Abstract
Single-nucleotide polymorphisms in the human leukocyte antigen (HLA)-DQB1 gene are associated with chronic inflammatory and immunological diseases. Host genetic factors have a key role in the development of chronic hepatitis B (CHB). The aim of the present study was to investigate the association between the HLA-DQB1 polymorphisms and the susceptibility to CHB. PubMed, Embase, CNKI and Wanfang databases were searched for the studies that reported the association of the HLA-DQB1 polymorphisms with CHB between January 1, 1966 and July 30, 2015. HLA-DQB1 polymorphism-specific odds ratio (OR) and 95% confidence intervals (95% CI) were pooled and calculated in the fixed effects model using the Mantel-Haenszel method. Q-test and I2 test were performed to examine the heterogeneity. Begg's funnel test and Egger's test were conducted to assess publication bias. All the statistical tests were two-tailed. Subsequent to searching the databases and screening according to the inclusion criteria, 7 case-control studies were available in the present meta-analysis, including 815 CHB patients and 731 control subjects for the HLA-DQB1 polymorphisms. In conclusion, the statistically significant pooled OR of the HLA-DQB1 polymorphisms were obtained for the HLA-DQB1 loci (*0201, case vs. control: I2=36.5%; P-value of heterogeneity=0.15; OR, 1.29; 95% CI, 1.02–1.64; P=0.0301; *0301, case vs. control: I2=0%; P-value of heterogeneity=0.899; OR, 1.37; 95% CI, 1.12–1.69; P=0.002; *0502, case vs. control: I2=24.9%; P-value of heterogeneity=0.239; OR, 1.50; 95% CI, 1.02–2.20; P=0.04), which were associated with an increased risk of CHB. Similar significant results were observed and acquired in the following HLA-DQB1 loci (*0303, case vs. control: I2=0%; P-value of heterogeneity=0.986; OR, 0.77; 95% CI, 0.62–0.95; P=0.017; *0604, case vs. control: I2=0%; P-value of heterogeneity=0.594; OR, 0.38; 95% CI, 0.20–0.74; P=0.003), which were associated with a decreased risk of CHB. No significant association was observed for the other HLA-DQB1 family loci. The present meta-analysis demonstrated that the HLA-DQB1 loci (*0201, *0301 and *0502) polymorphisms were significantly associated with an increased risk of CHB. However, HLA-DQB1 loci polymorphisms (*0303 and *0604) were associated with a decreased risk of CHB. These results support the hypothesis that polymorphisms of the HLA-DQB1 allele families may affect the susceptibility or resistance to CHB.
Keywords: chronic hepatitis B, human leukocyte antigens/alleles, human leukocyte antigen haplotypes, meta-analysis
Introduction
Chronic hepatitis B (CHB) imposes a major health and economic burden as to 2 billion people worldwide have a history of hepatitis B virus (HBV) infection and ~360 million suffering from chronic HBV infection despite its declining incidence, leading the main cause of chronic diseases-related malfunction (1,2). CHB may increase the risk of developing liver cirrhosis, severe liver failure and hepatocellular carcinoma, although primary HBV infections usually have a self-limited course in adults (3). Due to the residual virus and weakened immunity to reinfection, ~20% do not recover but progress to liver cirrhosis and 5% develop hepatocellular carcinoma through persistent infections (3,4). However, the precise mechanisms leading to the chronicity of HBV infection remain to be elucidated at the molecular level. Infection may spread in a variety of ways including vertical (mother-to-child transmission) and horizontal transmission (lesions, bites, sanitary habits, sexual contact, medical exposure and drug use). In Asian countries, over half of the CHB patients were infected via vertical transmission and subsequently became HBV carriers (5). In adolescence, ~5% of the primary HBV carriers exhibit a long-term liver dysfunction and progress to chronic hepatitis (6). CHB significantly increases the probability of liver cirrhosis and primary hepatocellular cancer in the decades following the initial diagnosis and treatment (7,8).
Currently, CHB remains a major concern regarding the issue of public health. However, the detailed pathogenesis of such a disease remains to be elucidated. In addition to the differences in the viral and environmental factors, the variations of host genetic factors are proved to dominate the pathological states of CHB development and progression. A number of genetic studies provide evidence that variations at the genetic level contribute to the development of chronic hepatitis (9–11). In addition to the aforementioned evidence, extensive epidemiological studies have shown that the variations of genetic factors, including cytokines (12–14), human leukocyte antigen (HLA) (15–18) and immune response-associated genes (19–21), could evidently affect the clinical outcomes of primary HBV infection. The HLA complex is the first discovered genetic factor exhibiting a definite correlation with HBV infection. HLA polymorphisms are usually associated with immune response variability. The genotype of the HLA genes may affect the progression or regression of HBV infection. The main function of HLA-II molecules is to present specific antigens to cluster of differentiation 4+ (CD4+) T cells, which regulate the immune response of CD8+ cytotoxic T lymphocytes (CTL) and are important to the production of specific-neutralizing antibodies. The process of HBV clearance is governed by eliminating infected cells via CTL and protecting additional cells from persistent infection via neutralizing antibody.
Therefore, it appears biologically viable to assume that variability in the interaction between HLA-II molecules and HBV antigens may be extremely important. This is verified by the evidence that patients with acute HBV infections showed superior HLA-II restricted CD4+ T-cell immune responses to the hepatitis B core antigen compared with chronic hepatitis patients (22). HLA class II gene polymorphisms are associated with various diseases, particularly for autoimmune disorders (23). However, the association of the HLA class II gene polymorphism with human diseases exhibits ethnical and geographic variability (24). Acute hepatitis B patients with strong HLA class I and II-restricted T-cell responses will not suffer from persistent HBV infection, while those without these responses may progress to CHB (25–27). Shi et al (28) indicated that HLA-II genes polymorphisms may be a crucial factor in affecting the outcome of HBV infection.
Kamatani et al (29) demonstrated that variants in the HLA-DP locus were strongly associated with CHB in the Asian population by conducting a genome-wide association study (GWAS). HLA-DQB1 polymorphisms have recently been proved to affect immune responses of patients, and thus influence the clinical outcome of numerous diseases (30,31). A previous GWAS study conducted by Mbarek et al (32) suggested that there was a strong association between the HLA-DQB1 polymorphism (rs2856718) and CHB. HLA-DQB1*0301 is also correlated with susceptibility to CHB (33–35), whereas HLA-DQB1*0201 is proved to be a HBV-resistance gene in Xinjiang Uygur (35), and HLA-DQB1*0501 has been revealed to be associated with persistent response to interferon treatment in chronic hepatitis C patients (36). Li et al (37) suggested that HLA-DQB1*0302 could reduce the incidence of hepatocellular carcinoma by inhibiting the replication of HBV.
However, according to previous studies, it remains unclear whether HLA-DQB1 polymorphisms are associated with the susceptibility to CHB due to small sample size and small phenotypic effects of HLA-DQB1 locus. Therefore, the present study conducted a comprehensive meta-analysis to evaluate the potential association between HLA-DQB1 polymorphisms and susceptibility to CHB. HLA-DQB1 polymorphisms were quantitatively summarized in serum samples from patients with chronic hepatitis B infection. The case-control studies were adopted to evaluate whether HLA-DQB1 polymorphisms are associated with the risk of chronic HBV infection by a comparison of the frequency distribution differences in 13 HLA-DQB1 locus between the CHB and healthy control groups.
Materials and methods
Search strategy and selection criteria
The PubMed, Embase, CNKI and Wanfang databases were searched for studies that reported on the association of HLA-DQB1 polymorphisms with CHB between January 1, 1966 and July 30, 2015, using Medical Subject Heading terms ‘major histocompatibility complex, class II, DQβ1’ and ‘polymorphisms’ and ‘chronic hepatitis B’ or ‘chronic hepatitis B infection’ or ‘chronic hepatitis’ and corresponding free words. The Cochrane library (http://www.cochrane.org) was also searched using the term ‘major histocompatibility complex, class II, DQβ1’, ‘polymorphisms’ and ‘chronic hepatitis B’ or ‘chronic hepatitis B infection’. Furthermore, the citations of the retrieved studies were reviewed in order to search for additional studies in association with the present meta-analysis. Included studies met the following criteria: i) Case-control studies, nested case-control studies or cohort studies; ii) studies investigating the correlation between HLA-DQB1 polymorphisms and CHB, and the exposed risk factor should be HLA-DQB1 polymorphisms; iii) relevant genotype frequencies, or odds ratio (OR) and 95% confidence interval (CI) should be reported; iv) full-text studies so that detailed information could be acquired. Excluded studies were: i) Studies without healthy control subjects; ii) duplicated publications; iii) studies that involved <20 participants. When there was more than one study on the same subjects, only the most recent study was used.
Data extraction
Data extraction was independently performed by two experienced investigators (J. Huang and Z. Zhou) and examined carefully by the other investigators. The concordance rate of the investigators was 95.6%. Disagreement was resolved by consensus. The following data was extracted from the included studies: The first author's name, date of publication, region, ethnicity, design method, genotyping, case and control subjects number, and genotype frequencies. Data were collected only for subjects whose HLA-DQB1 polymorphisms status had been detected in CHB and its control.
Assessment of study quality
Two investigators (J. Huang and Z. Zhou) independently assessed the quality of each included study according to a 12-point scoring system (38). Study design, number of cases, source of subjects, genotyping method and matching method of case and control were examined in the assessment of study quality. Studies, which met each of the following criteria (a prospective study, >100 cases, including community-based participants, DNA sequencing was used to detect HLA-DQB1 polymorphisms, and matched for age and gender), were scored on a 2-point scale. Studies with a total score of ≥8 were defined as high-quality studies, 5–7 were defined as medium-quality studies, and ≤4 were regard as low-quality studies. These cut-off values were confirmed based on the quality scores distribution of all studies. The Spearmans rank correlation coefficient of consensus between each of the two reviewers on the total quality assessment for all the associated studies was 0.97. In addition, disagreements were settled by consultation.
Statistical analysis
The meta-analysis was performed using the software Stata 12.0 (Stata Corporation, College Station, TX, USA). OR and its corresponding 95% CI were adopted as the effect measures to conduct the meta-analysis. The Q-test, P-value and I2 test was used to evaluate heterogeneity among studies (39,40). When the P-value of heterogeneity value was >0.05 and I2<50%, a fixed-effects model was adopted to calculate OR and its 95% CI, otherwise a random-effects model was used. The combined OR was calculated by two-sided Z-test, and P<0.05 was considered to indicate a statistically significance difference. Sensitivity analysis was performed to assess the reliability and stability of the overall results. Publication bias test was performed using Begg's funnel plots and the Egger's regression plots (41,42).
Results
Characteristics of eligible studies
Subsequent to searching the previously defined databases, a total of 13 studies were selected according to the established search strategy. Six studies that were not eligible, as shown by the data provided in the abstract and text, were excluded. Finally, a total of 7 case-control studies were available in this meta-analysis, including 815 CHB patients and 731 control subjects for HLA-DQB1 polymorphisms (34,35,37,43–46). The study search and selection process is shown in Fig. 1. The detailed characteristics of the 7 included studies are shown in Table I. The publication year of the included studies ranged between 2003 and 2015. The distribution of the HLA-DQB1 polymorphisms in CHB is shown in Table II. All the studies used blood samples for HLA-DQB1 genotyping. All the quality scores of the included studies were >7 (moderate-high quality) (38).
Figure 1.
Flow diagram for the study selection.
Table I.
Characteristics of the studies included in the meta-analysis.
| First author (year) | Region | Ethnicity | Design | Genotyping | Case (n) | Control (n) | Refs. |
|---|---|---|---|---|---|---|---|
| Jiang et al (2003) | China | Asian | PB | PCR/SSP | 52 | 106 | (34) |
| Park et al (2003) | Korean | Asian | PB | PCR/RFLP/SSCP | 135 | 100 | (43) |
| Xi-Lin et al (2006) | China | Asian | HB | PCR/SSP | 139 | 134 | (44) |
| Liu and Cheng (2007) | China | Asian | PB | PCR/SSP | 168 | 100 | (45) |
| Zhu et al (2007) | China | Asian | HB | PCR/SSP | 151 | 133 | (46) |
| Zhang et al (2015) | China | Asian | HB | PCR/SSP | 110 | 100 | (35) |
| Li et al (2015) | China | Asian | HB | PCR/SSP | 60 | 58 | (37) |
HB, hospital-based; PB, population-based; PCR, polymerase chain reaction; SSP, sequence-specific primer; SSCP, single-strand conformation polymorphism; RFLP, restriction fragment length polymorphism.
Table II.
Distribution of the HLA-DQB1 polymorphisms in chronic hepatitis B.
| HLA-DQB1 loci | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author (year) | 0201 | 0301 | 0302 | 0303 | 0401 | 0402 | 0501 | 0502 | 0503 | 0601 | 0602 | 0603 | 0604 | Refs. |
| Jiang et al (2003) | (34) | |||||||||||||
| Case, n | 10 | 37 | 6 | 15 | 5 | 1 | 3 | 7 | 2 | 7 | 4 | 2 | 2 | |
| Control, n | 23 | 40 | 14 | 35 | 11 | 2 | 9 | 20 | 6 | 20 | 12 | 5 | 7 | |
| Park et al (2003) | (43) | |||||||||||||
| Case, n | 31 | 43 | 27 | 23 | 22 | 5 | 12 | 9 | 17 | 31 | 27 | 2 | 4 | |
| Control, n | 13 | 26 | 18 | 24 | 9 | 11 | 17 | 3 | 11 | 12 | 18 | 5 | 14 | |
| Xi-Lin et al (2006) | (44) | |||||||||||||
| Case, n | 48 | 71 | 10 | 58 | 8 | NA | 12 | 17 | 8 | 24 | 21 | 1 | 0 | |
| Control, n | 45 | 55 | 9 | 74 | 10 | NA | 9 | 11 | 14 | 25 | 13 | 0 | 3 | |
| Liu and Cheng (2007) | (45) | |||||||||||||
| Case, n | 63 | 10 | 14 | 14 | 9 | 5 | 7 | 9 | 16 | 45 | 67 | 7 | 7 | |
| Control, n | 17 | 5 | 8 | 12 | 10 | 5 | 2 | 3 | 8 | 15 | 52 | 3 | 7 | |
| Zhu et al (2007) | (46) | |||||||||||||
| Case, n | 51 | 79 | 12 | 61 | 8 | NA | 15 | 18 | NA | 26 | 22 | NA | NA | |
| Control, n | 38 | 56 | 20 | 61 | 10 | NA | 18 | 3 | NA | 15 | 25 | NA | NA | |
| Zhang et al (2015) | (35) | |||||||||||||
| Case, n | 20 | 30 | 9 | 22 | 3 | NA | 5 | 7 | NA | NA | 10 | 1 | 1 | |
| Control, n | 11 | 17 | 8 | 31 | 4 | NA | 6 | 5 | NA | NA | 9 | 2 | 1 | |
| Li et al (2015) | (37) | |||||||||||||
| Case, n | 3 | 42 | 2 | 13 | 6 | 1 | 6 | 7 | 4 | 11 | 8 | 1 | NA | |
| Control, n | 8 | 27 | 8 | 17 | 3 | 0 | 6 | 8 | 3 | 16 | 7 | 1 | NA | |
NA, not available; HLA, human leukocyte antigen.
Quantitative data synthesis
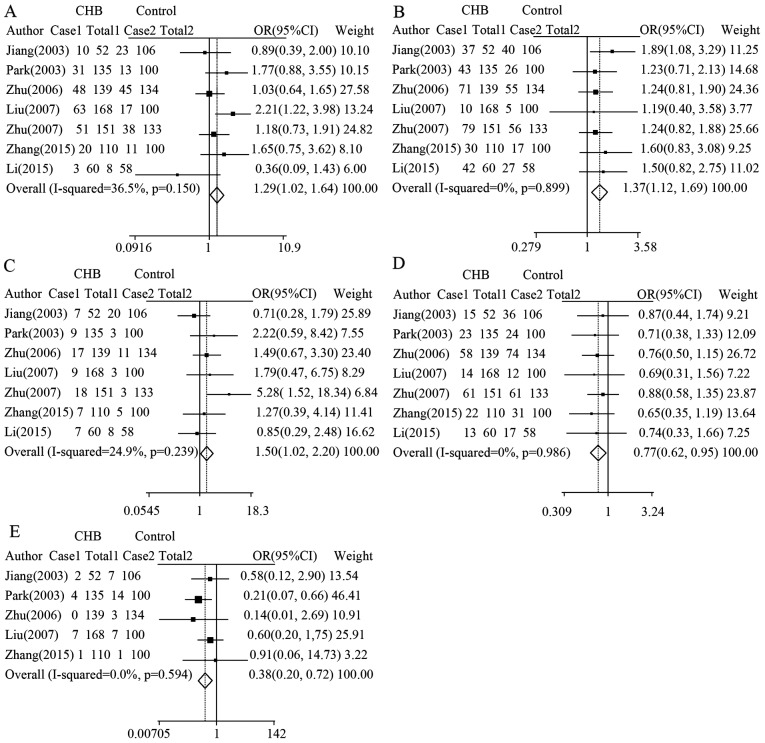
In conslusion, statistically significant pooled OR of HLA-DQB1 polymorphisms were obtained for HLA-DQB1 loci [*0201, case vs. control: I2=36.5%; P-value of heterogeneity=0.15; OR, 1.29; 95% CI, 1.02–1.64; P=0.0301 (Fig. 2A and Table III); *0301, case vs. control: I2=0%; P-value of heterogeneity=0.899; OR, 1.37; 95% CI, 1.12–1.69; P=0.002 (Fig. 2B and Table III); *0502, case vs. control: I2=24.9%; P-value of heterogeneity=0.239; OR, 1.50; 95% CI, 1.02–2.20; P=0.04 (Fig. 2C and Table III)], which were associated with increased risk of CHB. Similar significant results were observed and acquired in the following HLA-DQB1 loci [*0303, case vs. control: I2=0%; P-value of heterogeneity=0.986; OR, 0.77; 95% CI, 0.62–0.95; P=0.017 (Fig. 2D and Table III); *0604, case vs. control: I2=0%; P-value of heterogeneity=0.594; OR, 0.38; 95% CI, 0.20–0.74; P=0.003 (Fig. 2E and Table III)], which were associated with a decreased risk of CHB. No significant association was observed for the other HLA-DQB1 family loci (Table III).
Figure 2.
Forest plots showing the association of the HLA-DQB1 polymorphisms with the risk of CHB. (A) *0201; (B) *0301; (C) *0502; (D) *0303 and (E) *0604. HLA, human leukocyte antigen; CHB, chronic hepatitis B; OR, odds ratio; CI, confidence interval.
Table III.
Results of Q-test and I2 test for HLA-DQB1 polymorphisms in chronic hepatitis B.
| HLA-DQB1 loci | Q-value | I2, % | P-value | OR (95% CI) | Pooled P-value |
|---|---|---|---|---|---|
| 0201 | 9.45 | 36.5 | 0.150 | 1.29 (1.02–1.64) | 0.031 |
| 0301 | 2.21 | 0.0 | 0.899 | 1.37 (1.12–1.69) | 0.002 |
| 0302 | 5.12 | 0.0 | 0.523 | 0.84 (0.60–1.16) | 0.290 |
| 0303 | 0.98 | 0.0 | 0.986 | 0.77 (0.62–0.95) | 0.017 |
| 0401 | 53.23 | 88.7 | <0.001 | 0.47 (0.15–1.43) | 0.182 |
| 0402 | 2.05 | 0.0 | 0.562 | 0.53 (0.25–1.10) | 0.088 |
| 0501 | 3.81 | 0.0 | 0.702 | 0.82 (0.57–1.19) | 0.293 |
| 0502 | 7.98 | 24.9 | 0.239 | 1.50 (1.02–2.20) | 0.040 |
| 0503 | 2.17 | 0.0 | 0.704 | 0.93 (0.60–1.45) | 0.741 |
| 0601 | 7.39 | 32.3 | 0.193 | 1.24 (0.93–1.64) | 0.138 |
| 0602 | 3.67 | 0.0 | 0.721 | 0.93 (0.72–1.20) | 0.565 |
| 0603 | 2.84 | 0.0 | 0.725 | 0.79 (0.38–1.66) | 0.536 |
| 0604 | 2.78 | 0.0 | 0.594 | 0.38 (0.20–0.74) | 0.003 |
OR, odds ratio; CI, confidence interval, HLA, human leukocyte antigen.
Sensitivity analysis
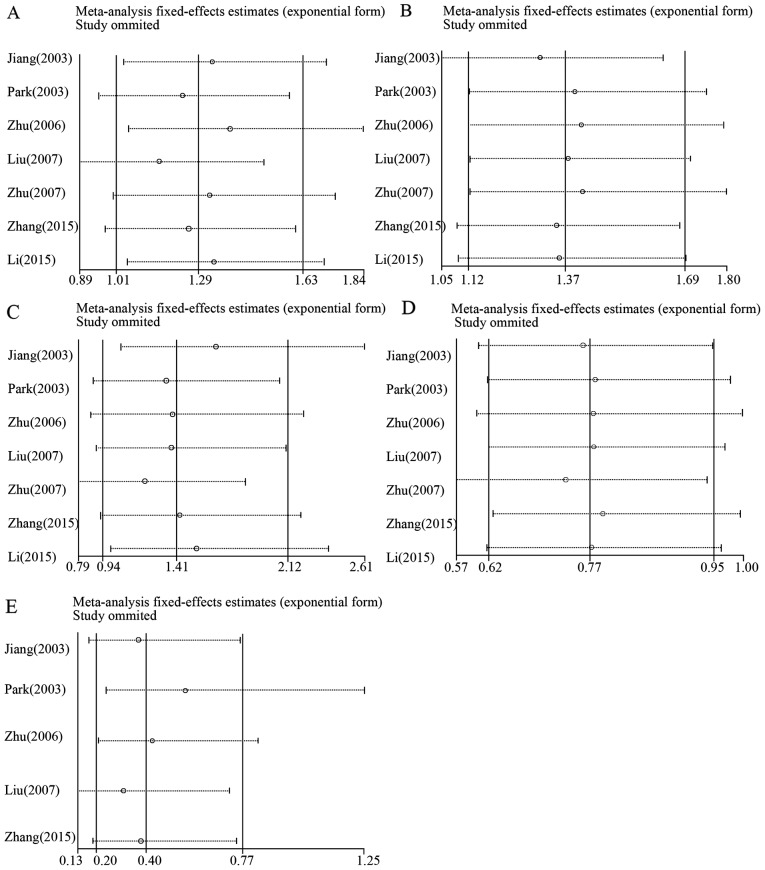
Sensitivity analysis was performed by removing one study at a time to detect the source of heterogeneity. There was no evident heterogeneity in all the HLA-DQB1 family loci. Additionally, there was no valid evidence to support that any study independently influenced the combined OR, which indicated that the overall results of this study are robust and convincing, as shown in the plots for sensitivity analysis (Fig. 3).
Figure 3.
Sensitivity analysis for heterogeneity. (A) *0201; (B) *0301; (C) *0502; (D) *0303 and (E) *0604.
Publication bias
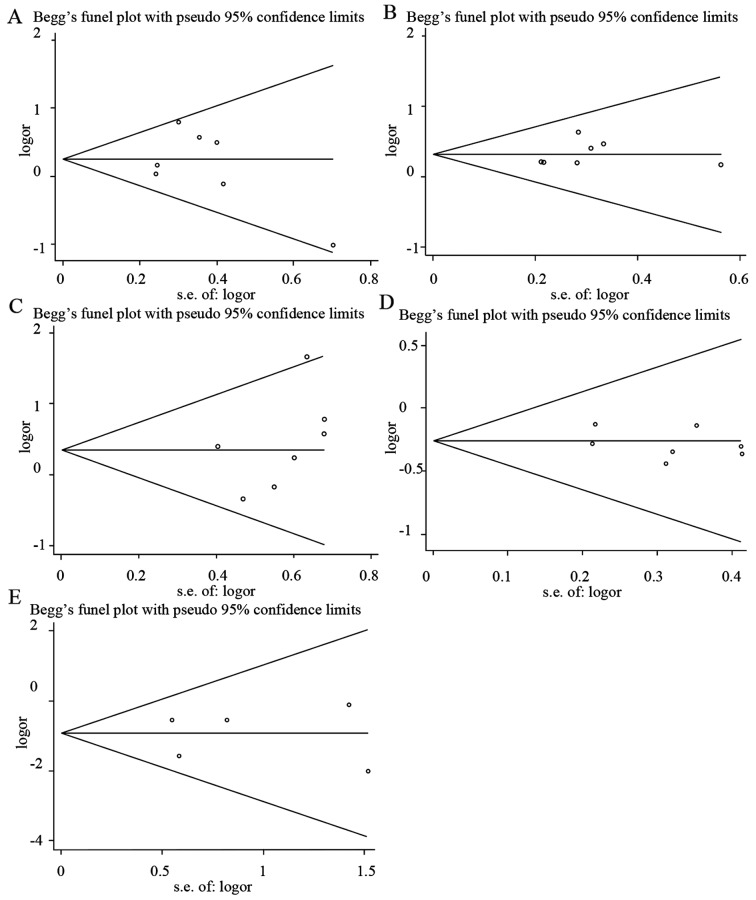
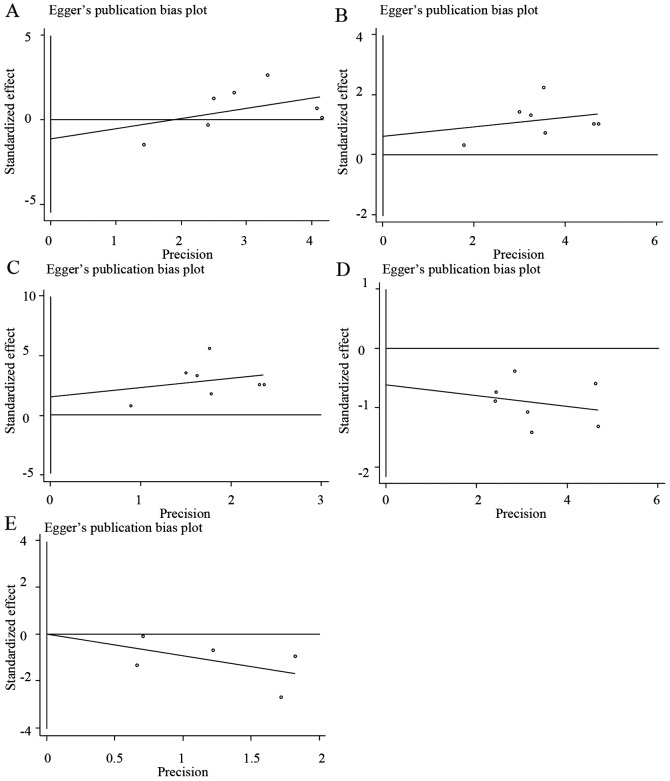
Begg's funnel plots and Egger's regression plots were used to detect the publication bias of all the HLA-DQB1 loci. As illustrated in Fig. 4, the funnel plots did not show any evidence of significant asymmetry and suggested that no publication bias existed [*0201, Z=0.6, P=0.548 (Fig. 4A and Table IV); *0301, Z=1.5, P=0.133 (Fig. 4B and Table IV); *0502, Z=1.5, P=0.133 (Fig. 4C and Table IV); *0303, Z=0, P=1 (Fig. 4D and Table IV); *0604, Z=0.24, P=0.86 (Fig. 4E and Table IV)]. Egger's test also indicated that there was no statistically significant publication bias [*0201, T=−0.66, P=0.54 (Fig. 5A and Table IV); *0301, T=0.63, P=0.554 (Fig. 5B and Table IV); *0502, T=1.18, P=0.291 (Fig. 5C and Table IV); *0303, T=−1.03, P=0.349 (Fig. 5D and Table IV); *0604, T=0.01, P=0.996 (Fig. 5E and Table IV)].
Figure 4.
Begg's funnel plots for publication bias test. (A) *0201; (B) *0301; (C) *0502; (D) *0303 and (E) *0604.
Table IV.
Results of Beggs test and Eggers test for HLA-DQB1 polymorphisms in chronic hepatitis B.
| Beggs test | Eggers test | |||
|---|---|---|---|---|
| HLA-DQB1 loci | Z-value | P-value | T-value | Pooled P-value |
| 0201 | 0.60 | 0.548 | −0.66 | 0.540 |
| 0301 | 1.50 | 0.133 | 0.63 | 0.554 |
| 0302 | 1.50 | 0.133 | −1.24 | 0.270 |
| 0303 | <0.01 | 1.000 | −1.03 | 0.349 |
| 0401 | <0.01 | 1.000 | −0.25 | 0.811 |
| 0402 | 1.70 | 0.089 | 3.57 | 0.070 |
| 0501 | 0.90 | 0.368 | 1.30 | 0.249 |
| 0502 | 1.50 | 0.133 | 1.18 | 0.291 |
| 0503 | 0.73 | 0.462 | −0.14 | 0.897 |
| 0601 | 0.38 | 0.707 | −1.24 | 0.282 |
| 0602 | 0.60 | 0.548 | 0.80 | 0.459 |
| 0603 | 0.38 | 0.707 | 0.22 | 0.836 |
| 0604 | 0.24 | 0.806 | 0.01 | 0.996 |
HLA, human leukocyte antigen.
Figure 5.
Egger's regression plots for publication bias test. (A) *0201; (B) *0301; (C) *0502; (D) *0303 and (E) *0604. OR, odds ratio; s.e., standard error.
Discussion
To the best of our knowledge, this is the first study investigating the association of HLA-DQB1 alleles with CHB. Numerous studies have suggested the associations of HLA gene polymorphisms with inflammatory diseases and autoimmune diseases, such as HBV infection (32,47), hepatitis C virus infection (48,49), systemic lupus erythematosus (50) and rheumatoid arthritis (51). However, the majority of these studies focus on the correlation between the HLA antigen and CHB based on a small sample size and HLA serotyping that has limited resolution; therefore, those results may be inaccurate and inconsistent for the distribution of numerous HLA-DQB1 loci. Along with the development of genotyping methods, HLA-genotyping is becoming more precise in the identification of the HLA-DQB1 loci, and more accurate in the identification of the peptide-binding site of MHC II molecules. Therefore, HLA genotyping methods are being used more frequently in the study of immunogenetics.
Recent studies on the correlation between HLA-DQB1 polymorphisms and CHB have been inconsistent and inconclusive. Jiang et al (34) reported that HLA-DQB1*0301 are closely associated with susceptibility to CHB, while other HLA-DQB1 alleles are not. Thus, it is plausible that the HLA-DQB1*0301 allele may be a risk factor for the development of CHB (OR, 3.9). Park et al (43) insisted that HLA-DQB1*0402 and DQB1*0604 alleles have a certain protective effect on the occurrence of CHB (OR, 0.3; and OR, 0.1, respectively). Therefore, these alleles may be considered as good prognostic factors. Liu and Cheng (45) observed that the HLA-DQB1*0201 and DQB1*0601 alleles have significant susceptible effect on chronic HBV infection (OR, 2.93; and OR, 2.07, respectively). However, Xi-Lin et al (44) identified that the HLA-DQB1*0303 and DQB1*0503 alleles are independently resistant genetic factors to CHB (OR, 0.65; and OR, 0.35, respectively). Zhu et al (46) further observed that the HLA-DQB1*0502 allele is significantly associated with the clinical outcome of HBV infection (OR, 18) and is a host genetic risk factor for HBV infection.
The present study showed that five specific HLA-DQB1 loci are associated with an increased or decreased risk of CHB. Among the 13 specific HLA-DQB1 alleles, DQB1*0201, DQB1*0301 and DQB1*0502 were significantly associated with the increased risk of CHB. The pooled OR was 1.29 (95% CI, 1.02–1.64; P=0.0301), 1.37 (95% CI, 1.12–1.69; P=0.002) and 1.50 (95% CI, 1.02–2.20; P=0.04), respectively. However, DQB1*0303 and DQB1*0604 were significantly associated with a decreased risk of CHB. The pooled OR was 0.77 (95% CI, 0.62–0.95; P=0.017) and 0.38 (95% CI, 0.20–0.74; P=0.003), respectively. No significant association was observed for the other HLA-DQB1 family alleles. The overall results indicate that HLA-DQB1*0201, HLA-DQB1*0301 and HLA-DQB1*0502 alleles may have a significantly higher risk for CHB, while HLA-DQB1*0303 and HLA-DQB1*0604 may have a significantly protective effect for CHB.
The study by Zhang et al (35) suggested that HLA-DQB1*0303 is a resistance gene of CHB in Xinjiang Uygur, while HLA-DQB1*0301 is associated with continuous infection of HBV. The HLA-DQB1*0201 distribution frequency in the low copy group was significantly higher than that of the high copy group (OR, 1.939; P<0.05), and thus assumed that DQB1*0201 may contribute to the clearance of HBV (35). In addition, Li et al (52) reported that the HLA-DQB1*0501, HLA-DQB1*0601 and HLA-DQB1*0602 alleles are associated with significantly increased immunological responses to the hepatitis B vaccine in healthy people (OR, 1.85; OR, 2.35; and OR, 2.34, respectively), while HLA-DQB1*0201 is adverse (OR, 0.27). The mechanisms underlying these effects on CHB are not fully elucidated, but larger-scale studies provide a promise of further confirmation. Jiang et al (53) identified five novel susceptibility loci for CHB using a GWAS with 2,514 CHB cases and 1,130 normal controls from eastern China, and four of them are located in the human leukocyte antigen (HLA) region at 6p21.3. Additionally, the study validated seven previously reported CHB susceptibility loci, including rs2856718 at HLA-DQB1, rs7453920 at HLA-DQB2, rs3077 at HLA-DPA1, rs9277535 at HLA-DPA2, rs3130542 at HLA-C, rs1419881 at TCF19, and rs652888 at EHMT2 (53). All are located in the HLA region.
CHB development is preceded by acute inflammation and immune responses. Whether antigen-presenting cells are able to identify HBV antigens may be critical for the development of CHB. The correlation of specific HLA-DQB1 alleles with resistance or susceptibility to CHB is possibly attributed to a direct effect of HLA-DQB1 molecule as an antigen-presenting unit or possibly owing to a neighboring-related gene (53). We assume that the host immune response status of the patients with CHB and carrying HLA-DQB1 polymorphisms are changed. T-cells are often activated under certain conditions such as infection, depression and fatigue. Accompanied by the removal of HBV, liver damage was triggered and a range of clinical symptoms occurred such as fever, anorexia, abnormally elevated aminotransferases and icterus, inducing the formation of CHB. With regards to the HLA-DQB1 loci, it may be plausible that HLA molecule mediates the function of host antigen-presenting cells and induces cytotoxic T-lymphocyte responses.
However, due to the potential heterogeneity of HBV, the results of the present study should be explained with caution. These retrospective studies are more prone to bias than prospective randomized clinical trial (RCT) studies. The information of CHB patients complicated by HCC were not specially extracted and analyzed. The association of HLA-DQB1 loci with HCV infection was not included in the meta-analysis. The overall sample size was relatively small due to the limited number of original studies.
In conclusion, the present meta-analysis suggests that HLA-DQB1*0201, DQB1*0301 and DQB1*0502 are risk factors for CHB, while HLA-DQB1*0303 and DQB1*0604 are protective factors. These results are compatible with the published studies regarding the correlation between HLA-DQB1 loci and other inflammatory disorders. Future large scale studies of HLA-DQB1 should be used to provide strong evidence for a genetic contribution to CHB.
References
- 1.Ocama P, Opio CK, Lee WM. Hepatitis B virus infection: Current status. Am J Med. 2005;118:1413. doi: 10.1016/j.amjmed.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 3.Ganem D, Prince AM. Hepatitis B virus infection - natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 4.Wright TL, Lau JY. Clinical aspects of hepatitis B virus infection. Lancet. 1993;342:1340–1344. doi: 10.1016/0140-6736(93)92250-W. [DOI] [PubMed] [Google Scholar]
- 5.Pungpapong S, Kim WR, Poterucha JJ. Natural history of hepatitis B virus infection: An update for clinicians. Mayo Clin Proc. 2007;82:967–975. doi: 10.4065/82.8.967. [DOI] [PubMed] [Google Scholar]
- 6.Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y, Mayumi M. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med. 1976;294:746–749. doi: 10.1056/NEJM197604012941402. [DOI] [PubMed] [Google Scholar]
- 7.Chemin I, Zoulim F. Hepatitis B virus induced hepatocellular carcinoma. Cancer Lett. 2009;286:52–59. doi: 10.1016/j.canlet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 8.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45–S55. doi: 10.1002/hep.22898. [DOI] [PubMed] [Google Scholar]
- 9.Lin TM, Chen CJ, Wu MM, Yang CS, Chen JS, Lin CC, Kwang TY, Hsu ST, Lin SY, Hsu LC. Hepatitis B virus markers in Chinese twins. Anticancer Res. 1989;9:737–741. [PubMed] [Google Scholar]
- 10.Tong H, Bock CT, Velavan TP. Genetic insights on host and hepatitis B virus in liver diseases. Mutat Res Rev Mutat Res. 2014;762:65–75. doi: 10.1016/j.mrrev.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Su M, Zeng Y, Chen J, Jiang L, Chen T, Liu C, Yang B, Ou Q. Studies on the association of single nucleotide polymorphisms of HLA-DP and DQ genes with the outcome of chronic hepatitis B virus infection. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2014;31:765–769. doi: 10.3760/cma.j.issn.1003-9406.2014.06.019. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 12.Ben-Ari Z, Mor E, Papo O, Kfir B, Sulkes J, Tambur AR, Tur-Kaspa R, Klein T. Cytokine gene polymorphisms in patients infected with hepatitis B virus. Am J Gastroenterol. 2003;98:144–150. doi: 10.1111/j.1572-0241.2003.07179.x. [DOI] [PubMed] [Google Scholar]
- 13.Höhler T, Kruger A, Gerken G, Schneider PM, Meyer Zum Büschenefelde KH, Rittner C. A tumor necrosis factor-alpha (TNF-alpha) promoter polymorphism is associated with chronic hepatitis B infection. Clin Exp Immunol. 1998;111:579–582. doi: 10.1046/j.1365-2249.1998.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Migita K, Maeda Y, Abiru S, Nakamura M, Komori A, Miyazoe S, Nakao K, Yatsuhashi H, Eguchi K, Ishibashi H. Polymorphisms of interleukin-1beta in Japanese patients with hepatitis B virus infection. J Hepatol. 2007;46:381–386. doi: 10.1016/j.jhep.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Thio CL, Carrington M, Marti D, O'Brien SJ, Vlahov D, Nelson KE, Astemborski J, Thomas DL. Class II HLA alleles and hepatitis B virus persistence in African Americans. J Infect Dis. 1999;179:1004–1006. doi: 10.1086/314684. [DOI] [PubMed] [Google Scholar]
- 16.Kummee P, Tangkijvanich P, Poovorawan Y, Hirankarn N. Association of HLA-DRB1*13 and TNF-alpha gene polymorphisms with clearance of chronic hepatitis B infection and risk of hepatocellular carcinoma in Thai population. J Viral Hepat. 2007;14:841–848. doi: 10.1111/j.1365-2893.2007.00880.x. [DOI] [PubMed] [Google Scholar]
- 17.Hwang SH, Sohn YH, Oh HB, Hwang CY, Lee SH, Shin ES, Lee KJ. Human leukocyte antigen alleles and haplotypes associated with chronicity of hepatitis B virus infection in Koreans. Arch Pathol Lab Med. 2007;131:117–121. doi: 10.5858/2007-131-117-HLAAAH. [DOI] [PubMed] [Google Scholar]
- 18.Laaribi AB, Zidi I, Hannachi N, Ben Yahia H, Chaouch H, Bortolotti D, Zidi N, Letaief A, Yacoub S, Boudabous A, et al. Association of an HLA-G 14-bp Insertion/Deletion polymorphism with high HBV replication in chronic hepatitis. J Viral Hepat. 2015;22:835–841. doi: 10.1111/jvh.12395. [DOI] [PubMed] [Google Scholar]
- 19.Thio CL, Mosbruger TL, Kaslow RA, Karp CL, Strathdee SA, Vlahov D, O'Brien SJ, Astemborski J, Thomas DL. Cytotoxic T-lymphocyte antigen 4 gene and recovery from hepatitis B virus infection. J Virol. 2004;78:11258–11262. doi: 10.1128/JVI.78.20.11258-11262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou J, Lu L, Yuen MF, Lam TW, Chung CP, Lam CL, Zhang B, Wang S, Chen Y, Wu SH, et al. Polymorphisms of type I interferon receptor 1 promoter and their effects on chronic hepatitis B virus infection. J Hepatol. 2007;46:198–205. doi: 10.1016/j.jhep.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Chong WP, To YF, Ip WK, Yuen MF, Poon TP, Wong WH, Lai CL, Lau YL. Mannose-binding lectin in chronic hepatitis B virus infection. Hepatology. 2005;42:1037–1045. doi: 10.1002/hep.20891. [DOI] [PubMed] [Google Scholar]
- 22.Cao T, Desombere I, Vanlandschoot P, Sällberg M, Leroux-Roels G. Characterization of HLA DR13-restricted CD4(+) T cell epitopes of hepatitis B core antigen associated with self-limited, acute hepatitis B. J Gen Virol. 2002;83:3023–3033. doi: 10.1099/0022-1317-83-12-3023. [DOI] [PubMed] [Google Scholar]
- 23.Battelino T, Ursic-Bratina N, Dolzan V, Stopar-Obreza M, Pozzilli P, Krzisnik C, Vidan-Jeras B. The HLA-DRB, -DQB polymorphism and anti-insulin antibody response in Slovenian patients with type 1 diabetes. Eur J Immunogenet. 2003;30:223–227. doi: 10.1046/j.1365-2370.2003.00395.x. [DOI] [PubMed] [Google Scholar]
- 24.Crespí C, Milà J, Martínez-Pomar N, Etxagibel A, Muñoz-Saa I, Priego D, Luque A, Pons J, Picornell A, Ramon M, et al. HLA polymorphism in a Majorcan population of Jewish descent: Comparison with Majorca, Minorca, Ibiza (Balearic Islands) and other Jewish communities. Tissue Antigens. 2002;60:282–291. doi: 10.1034/j.1399-0039.2002.600402.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen WN, Oon CJ. Mutation ‘hot spot’ in HLA class I-restricted T cell epitope on hepatitis B surface antigen in chronic carriers and hepatocellular carcinoma. Biochem Biophys Res Commun. 1999;262:757–761. doi: 10.1006/bbrc.1999.1267. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy PT, Sandalova E, Jo J, Gill U, Ushiro-Lumb I, Tan AT, Naik S, Foster GR, Bertoletti A. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology. 2012;143:637–645. doi: 10.1053/j.gastro.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Han Y, Jiang ZY, Jiao LX, Yao C, Lin QF, Ma N, Ju RQ, Yang F, Yu JH, Chen L. Association of human leukocyte antigen-DRB1 alleles with chronic hepatitis B virus infection in the Han Chinese of Northeast China. Mol Med Rep. 2012;5:1347–1351. doi: 10.3892/mmr.2012.800. [DOI] [PubMed] [Google Scholar]
- 28.Shi C, Qian YH, Su J, Luo SS, Gu J, You H, Cui Q, Lin YD, Dong MH, Yu RB. Genetic variation in the LMP/TAP gene and outcomes of hepatitis B virus infection in the Chinese population. Epidemiol Infect. 2011;139:674–682. doi: 10.1017/S0950268810001299. [DOI] [PubMed] [Google Scholar]
- 29.Kamatani Y, Wattanapokayakit S, Ochi H, Kawaguchi T, Takahashi A, Hosono N, Kubo M, Tsunoda T, Kamatani N, Kumada H, et al. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet. 2009;41:591–595. doi: 10.1038/ng.348. [DOI] [PubMed] [Google Scholar]
- 30.Ferstl B, Zacher T, Lauer B, Blagitko-Dorfs N, Carl A, Wassmuth R. Allele-specific quantification of HLA-DQB1 gene expression by real-time reverse transcriptase-polymerase chain reaction. Genes Immun. 2004;5:405–416. doi: 10.1038/sj.gene.6364108. [DOI] [PubMed] [Google Scholar]
- 31.Muro M, Herrero N, Marin L, Torío A, Minguela A, Sánchez-Bueno F, García-Alonso AM, Alvarez-López MR. Polymorphism in the upstream regulatory region of the HLA-DQB1 gene in liver graft recipients. Hum Biol. 2001;73:845–854. doi: 10.1353/hub.2001.0088. [DOI] [PubMed] [Google Scholar]
- 32.Mbarek H, Ochi H, Urabe Y, Kumar V, Kubo M, Hosono N, Takahashi A, Kamatani Y, Miki D, Abe H, et al. A genome-wide association study of chronic hepatitis B identified novel risk locus in a Japanese population. Hum Mol Genet. 2011;20:3884–3892. doi: 10.1093/hmg/ddr301. [DOI] [PubMed] [Google Scholar]
- 33.Meng XQ, Chen HG, Ma YL, Liu KZ. Influence of HLA class II molecules on the outcome of hepatitis B virus infection in population of Zhejiang Province in China. Hepatobiliary Pancreat Dis Int. 2003;2:230–233. [PubMed] [Google Scholar]
- 34.Jiang YG, Wang YM, Liu TH, Liu J. Association between HLA class II gene and susceptibility or resistance to chronic hepatitis B. World J Gastroenterol. 2003;9:2221–2225. doi: 10.3748/wjg.v9.i10.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Zhao F, Lan L, Qin Z, Jun L. Correlation of HLA-DQB1 gene polymorphism of Xinjiang Uygur with outcome of HBV infection. Int J Clin Exp Med. 2015;8:6067–6072. [PMC free article] [PubMed] [Google Scholar]
- 36.Yu ML, Dai CY, Chen SC, Chiu CC, Lee LP, Lin ZY, Hsieh MY, Wang LY, Chuang WL, Chang WY. Human leukocyte antigen class I and II alleles and response to interferon-alpha treatment, in Taiwanese patients with chronic hepatitis C virus infection. J Infect Dis. 2003;188:62–65. doi: 10.1086/375554. [DOI] [PubMed] [Google Scholar]
- 37.Li QJ LX, Zhang LY, Zhao FL. Correlation between polymorphisms in the human leukocyte antigen-DQB1 alleles and hepatitis B with primary hepatocellular carcinoma. Chin Zhonghua Gan Zang Bing Za Zhi. 2015;23:270–274. doi: 10.3760/cma.j.issn.1007-3418.2015.04.008. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Zhang H, Gu C, Yin J, He Y, Xie J, Cao G. Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: A meta-analysis. J Natl Cancer Inst. 2009;101:1066–1082. doi: 10.1093/jnci/djp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 40.Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. 2011;11:41. doi: 10.1186/1471-2288-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 42.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park MH, Song EY, Ahn C, Oh KH, Yang J, Kang SJ, Lee HS. Two subtypes of hepatitis B virus-associated glomerulonephritis are associated with different HLA-DR2 alleles in Koreans. Tissue Antigens. 2003;62:505–511. doi: 10.1046/j.1399-0039.2003.00141.x. [DOI] [PubMed] [Google Scholar]
- 44.Xi-Lin Z, Te D, Jun-Hong L, Liang-Ping L, Xin-Hui G, Ji-Rong G, Chun-Yan G, Zhuo L, Ying L, Hui L. Analysis of HLA-DQB1 gene polymorphisms in asymptomatic HBV carriers and chronic hepatitis B patients in the Chinese Han population. Int J Immunogenet. 2006;33:249–254. doi: 10.1111/j.1744-313X.2006.00607.x. [DOI] [PubMed] [Google Scholar]
- 45.Liu C, Cheng B. Association of polymorphisms of human leucocyte antigen-DQA1 and DQB1 alleles with chronic hepatitis B virus infection, liver cirrhosis and hepatocellular carcinoma in Chinese. Int J Immunogenet. 2007;34:373–378. doi: 10.1111/j.1744-313X.2007.00702.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhu XL, Du T, Li JH, Lu LP, Guo XH, Gao JR, Gou CY, Li Z, Liu Y, Li H. Association of HLA-DQB1 gene polymorphisms with outcomes of HBV infection in Chinese Han population. Swiss Med Wkly. 2007;137:114–120. doi: 10.4414/smw.2007.11428. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Jia J, Dong J, Yu F, Ma N, Li M, Liu X, Liu W, Li T, Liu D. HLA-DQ polymorphisms with HBV infection: Different outcomes upon infection and prognosis to lamivudine therapy. J Viral Hepat. 2014;21:491–498. doi: 10.1111/jvh.12159. [DOI] [PubMed] [Google Scholar]
- 48.Hong X, Yu RB, Sun NX, Wang B, Xu YC, Wu GL. Human leukocyte antigen class II DQB1*0301, DRB1*1101 alleles and spontaneous clearance of hepatitis C virus infection: A meta-analysis. World J Gastroenterol. 2005;11:7302–7307. doi: 10.3748/wjg.v11.i46.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yue M, Xu K, Wu MP, Han YP, Huang P, Peng ZH, Wang J, Su J, Yu RB, Li J, et al. Human leukocyte antigen class II alleles are associated with hepatitis C virus natural susceptibility in the Chinese population. Int J Mol Sci. 2015;16:16792–16805. doi: 10.3390/ijms160816792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castaño-Rodríguez N, Diaz-Gallo LM, Pineda-Tamayo R, Rojas-Villarraga A, Anaya JM. Meta-analysis of HLA-DRB1 and HLA-DQB1 polymorphisms in Latin American patients with systemic lupus erythematosus. Autoimmun Rev. 2008;7:322–330. doi: 10.1016/j.autrev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Laivoranta-Nyman S, Möttönen T, Hermann R, Tuokko J, Luukkainen R, Hakala M, Hannonen P, Korpela M, Yli-Kerttula U, Toivanen A, et al. FIN-RACo Trial Group: HLA-DR-DQ haplotypes and genotypes in Finnish patients with rheumatoid arthritis. Ann Rheum Dis. 2004;63:1406–1412. doi: 10.1136/ard.2003.009969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li ZK, Nie JJ, Li J, Zhuang H. The effect of HLA on immunological response to hepatitis B vaccine in healthy people: A meta-analysis. Vaccine. 2013;31:4355–4361. doi: 10.1016/j.vaccine.2013.06.108. [DOI] [PubMed] [Google Scholar]
- 53.Jiang DK, Ma XP, Yu H, Cao G, Ding DL, Chen H, Huang HX, Gao YZ, Wu XP, Long XD, et al. Genetic variants in five novel loci including CFB and CD40 predispose to chronic hepatitis B. Hepatology. 2015;62:118–128. doi: 10.1002/hep.27794. [DOI] [PubMed] [Google Scholar]