after initial exposure to the virus is infectious mononucleosis with sore throat, fatigue, lymphadenopathy and fever of 1–2 weeks duration.2 3 5 Hepatosplenomegaly and elevated transaminases and ALP are also commonly seen with the initial infection.3
Primary infection with EBV was recently associated with an atypical presentation: the development of AAC.
Agergaard and Larsen conducted a review of 27 cases, in 2015. With respect to general characteristics of the patients, the authors reported that 26 were female, with ages ranging from 4 to 53 years. Twenty of the patients were of European origin (including those from Turkey and Denmark). Clinically pertinent findings included abdominal pain in all the patients, fever (37.5–40°C) in 24 of the patients and pharyngitis in 16 patients (with an additional patient having a history of tonsillopharyngitis in the previous 7 days). Sixteen patients had lymphadenopathy on admission, and five more patients developed lymphadenopathy during their hospital stay. Common laboratory findings were elevated ALT (25 patients), ALP (22 patients) and bilirubin (19 patients). None of the patients had an elevated CRP level suggestive of bacterial superimposed infection. Finally, radiographically, in all patients, the gallbladder wall thickness was over 3.5 mm or reported as thickened or diffusely oedematous; and 18 patients had a positive sonographic Murphy's sign. Seventeen patients were administered antibiotics at one point during the course of their illness.3 In only one patient, known to have ulcerative colitis and on immunomodulator therapy, was laparoscopic cholecystectomy performed due to severe septic AAC.3 6 Of the 19 patients with data available on length of admission, 14 required hospitalisation for 7 or more days.3
Pawłowska-Kamieniak et al also published a case report, in 2015, of EBV infection associated with AAC in a 17-year-old Polish girl, who presented with right upper quadrant pain, fever and nausea. There was no lymphadenopathy on physical examination, however, the authors did mention inflammatory changes of the adenoid glands. Similarly to previously reported cases, the patient had elevated liver function tests, hyperbilirubinaemia and a thickened gallbladder wall (7 mm). She was treated with antibiotics, ursodeoxycholic acid, analgesics and relaxants. She was discharged home after 2 weeks, with no complications.1
Yi et al published a retrospective case series, in July 2015, that analysed ultrasonographic characteristics of primary EBV infection in the paediatric population and evaluated the clinical course of EBV-associated gallbladder disease. The authors reviewed abdominal sonographic findings of 94 patients of a total of 287 diagnosed with primary EBV infection (positive EBV VCA IgM and clinical characteristics).
Individuals with hepatobiliary dysfunction secondary to underlying diseases were excluded from the initial sample of 287 patients. A gallbladder wall thickness greater than 3.5 mm was found in 24 (25.3%) of the 94 patients, 14 of whom were female.7 The prevalence figures need to be used judiciously since the patients initially included were those with hepatobiliary manifestations (abdominal pain, jaundice and liver function test abnormalities), and some patients were excluded due to lack of available imaging. Statistically significant findings in the thickened gallbladder group included lower platelet counts, higher alanine aminotransferase, g-GT and direct bilirubin levels, and the need for prolonged hospitalisation. There was, however, no significant difference in the number of patients requiring admission to the intensive care unit between the thickened gallbladder wall and the normal gallbladder wall groups. Sonographically, patients in the thickened gallbladder group were significantly more likely to have hepatomegaly, splenomegaly, increased periportal echogenicity and enlarged periportal lymph nodes.7
The exact pathogenesis by which primary EBV causes AAC remains unknown, however, a genetic and/or gender predisposition may play a role.3 EBV-associated AAC seems to show a female predominance. If findings from Agergaard and Larsen's review, Pawłowska-Kamieniak et al's1 report and our present case are combined, then 28 of a total of 29 cases of AAC secondary to primary EBV infection occurred in females.3 In the retrospective case series published by Yi et al, primary EBV-associated acute gallbladder disease occurred in 14 females versus 10 males. The difference in the sex distribution, however, was not statistically significant.7 Bile stasis and increased lithogenicity of bile are thought to contribute to development of AAC.4 5 A mechanism that has been hypothesised is that EBV-induced hepatitis results in cholestasis, gallbladder inflammation and AAC.2 4 5 In younger children who develop AAC secondary to EBV, Attilakos et al8 theorised Gilberts syndrome to be a possible predisposition, since the bilirubin monoglucoronide excreted in bile is less water-soluble and may contribute to cholestasis.
In Agergaard and Larsen's review, none of the previously documented cases of uncomplicated AAC associated with EBV required surgical intervention. Furthermore, patients who did not receive antibiotics had a similar clinical course and outcome to those treated with antibiotics.3 In the case series by Yi et al,7 only one patient with a prominently thickened gallbladder wall and AAC underwent emergent surgery prior to diagnosis of a primary EBV infection.
Our patient's presentation and clinical course was very similar to previously reported cases of EBV infection associated with AAC. Important to note is that our patient had neither pharyngitis nor lymphadenopathy on physical examination. She did, however, present to our emergency department after 10 days of fever, and therefore these symptoms may have been present prior to her admission. She recovered without surgical intervention. Antibiotics were administered during the course of her illness, however, she improved within hours of their initiation. Our decision to begin antibiotics was based on clinical findings, mainly the persistence of fever and abdominal pain. An objective value, such as a procalcitonin level or a rising CRP, would have strengthened the need for antibiotic administration. Therefore, retrospectively, their use may have been of questionable benefit.
Learning points.
Acute acalculous cholecystitis (AAC) is emerging as a new possible association with primary Epstein-Barr virus (EBV) infection, and may be more common than previously thought.
In a patient with primary EBV, elevated liver function tests and right upper quadrant pain, AAC should be considered as a diagnosis.
Furthermore, primary EBV infection should not be overlooked in a patient, especially if female, who presents with abdominal pain and signs of cholecystitis.
Treatment should aim at being supportive, and it seems reasonable to suggest that antibiotics might not be necessary in uncomplicated AAC associated with primary EBV infection. Surgical intervention has rarely been required in cases of uncomplicated AAC associated with primary EBV infection.
The exact pathogenesis remains unknown and still requires investigation.
Footnotes
Contributors: ZS conducted the literature review, and drafted and revised the manuscript. NM conducted the literature review, and drafted and revised the manuscript. SR managed the case and worked on final revision of the manuscript. MM managed the case and, as the contributing author, takes full responsibility.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Pawłowska-Kamieniak A, Mroczkowska-Juchkiewicz A, Gołyska D et al. Acute acalculous cholecystitis in a 17-year-old girl with Epstein-Barr virus infection. Prz Gastroenterol 2015;10:54–6. 10.5114/pg.2015.48998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gagneux-Brunon A, Suy F, Pouvaret A et al. Acute acalculous cholecystitis, a rare complication of Epstein–Barr virus primary infection: report of two cases and review. J Clin Virol 2014;61:173–5. 10.1016/j.jcv.2014.05.019 [DOI] [PubMed] [Google Scholar]
- 3.Agergaard J, Larsen CS. Acute acalculous cholecystitis in a patient with primary Epstein-Barr virus infection: a case report and literature review. Int J Infect Dis. 2015;35:67–72. 10.1016/j.ijid.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 4.Iaria C, Arena L, Di Maio G et al. Acute acalculous cholecystitis during the course of primary Epstein-Barr virus infection: a new case and a review of the literature. Int J Infect Dis 2008;12:391–5. 10.1016/j.ijid.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 5.Alkhoury F, Diaz D, Hidalgo J. Acute acalculous cholecystitis (AAC) in the pediatric population associated with Epstein–Barr Virus (EBV) infection. Case report and review of the literature. Int J Surg Case Rep 2015;11:50–2. 10.1016/j.ijscr.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagel S, Bruns T, Kantowski M et al. Cholestatic hepatitis, acute acalculous cholecystitis, and hemolytic anemia: primary Epstein-Barr virus infection under azathioprine. Inflamm Bowel Dis 2009;15:1613–16. 10.1002/ibd.20856 [DOI] [PubMed] [Google Scholar]
- 7.Yi DY, Kim JY, Yang HR. Ultrasonographic Gallbladder Abnormality of Primary Epstein-Barr Virus Infection in Children and Its Influence on Clinical Outcome. Medicine (Baltimore) 2015;94:e1120 10.1097/MD.0000000000001120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attilakos A, Prassouli A, Hadjigeorgiou G et al. Acute acalculous cholecystitis in children with Epstein-Barr virus infection: a role for Gilbert's syndrome? Int J Infect Dis 2009;13:e161–4. 10.1016/j.ijid.2008.08.009 [DOI] [PubMed] [Google Scholar]


