Abstract
Pyomyositis (PM) is a common masquerading disease that is frequently misdiagnosed. A concurrent state of immunodeficiency is observed in up to 75% of tropical PM cases. PM in systemic lupus erythaematosus (SLE) is a relatively rare disease. I report a case of PM that was caused by Klebsiella pneumoniae in a patient with SLE who presented with leg pain, fever and a lupus flare-up. The patient was correctly diagnosed using a CT scan. Immediate surgical drainage was performed, and empirical antibiotics were administered. The patient was discharged while in a recovering condition. The clinical features, the results of radiographic investigations and the management of PM in SLE are synopsised in this article to underscore the importance of considering this relatively rare disease during differential diagnosis in patients with SLE with muscle pain with or without fever. I also emphasise the need to exclude mycobacterial infection in patients with SLE with PM.
Background
Pyomyositis (PM) usually presents as a muscle abscess caused by Staphylococcus aureus (>90% in tropical areas). Three sequential clinical stages of PM were described by Chiedozi. The first is the invasive stage, in which muscle pain is followed by oedema, worsening pain and low-grade fever, with signs of a woody consistency. The second and most common presentation is the suppurative stage (>90% of cases). This occurs 10–21 days after onset and presents as a high fever and severe muscle tenderness. Abscess formation can now be detected. If treatment is delayed, the patient will advance to the last and most severe stage, the late stage. In this stage, patients become septic. Acute renal failure, metastatic abscesses, septic shock and death are likely in this third stage.1 PM typically presents in patients who reside in tropical areas; in patients who are immunocompromised, such as those with diabetes mellitus, haematological malignancy, chronic renal failure, asplenia, HIV or autoimmune diseases; in patients receiving chemotherapy or immunosuppressive drugs and individuals who abuse intravenous drugs.2 Systemic lupus erythaematosus (SLE) is an autoimmune disease that renders patients prone to infection because of their impaired immunological response and the immunosuppressive drugs used to treat SLE, such as steroids, cyclophosphamide and mycophenolate mofetil. Diagnosis of PM in patients with SLE is challenging because the infection can mimic an SLE flare-up and/or exacerbate SLE disease activity. In addition, infectious myositis can sometimes mimic inflammatory myositis. Although the pathogens that infect patients with SLE are often the same as those found in the general population, the clinical manifestations of infections can be atypical in patients with SLE.3 I report a case of PM that was caused by Klebsiella pneumoniae in a patient with SLE. I review and analyse the literature regarding PM in SLE. On the basis of an extensive literature search, I believe that this is the first reported case of K. pneumoniae PM in a patient with SLE.
Case presentation
In September 2015, a 14-year-old girl was admitted to Mae Sot General Hospital with tenderness in her left leg and a fever that had persisted for 2 weeks. She denied any history of previous trauma, strenuous exercise or genitourinary disorders. She had been diagnosed with SLE when she was 11.6 years old and had fulfilled the SLE diagnostic criteria of the Systemic Lupus International Collaborating Clinics (SLICC), including a photosensitive rash, oral ulcers, polyarthritis, lupus nephritis (class III), 1:1280 antinuclear antibody titres and a positive direct Coombs test. At that time, the patient's SLE Disease Activity Index 2000 (SLEDAI-2K) score was 23. Two months later, a seizure revealed neurological involvement. Administration of prednisolone was initiated as soon as SLE was diagnosed and was slowly tapered according to the severity of the patient's proteinuria. A full six courses of pulse cyclophosphamide (a cumulative dose of 4.05 g) was completed 2 years before the recent admission, and azathioprine (50 mg/day) was subsequently maintained for 1 year. The patient's medications at the time of admission were prednisolone (5 mg/day) and phenytoin (100 mg/day). On admission, her SLEDAI-2K score was 27, temperature was 38.2°C, pulse was 110 bpm, blood pressure was 103/63 mm Hg and her weight was 48 kg. Her left knee was slightly flexed, her whole left leg was swollen, and the calf was bulging and severely tender, which limited any mobilisation of the left knee and lower extremity.
Investigations
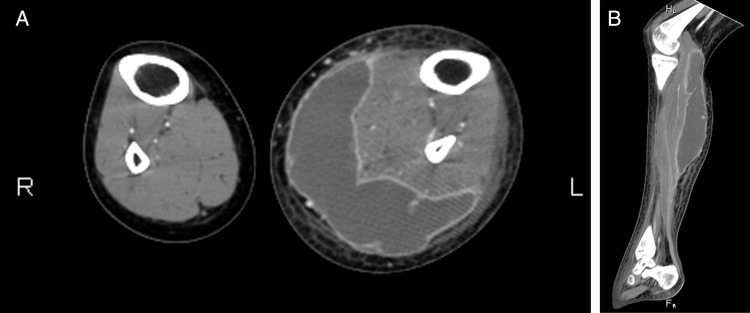
Laboratory tests showed that the patient's white cell count (WCC) was 11.4×103/µL (5.0–10.0×103) and consisted of 86.0% neutrophils. Her haemoglobin level was 10.1 g/dL (12.0–16.0) and platelet count was 309×103/µL (140–450×103). Normal renal and liver function was observed, except for an albumin level of 3.2 g/dL (3.3–4.5). A lupus flare was detected based on hypocomplementaemia (the C3 level was 0.31 g/L (0.79–1.52)) and elevated anti-double-stranded DNA antibodies (positive 1:8, as assessed in a latex agglutination test). Her erythrocyte sedimentation rate was high (87 mm/h (0–20)). A urinary analysis indicated the presence of proteinuria (1+), red blood cells (10–20/high-power field (HPF)), WCCs 5–10/HPF and 24 h urine protein of 1.073 g/d. Doppler ultrasound of the left lower and extremity-excluded deep vein thrombosis. CT scan revealed a multiloculated, rim-enhanced hypodense lesion in the muscle of the left posterior leg. This lesion measured approximately 4.5×9.5×19.0 cm (figure 1). The diagnosis was PM in the left gastrocnemius coupled with an SLE flare.
Figure 1.
Axial (A) and sagittal (B) views obtained in a CT scan of the patient's lower extremities showing a multiloculated, rim-enhanced hypodense lesion in the muscle of the left posterior leg. There was perilesional swelling and stranding of the subcutaneous fat, and the bony structure was intact.
Differential diagnosis
A differential diagnosis of PM in a patient with SLE may include a fever of unknown origin, sciatica, septic arthritis, osteomyelitis, cellulitis, fasciitis, deep vein thrombosis and inflammatory myositis.
Treatment
Surgical drainage was immediately performed. Pus collection was observed and Gram-negative bacilli were identified. Analysis of the pus cultures identified pure K. pneumoniae growth, but blood culture was negative. The administered antibiotics were changed to ceftriaxone (2 g/day) and the patient completed a 2-week course of these intravenous antibiotics.
Outcome and follow-up
A CT scan of the lower extremities was repeated on the 10th postoperative day because of a persistent fever, but no pus collection was demonstrated. The steroid dose was then increased to control the lupus flare-up. The fever subsided 2 days later, and delayed primary closure was performed without postoperative complications. The patient was discharged on day 22 after admission while in a recovering condition and was administered a home medication regimen of prednisolone at a dosage of 45 mg/day. At the last follow-up, 2 months later, her health had improved. The prednisolone dose was reduced to 20 mg/day because of her improved proteinuria, and hydroxychloroquine (100 mg/day) was added to provide an immunoprotective effect.
Discussion
PubMed and Google Scholar were investigated in September 2015, using the following keywords in several combinations: PM, myositis, muscle abscess; quadriceps, glutaeal, iliopsoas, retroperitoneal, leg, extremity; tuberculosis, Mycobacterium; and SLE, lupus and immunocompromised. The reference lists of the recovered articles were reviewed to identify additional studies.
A total of 25 patients with SLE with PM (including the present case) were identified.4–26 Since PM is strictly limited to cases in which a primary muscle abscess arises within the skeletal muscles,2 cases were excluded if they involved intermuscular abscesses; abscesses extending into muscles from adjoining tissue, such as bone or subcutaneous tissues;15 and abscesses secondary to previous septicaemia.26 Of the remaining cases, 23 were analysed.4–25 Tables 1–3 summarise the clinical characteristics of patients with SLE with PM. The most commonly presenting symptoms were muscle and/or adjacent joint tenderness (100%, n=22) and fever (78.9%, n=15/19). Commonly involved muscles were the quadriceps (n=7), iliopsoas (n=4), glutaei (n=4), calves, gastrocnemius (n=4) and the thigh or posterior thigh (n=3).
Table 1.
Summarised clinical features of pyomyositis in systemic lupus erythaematosus
| Reference/year | Age (year) | Current therapy | Location(s) | Organism(s) | Outcome |
|---|---|---|---|---|---|
| Ushijima et al4/1985 | 14 | PRDL | Glutaeal, quadriceps | S. aureus | Recovery |
| Shames and Fast5/1989 | 59 | PRED | Gluteal | S. aureus | Recovery |
| Shamiss et al6/1990 | 19 | Steroid | Posterior thighs, calves | Salmonella enteritidis | Recovery |
| Bonafede et al7/1992 | 31 | None | Upper arm, thigh | S. aureus | Recovery |
| Dede et al8/1993 | 23 | PRDL+CTX | Calf | S. aureus | Recovery |
| Yoshino et al9/1994 | 44 | MePRDL | Glutaeal | S. aureus | Recovery |
| Belzunegui et al10/1995 | 27 | PRED+CTX | Erector spinae | M. tuberculosis | Recovery |
| Gordon et al11/1995 | 48 | Steroid | Pectoralis major and minor, subclavius, intercostal muscles | S. aureus | Death |
| Claudepierre et al12/1996 | 32 | PRED+HCQ | Quadriceps | S. aureus | Recovery |
| Ushida et al13/2001 | 21 | PRDL+MZR | Psoas | S. aureus | Death |
| Teh et al14/2002 | 25 | PRDL+MMF | Thigh, calf | M. haemophilum | Recovery |
| García Hernández et al15/2003 | 33 | None | Iliopsoas | S. aureus | Recovery |
| Jidpugdeebodin and Punyagupta16/2004 | 31 | PRDL+CTX+MePRDL | Shoulder, axilla, arm, forearm | Salmonella serogroup B | Recovery |
| Ravindran and Duke17/2009 | 34 | HCQ | Pronator teres | S. aureus | Recovery |
| Collier et al18/2010 | NA | PRDL | Sternocleidomastoid | NA* | Recovery |
| El Baaj et al19/2010 | 47 | PRED+HCQ | Quadriceps | E. coli | Recovery |
| Manzoor20/2010 | 23 | NA | Rectus femoris | S. aureus | Recovery |
| Sokolove et al21/2010 | 39 | PRED | Quadriceps | M. tuberculosis | Recovery |
| Souza et al22/2011 | 25 | NA | Iliacus | S. aureus | Recovery |
| Blay et al23/2014 | 16 | PRED+HCQ+AZA | Vastus intermedius | NA† | Recovery |
| Chebbi et al24/2014 | 52 | PRDL+HCQ+MMF | Iliacus, glutaeal | S. aureus | Recovery |
| Simopoulou et al25/2014 | 46 | PRDL | Vastus lateralis | S. aureus and M. tuberculosis | Recovery |
| Present case/2016 | 14 | PRDL | Gastrocnemius | K. pneumoniae | Recovery |
*The patient's condition was improved by treatment with benzylpenicillin given at 2.4 g 4 times per day.
†The patient responded well to clindamycin plus intravenous cefepime for 1 week and oral clindamycin for an additional week.
AZA, azathioprine; CTX, cyclophosphamide; E. coli, Escherichia coli; HCQ, hydroxychloroquine; K. pneumoniae, Klebsiella pneumoniae; MePRDL, methylprednisolone; M. haemophilum, Mycobacterium haemophilum; M.tuberculosis, Mycobacterium tuberculosis; MMF, mycophenolate mofetil; MZR, mizoribine; NA, not available; PRDL, prednisolone; PRED, prednisone; S. aureus, Staphylococcus aureus.
Table 2.
Clinical characteristics, radiographic investigations, and studies leading to a definitive diagnosis of and organisms in pyomyositis in systemic lupus erythaematosus
| Clinical characteristic | n (%) |
|---|---|
| Female | 23/23 (100.0) |
| Temperate zone pyomyositis: tropical zone pyomyositis | 18:5/23 (78.3:21.7) |
| Affected sides | 22 |
| Right | 11 (50.0) |
| Left | 8 (36.4) |
| Bilateral | 3 (13.6) |
| Radiographic investigations | 20 |
| Ultrasound | 7 (35.0) |
| CT | 12 (60.0) |
| MRI | 9 (45.0) |
| Studies leading to a definitive diagnosis | 23 |
| Pus culture | 19 (82.6) |
| Tissue culture | 2 (8.7) |
| Blood culture | 1 (4.3) |
| No specimen (therapeutic diagnosis) | 1 (4.3) |
| Organisms | 23 |
| Staphylococcus aureus | 13 (56.5) |
| Salmonella spp | 2 (8.7) |
| Mycobacteria | 3 (13.0) |
| Other (Escherichia coli or Klebsiella pneumoniae) | 2 (8.7) |
| Unknown non-mycobacteria | 2 (8.7) |
| Mixed Staphylococcus aureus and Mycobacterium tuberculosis | 1 (4.3) |
Table 3.
Non-mycobacterial versus mycobacterial* pyomyositis in systemic lupus erythaematosus
| Non-mycobacteria (range) | n | Mycobacteria (range) | n | Total | n | |
|---|---|---|---|---|---|---|
| Median age | 31.0 (14–59) | 18 | 33.0 (25–46) | 4 | 31.0 (14–59) | 22 |
| Median duration of muscle tenderness before admission (d) | 14.0 (3–30) | 15 | 30.0 (30–90) | 3 | 14.0 (3–90) | 18 |
| History of nephritis | 71.4% | 10/14 | 66.7% | 2/3 | 70.6% | 12/17 |
| History of neurological involvement | 50.0% | 7/14 | 66.7% | 2/3 | 52.9% | 9/17 |
| Antiphospholipid antibodies | 0.0% | 0/13 | 50.0% | 2/4 | 11.8% | 2/17 |
| Previous pulse CTX | 25.0% | 4/16 | 50.0% | 2/4 | 30.0% | 6/20 |
| Current steroid therapy | 82.4% | 14/17 | 100.0% | 4/4 | 85.7% | 18/21 |
| PRED-equivalent doses over 10 mg/day | 50.0% | 7/14 | 100.0% | 4/4 | 61.1% | 11/18 |
| Median WCC (×103/µL) | 9.0 (2.5–19.3) | 15 | 11.7 (8.5–14.9) | 2 | 9.0 (2.5–19.3) | 17 |
| Median ESR (mm/h) | 89.0 (36–124) | 9 | 66.0 (40–92) | 3 | 83.5 (36–124) | 12 |
| Median CRP (mg/L) | 86.4 (1–260) | 7 | 117.6 (23–220) | 4 | 86.4 (1–260) | 11 |
| Drainage | 84.2% | 16/19 | 25.0% | 1/4 | 73.9% | 17/23 |
| Mortality rate | 10.5% | 2/19 | 0.0% | 0/4 | 8.7% | 2/23 |
*Including a case of mycobacterial and Staphylococcus aureus coinfection.25
CRP, C reactive protein; CTX, cyclophosphamide; ESR, erythrocyte sedimentation rate; PRED, prednisone; WCC, white cell count.
The results of the radiographic investigations performed in cases of PM in SLE are illustrated in table 2. CT and MRI commonly confirmed the US results and showed one or more collections with or without adjacent muscular swelling, stranding of subcutaneous fat and skin thickening; this was true in all but four cases.14 18 21 25 Of these cases, 13.0% were invasive (n=3),14 18 21 78.3% were suppurative (n=18)4–10 12 13 15 17 19 20 22–25 and 8.7% were late stage PM (n=2).11 16 At least 47.8% (n=11/23) met the criteria for sepsis,4 5 11 15 16 19 21 22 24 25 and bacteraemia was evident in 7 of 13 documented blood culture results.5 6 16 17 21 22 24
The pathogens that caused PM are summarised in table 2. Laboratory results showed anaemia, with a median haemoglobin level of 10.1 g/dL (range 8.3–11.4 g/dL, n=12) and a WCC>12×103 (n=6) or <4×103/µL (n=1) in 41.2% of the cases (n=7/17). Hypocomplementaemia was identified in eight of nine reported cases (including the present case).4 9 13 14 19 21 23 Of these, seven showed symptoms or signs of active SLE.4 9 13 14 19 21 23
Surgical drainage, percutaneous drainage and no drainage were the definitive treatments in 13 (56.5%), 4 (17.4%) and 6 (26.1%) patients, respectively. Major complications included osteomyelitis (n=3), death (n=2), septic arthritis (n=1), necrotising fasciitis with sepsis (n=1) and bone infarction (n=1). In the two fatal cases, one patient in the suppurative stage was thought to have died from a lupus flare-up (wide brainstem infarction and a haemorrhage spot),13 and the other from pneumonia and sepsis with multiple organ failure during the late stage of PM.11
Although this disease is classically thought of as a muscle abscess, the hallmark of the disease is not the abscess but, rather, a finding of myositis in a biopsy specimen from the affected muscle. Myositis is therefore the preferred diagnostic criterion for some authors.27 In healthy hosts, PM should alert the clinician to the possibility of subclinical immunodeficiency, and tests for HIV, diabetes, rheumatological disorders and malignancy, along with assessments of immunoglobulin levels, should be performed.2 PM is extremely rare in patients with SLE, with a prevalence of 0.35% (1/289).23 The diagnosis is therefore frequently missed or delayed as a result of a lack of specific signs and symptoms, a lack of awareness of the disease, atypical manifestations and a varied range of differential diagnoses.17 In certain cases, sciatica,5 deep vein thrombosis,6 fasciitis,7 aseptic necrosis of the femoral head,9 recurrent cellulitis,14 inflammatory myositis,21 or fever with nodular lesions25 is diagnosed prior to a PM diagnosis. In many cases, the invasive stage is prolonged in mycobacterial infections14 21 25 but shortened in non-mycobacterial infections.15–17 22 24 A lupus flare-up, which sometimes precedes or is accompanied by muscle tenderness and requires intense immunosuppressive therapy, can complicate the treatment of PM or even cause death.13 The interaction between infection and underlying SLE activity is well established: infection can mimic the clinical presentation of SLE, frequently inducing lupus flares and exacerbating disease activity. Furthermore, infectious myositis can masquerade as inflammatory myositis and drive clinicians towards treating the patient with immunosuppressive drugs, which delays appropriate treatment. Pain out of proportion to weakness, normal muscle enzyme levels and a failure to respond to high-dose immunosuppressive agents suggest that inflammatory myositis is unlikely.21 If a diagnosis is delayed, then high rates of mortality (8.7%, which is much higher than the 0.5–2% rate for common PM2) and complications are observed (34.8% for major complications).
Leucocytosis (WCC >12×103/µL) and leukopaenia (WCC <4×103/µL) are infrequently observed in the immunosuppressive stage. At least 34.8% (n=8/23) of published cases showed evidence of lupus flares as hypocomplementaemia or elevated anti-double-stranded DNA antibodies. At least 30.4% (n=7/23) of the blood cultures were positive (compared with 20–30% in temperate regions)2 and, in one case, pathogenic bacteria were diagnosed based on blood culture alone.24 For cellulitis in immunocompromised hosts, blood cultures should be obtained to assess PM.28
Although S. aureus was the most commonly isolated pathogen (60.9%), mycobacteria and Gram-negative bacteria were both found in 17.4% of published cases. These results are in contrast with findings obtained in a previous study of immunocompromised hosts, which demonstrated a lower incidence of S. aureus PM (35–59%) and a higher incidence of Gram-negative bacilli PM (30%). The latter type of bacteria included Escherichia coli and K. pneumoniae .19 Acid-fast smears and mycobacterial cultures were added to microbiological studies in those cases, and antibiotics for Gram-negative bacilli were administered. Importantly, all three cases with skin nodules/abscesses were S. aureus PM. In PM coupled with underlying disease, empirical broad-spectrum antibiotics (eg, vancomycin plus one of the following: piperacillin-tazobactam, ampicillin-sulbactam, or carbapenem) and cefazolin or anti-staphylococcal penicillin is suggested by the recent Infectious Diseases Society of America (IDSA) guidelines for non-mycobacterial PM to treat PM caused by methicillin-susceptible S. aureus.28 Whereas a 2–3-week course of antibiotics is optimal for uncomplicated non-mycobacterial PM,28 metastatic infections require a longer duration of treatment.2 Generally, a 6-month course of front-line antituberculous drugs is adequate for treating mycobacterial PM without drug resistance.25 Although infection can lead to active SLE, immunosuppressive agents should be administered whenever an overlying lupus flare is suspected if there is very good source control and concurrent antimicrobial therapy. Excluding the invasive stage, in most cases of PM in SLE, medication plus early drainage of the abscess is preferred. The failure of a fever to normalise indicates metastatic infection (which should be excluded first), drug resistance, drug fever,2 or a lupus flare, as was observed in the present case.
With the exception of the current case, K. pneumoniae PM has not been reported in patients with SLE.. This bacterium generally causes PM in diabetics or in older adult patients at a mean age of 50.6 years. In these cases, PM frequently involves the psoas muscle and, in many cases, it is accompanied by a urinary tract infection.29 Gas production indicates a grave prognosis.30
Infective risk factors in SLE include a high SLEDAI score, high anti-DNA titres, hypocomplementaemia, lupus nephritis, neurological SLE, antiphospholipid antibodies, leukopaenia, steroid usage, prednisone equivalent doses of over 7.5–10 mg/day, methylprednisolone high-dose pulses and cyclophosphamide high-dose regimens.17 31 Many of these risks were present in this case and review (table 3).
In the literature, most clinicians treated PM in patients with SLE as non-mycobacterial PM because its prevalence is much higher (19 vs 4). Table 3 compares non-mycobacterial and mycobacterial PM in SLE according to their clinical characteristics. Statistical analyses were subject to major type 2 error for these comparisons. Most of the parameters were comparable, but the duration of muscle tenderness before arrival was longer (30 vs 14 days) and antiphospholipid antibodies and conservative treatment were more prominent in mycobacteria-infected cases.
Learning points.
Diagnoses of PM in SLE are often missed or delayed because of the rarity of and physicians' unfamiliarity with the condition, resulting in high morbidity and a high rate of complications.
In patients with SLE, especially those with a high risk of infections, a high index of suspicion of PM should be assumed when evaluating cases presenting with localised painful muscle/swelling with or without fever, and diagnostic imaging should be performed.
After a timely diagnosis of PM, management should involve early drainage of purulent discharge and immediate appropriate antibiotic therapy.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Chiedozi LC. Pyomyositis: review of 205 cases in 112 patients. Am J Surg 1979;137:255–9. 10.1016/0002-9610(79)90158-2 [DOI] [PubMed] [Google Scholar]
- 2.Chauhan S, Jain S, Varma S et al. Tropical pyomyositis (myositis tropicans): current perspective. Postgrad Med J 2004;80:267–70. 10.1136/pgmj.2003.009274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sciascia S, Cuadrado MJ, Karim MY. Management of infection in systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2013;27:377–89. 10.1016/j.berh.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 4.Ushijima H, Soda H, Okitsu S et al. Subcutaneous and/or intramuscular abscesses in two cases of systemic lupus erythematosus. Jpn J Clin Immunol 1985;8:47–53. 10.2177/jsci.8.47 [DOI] [Google Scholar]
- 5.Shames JL, Fast A. Gluteal abscess causing sciatica in a patient with systemic lupus erythematosus. Arch Phys Med Rehabil 1989;70:410–11. [PubMed] [Google Scholar]
- 6.Shamiss A, Thaler M, Nussinovitch N et al. Multiple Salmonella enteritidis leg abscesses in a patient with systemic lupus erythematosus. Postgrad Med J 1990;66:486–8. 10.1136/pgmj.66.776.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonafede P, Butler J, Kimbrough R et al. Temperate zone pyomyositis. West J Med 1992;156:419–23. [PMC free article] [PubMed] [Google Scholar]
- 8.Dede H, Ozdoğan H, Dumankar A et al. Tropical pyomyositis in a temperate climate. Br J Rheumatol 1993;32:435–6. 10.1093/rheumatology/32.5.435 [DOI] [PubMed] [Google Scholar]
- 9.Yoshino Y, Hirohata S, Takeuchi A et al. [Gluteal abscess caused by Staphylococcus aureus in a patient with systemic lupus erythematosus]. Ryumachi 1994;34:786–9. [PubMed] [Google Scholar]
- 10.Belzunegui J, Plazaola I, Uriarte E et al. Primary tuberculous muscle abscess in a patient with systemic lupus erythematosus. Br J Rheumatol 1995;34:1177–8. 10.1093/rheumatology/34.12.1177 [DOI] [PubMed] [Google Scholar]
- 11.Gordon BA, Martinez S, Collins AJ. Pyomyositis: characteristics at CT and MR imaging. Radiology 1995;197:279–86. 10.1148/radiology.197.1.7568838 [DOI] [PubMed] [Google Scholar]
- 12.Claudepierre P, Saint-Marcoux B, Allain J et al. Clinical images: value of magnetic resonance imaging in extensive pyomyositis. Arthritis Rheum 1996;39:1760 10.1002/art.1780391021 [DOI] [PubMed] [Google Scholar]
- 13.Ushida H, Koizumi S, Katoh K et al. [Systemic lupus erythematosus presenting as a brainstem infarction and hemorrhage during treating retroperitoneal abscess: a case report]. Nippon Hinyokika Gakkai Zasshi 2001;92:579–82. [DOI] [PubMed] [Google Scholar]
- 14.Teh CL, Kong KO, Chong AP et al. Mycobacterium haemophilum infection in an SLE patient on mycophenolate mofetil. Lupus 2002;11:249–52. 10.1191/0961203302lu175cr [DOI] [PubMed] [Google Scholar]
- 15.García Hernández FJ, Sánchez Román J, Ocaña Medina C et al. [Iliopsoas abscess and systemic lupus erythematosus]. An Med Interna 2003;20:198–200. [PubMed] [Google Scholar]
- 16.Jidpugdeebodin S, Punyagupta S. Salmonella crepitant pyomyositis in a patient with systemic lupus erythematosus. J Infect Dis Antimicrob Agents 2004;21: 11–15. [Google Scholar]
- 17.Ravindran V, Duke O. Non-tropical pyomyositis in a patient with systemic lupus erythematosus. Lupus 2009;18:379–80. 10.1177/0961203308100513 [DOI] [PubMed] [Google Scholar]
- 18.Collier S, Vig N, Collier J. Two cases of tropical pyomyositis of the sternocleidomastoid muscle occurring in the UK. Br J Oral Maxillofac Surg 2010;48:216–17. 10.1016/j.bjoms.2009.10.028 [DOI] [PubMed] [Google Scholar]
- 19.El Baaj M, Tabache F, Modden K et al. [Pyomyositis: an infectious complication in systemic lupus erythematous]. Rev Med Interne 2010;31:e4–6. 10.1016/j.revmed.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 20.Manzoor K. A ‘tropical disease’ in an immunocompromised patient. Darlington County Durham Med J 2010;4:7–9. [Google Scholar]
- 21.Sokolove J, Copland A, Shirvani S et al. A 39-year-old woman with lupus, myositis, and a recalcitrant vasculopathy. Arthritis Care Res (Hoboken) 2010;62:1351–6. 10.1002/acr.20236 [DOI] [PubMed] [Google Scholar]
- 22.Souza HC, Carvalho BN, Morais MG et al. Tropical pyomyositis in a patient with systemic lupus erythematosus and HTLV 1/2 infection. Rev Bras Reumatol 2011;51:97–103. 10.1590/S0482-50042011000100008 [DOI] [PubMed] [Google Scholar]
- 23.Blay G, Ferriani MP, Buscatti IM et al. Pyomyositis in childhood-systemic lupus erythematosus. Rev Bras Reumatol 2016;56:79–81. [DOI] [PubMed] [Google Scholar]
- 24.Chebbi W, Jerbi S, Kessomtini W et al. Pyogenic sacroiliitis and pyomyositis in a patient with systemic lupus erythematous. Case Rep Rheumatol 2014;2014:925961 10.1155/2014/925961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simopoulou T, Varna A, Dailiana Z et al. Tuberculous pyomyositis: a re-emerging entity of many faces. Clin Rheumatol 2016;35:1105–10. [DOI] [PubMed] [Google Scholar]
- 26.David DS, Brennan L, Grieco MH. Gas production with Salmonella typhimurium infection. Am J Med Sci 1971;262:255–60. 10.1097/00000441-197111000-00002 [DOI] [PubMed] [Google Scholar]
- 27.Shepherd JJ. Tropical myositis: is it an entity and what is its cause? Lancet 1983;2:1240–2. 10.1016/S0140-6736(83)91281-3 [DOI] [PubMed] [Google Scholar]
- 28.Stevens DL, Bisno AL, Chambers HF et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014;59:e10–52. 10.1093/cid/ciu444 [DOI] [PubMed] [Google Scholar]
- 29.Yahalom G, Guranda L, Meltzer E. Internal obturator muscle abscess caused by Klebsiella pneumoniae. J Infect 2007;54:e157–60. 10.1016/j.jinf.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 30.Chang CM, Ko WC, Lee HC et al. Klebsiella pneumoniae psoas abscess: predominance in diabetic patients and grave prognosis in gas-forming cases. J Microbiol Immunol Infect 2001;34:201–6. [PubMed] [Google Scholar]
- 31.Danza A, Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus 2013;22:1286–94. 10.1177/0961203313493032 [DOI] [PubMed] [Google Scholar]