Abstract
The microenvironment of a tumour is an important factor in ovarian cancer metastasis. The present study aimed to simulate the in vivo microenvironment of an ovarian carcinoma using a co-culture system consisting of human lymphatic endothelial cells (HLECs) and human ovarian carcinoma cells with directional high lymphatic metastasis (SKOV3-PM4s) in order to investigate the role of both cell types in ovarian carcinoma metastasis. The SKOV3-PM4s cultured in the HLEC-conditioned medium exhibited increased numbers of pseudopodia and mitotic figures, proliferated at a faster rate and exhibited enhanced invasion and migratory abilities. Furthermore, the HLECs cultured in SKOV3-PM4-conditioned medium exhibited significant morphological alterations and vacuolisation of the cytoplasm, as well as increased invasion, migratory and tube forming abilities. In addition, spontaneous fusion of the SKOV3-PM4s and HLECs was observed in the co-culture system using laser confocal microscopy. The gelatin zymography assay demonstrated that matrix metalloproteinase-2, which was downregulated in the SKOV3-PM4s, was upregulated in the co-culture system. The results of the present study suggested that the invasion ability of the SKOV3-PM4s was increased in the in vitro co-culture system of SKOV3-PM4 and HLECs. Therefore, alterations in the cell microenvironment may represent a novel strategy for ovarian cancer therapy.
Keywords: human ovarian carcinoma cells, human lymphatic endothelial cells, microenvironment, co-culture, lymphangiogenesis
Introduction
Ovarian cancer is the leading cause of mortality, and its morbidity ranks third among gynecological malignancies (1). Tumour metastasis, which is the primary cause of cancer-associated mortality, has been closely associated with the tumour cell microenvironment. The survival of micrometastases at distal sites to the primary tumour requires an ability to adapt to different microenvironments (2). In 1889, Stephen Paget's ‘seed and soil’ hypothesis laid the foundation for the concept of the tumour microenvironment (3). In an expansion of this hypothesis, Paget accurately predicted that tumour cells act as ‘seeds’ and are able to settle in a suitable ‘soil’ and grow; when the soil is remote tissues and organs, tumour cells form synergistic interactions with their microenvironment (4). A tumour microenvironment refers to the environment that is created by the tumour and regulated by tumour-induced interactions (5). It includes tumour, stromal, immune, inflammatory and endothelial cells, the blood and lymphatic vascular networks, micro-lymphatics, interstitial fluid and cell factors of the tumour cell and its microenvironment that co-evolve through iterative interactions to contribute to tumour progression (6–9). A previous study demonstrated that the tumour microenvironment was associated with a more aggressive cancer phenotype, and that it has a role in tumour progression and metastatic disease (10). In addition, it has been suggested that an improved understanding of the cellular and molecular pathways that operate within the tumour microenvironment is required in order to develop strategies for inhibiting tumour metastasis. Therefore, in order to control malignancy, it may be necessary to control the modifications within the tumour microenvironment that initiate and promote the growth, invasion and spread of cancer cells (4,11,12).
Lymphangiogenesis has been shown to be an important cause of metastasis; the microenvironment provides various lymphangiogenic factors, including vascular endothelial growth factor (VEGF)-C and -D, which promote lymphatic vessel formation (13) and are important for lymph node metastasis and metastatic tumour spread (6,14). Previous studies reported that patients with oesophageal squamous carcinomas and adenocarcinomas are at a higher risk of lymphatic vessel invasion and lymph node metastasis when they have high tumour lymphatic vessel densities (15,16).
In order to investigate the effects of the microenvironment on the lymph node metastasis of malignancies, and the underlying mechanisms that lead to tumour metastasis via lymphatic vessels, the present study established a co-culture system consisting of human lymphatic endothelial cells (HLECs) and human ovarian carcinoma cells (SKOV3s) with directional high lymphatic metastasis (SKOV3-PM4s). The SKOV3-PM4s were obtained by injecting SKOV3s into nude mice to derive a fourth generation subcell line from metastatic lymph nodes. Alterations in the biological characteristics of each cell line in different microenvironments were observed, and involved cell interactive culture systems containing conditioned media from two types of cells and an in vitro cell co-culture system. The results of the present study provided a theoretical basis for the mechanisms underlying the lymph node metastasis of ovarian cancer.
Materials and methods
Cell lines and plasmids
Human SKOV3-PM4s, human HLECs and the lentiviral pCDH-COPGFP plasmid, were obtained from the Oncology Laboratory at the Experimental Center of Guangxi Medical University (Nanning, China).
Fluorescent-labelled cell lines
The pCDH-COPGFP plasmid with an encoded green fluorescent protein (GFP gene was transfected into the SKOV3-PM4s, and SKOV3-PM4s stably expressing GFP were obtained. A stock solution containing 20 mg/ml fluorescent membrane dye (DiI; Biotium, Inc., Hayward, CA, USA) in N,N-dimethylformamide (DMF; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) was prepared, and the HLECs were labelled with a final working dilution of 30 µg/ml DiI in phosphate-buffered saline (PBS; Beyotime Institute of Biotechnology, Haimen, China) solution.
Preparation of the conditioned culture media and the establishment of the interactive culture system
Fluorescent-labelled SKOV3-PM4s and HLECs were initially plated into 75-m2 culture flasks at a density of 2.5×105 cells/ml. The supernatants were collected after 48 h, and the cell debris were removed by centrifugation at 1,500 × g for 10 min at 4°C. The supernatants were filtered using 0.22-µm membranes (Beyotime Institute of Biotechnology) and stored at −20°C until required for further experimentation. The cells were divided into four groups, as follows: i) SKOV4-PM4s cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C with 5% CO2; ii) SKOV3-PM4s cultured in the supernatant from the HLECs at 37°C with 5% CO2; iii) HLECs cultured in endothelial cell medium (Sciencell Research Laboratories, Carlsbad, CA, USA) at 37°C with 5% CO2; and iv) HLECs cultured in the supernatant from SKOV3-PM4s.
Establishment of the co-culture system
Fluorescent-labelled SKOV3-PM4 and HLEC cells (1×105 cells/ml) were added to Transwell® plates (EMD Millipore, Billerica, MA, USA) and glass-bottomed petri dishes, respectively, at 200µl, thereby establishing the fluorescent-labelled SKOV3-PM4-HLEC cell co-culture system.
Observations of cellular morphology
For groups A, B, C and D, the cell suspensions were adjusted to a density of 1×104 cells/ml, and 2 ml cell suspension was added to petri dishes in which coverslips had been placed. The coverslips were removed following incubation in an atmosphere containing 5% CO2 for 24 h at 37°C, and were fixed with 95% ethanol for 30 min and rinsed twice with PBS for hematoxylin-eosin (HE; Beyotime Institute of Biotechnology) staining.
Observations of cell proliferation and metastatic abilities
In order to determine the cell mitotic index of the SKOV3-PM4s and HLECs, the number of cells in the mitotic phase were calculated under a light microscope (CKX41-A22PHP; Olympus Corporation, Tokyo, Japan), based on the appearance of moderate cellular densities (at least 1,000 cells were counted). The cell mitotic index was determined using the following equation: Cell mitotic index (%) = (Number of cells with mitotic figures/total number of cells counted) × 100.
In order to determine the cell proliferation rate of the SKOV3-PM4s and HLECs, cell suspensions of groups A, B, C and D were seeded in 96-well plates (1×104 cells/ml). Once daily, the cells from three wells of each group underwent the 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide colorimetric assay (Beijing Solarbio Science & Technology Co., Ltd.), and the results of these assays over seven consecutive days were used to draw cell growth curves.
The invasion ability of the SKOV3-PM4s and HLECs was assessed using the Matrigel Invasion Assay (Sigma-Aldrich, St. Louis, MO, USA). Briefly, cells in the logarithmic growth phase from each of the four groups were adjusted to densities of 5×104 cells/ml. Subsequently, 200-µl cell suspensions from each group was seeded into the upper chamber of the Transwell® plate, and the cell supernatants of the other cells were added to the lower chamber. The membrane filters were removed following incubation for 16 h at 37°C and 5% CO2. The Matrigel was wiped with a cotton swab, and the cells were fixed in methanol for 20 min prior to staining with HE.
The Transwell migration assay was used to determine the migratory ability of the SKOV3-PM4s and HLECs. The experimental procedure was identical to that performed for the Matrigel Invasion Assay, with the exception that the surface of the polycarbonate membrane of the upper chamber lacked Matrigel.
Vessel formation assay
Cells from groups C and D were collected at the logarithmic growth phase and adjusted to a density of 4×105 cells/ml. Subsequently, 50-µl cell suspensions from each group were seeded into a 24-well plate coated with Matrigel and incubated at 37°C in an atmosphere containing 5% CO2. The tube formation abilities were observed under the microscope (CKX41-A22PHP; Olympus Corporation) between days 4 and 9.
Observations of the cell interactions
Fluorescent-labelled SKOV3-PM4s and HLECs were adjusted to densities of 1×105 cells/ml. Each cell type was plated together in 35-mm glass-bottom petri dishes (Ibidi GmbH, Planegg, Germany) for confocal microscopy (Nikon, Tokyo, Japan) analyses. Cell-cell interactions were observed following co-culturing for 12, 24 and 48 h.
Gelatin zymography method
The cellular supernatants of the fluorescent-labelled SKOV3-PM4s, HLECs and co-cultured SKOV3-PM4/HLECs were collected and were cultured in serum-free medium for 24 h, followed by centrifugation at 1,500 × g for 10 min at 4°C to remove cell debris. The sample volumes of each group were adjusted according to protein concentrations, obtained using a BCA Protein Assay (Beijing Solarbio Science & Technology Co., Ltd.). Subsequently, the cell supernatants were mixed with loading buffer (Beijing Solarbio Science & Technology Co., Ltd.), and the proteins in the cell supernatants were separated by 10% sodium dodecyl sulfate-polyacrylamide (Beyotime Institute of Biotechnology) gel electrophoresis containing 1.0 mg/ml gelatin. The gel was placed in eluent (Beijing Solarbio Science & Technology Co., Ltd.) containing 2.5% Triton X-100, 50 mmol/l Tris-HCl, 5 mmol/l CaCl2 and 1 µmol/l ZnCl2 (pH 7.6), and was eluted twice by agitation for 45 min, followed by twice washing for 20 min with eluent lacking Triton X-100. The gel was then incubated in incubation buffer (Beijing Solarbio Science & Technology Co., Ltd.) containing 50 mmol/l Tris-HCl, 5 mmol/l CaCl2, 1 µmol/l ZnCl2 and 0.02% Brij-35 (pH 7.6) at 37°C for 42 h. The gel was then transferred to 30 ml developing buffer and incubated for 2 h. Following this, the developing buffer (Beijing Solarbio Science & Technology Co., Ltd.) was replaced with add Coomassie brilliant blue R250 staining solution (Beijing Solarbio Science & Technology Co., Ltd.) and incubated for 20–30 min at room temperature. The gel was scanned using a GS-710 Calibrated Imaging Densitometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and analyzed using Phoretix 1D Pro software (CSL-Cleaver Scientific, Rugby, UK). Negative staining bands on the blue background were observed.
Statistical analyses
Statistical analyses were conducted using the SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation of three replicate assays. The results of cell growth curves for the four groups were compared using one-way analysis of variance. The mitotic figures were compared using the χ2 test. P<0.05 was considered to indicate a statistically significant difference.
Results
Changes in cell morphology
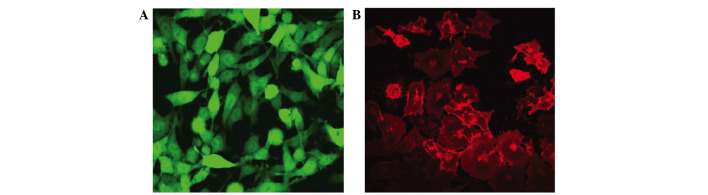
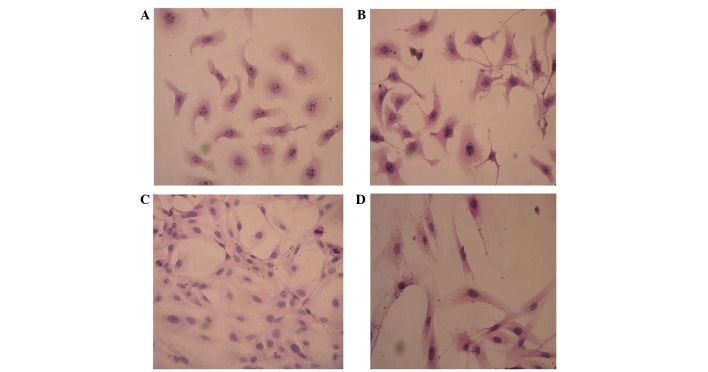
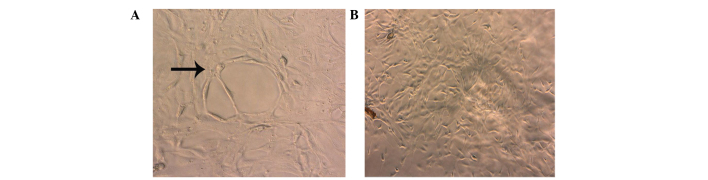
Stably expressing green fluorescent GFP-tagged SKOV3-PM4s and red fluorescent DiI-labelled HLECs were observed using an inverted fluorescence microscope (Fig. 1). The SKOV3-PM4s cultured in HLEC medium exhibited alterations in cell morphology, from immature round cells to multi-tentacle cells, and the nuclei increased in size and number. In addition, the number of pseudopodia and mitotic figures were increased (Fig. 2B). As compared with the HLECs cultured alone, the cell morphologies of the HLECs cultured in SKOV3-PM4 medium were oval with short spindles to fusiform and irregular polygon-shaped. Furthermore, the cells were larger, the nuclei were changed from small-to-large, and vacuoles were observed in the cells (Fig. 2D).
Figure 1.
Images of fluorescence-labeled cells were captured using an inverted fluorescent microscope (magnification, ×400). (A) Green fluorescent protein-tagged human ovarian carcinoma cells with directional high lymphatic metastasis. (B) Red fluorescent DiI-labelled human lymphatic endothelial cells.
Figure 2.
Cell morphologies were determined by staining with hematoxylin-eosin and observing under a microscope (magnification, ×400). Human ovarian carcinoma cells with directional high lymphatic metastasis (SKOV3-PM4s) were cultured in (A) normal medium or (B) the supernatant of human lymphatic endothelial cells (HLECs). The number of pseudopodia and mitotic figures were markedly increased in the SKOV3-PM4s cultured in the supernatant of HLECs, as compared with those cultured in normal medium. HLECs were cultured in (C) normal medium or (D) the supernatant of SKOV3-PM4s. HLECs cultured in the supernatant of SKOV3-PM4s were fusiform and irregular polygon-shaped in appearance, they were larger, the cell nuclei were changed from small-to-large and vacuoles were observed.
Comparisons of the mitotic indices and cell growth curves
The mitotic index is a measure of the percentage of cells undergoing mitosis. Mitosis is the division of somatic cells, in which the genetic information from one single cell is equally dispersed into two daughter cells (17). The mitotic indices of the SKOV3-PM4s cultured in the HLEC medium were significantly higher, as compared with those cultured in normal medium (P<0.05; Table I). The cell growth curves demonstrated that the growth rate of the SKOV3-PM4s cultured in the HLEC medium was accelerated, as compared with the growth of the SKOV3-PM4s grown in normal medium (Fig. 3). In addition, the growth rate of the HLEC cells cultured in the SKOV3-PM4s medium was increased marginally, as compared with the HLECs cultured in the normal medium (Fig. 3).
Table I.
Mitotic indexes of each group.
| Occurrence of mitosis | |||
|---|---|---|---|
| Group | Cell count | Cell count | Percentage (%) |
| SKOV3-PM4 | 1,071.33±56.72 | 53.67±2.08 | 5.01±0.17 |
| SKOV3-PM4 in HLEC medium | 1,045.00±65.85 | 86.00±2.00 | 8.23±0.34a |
| HLEC | 1,073.32±62.69 | 26.65±2.51 | 2.48±0.11 |
| HLEC in SKOV3-PM4 medium | 1,058.50±79.90 | 50.60±1.53 | 4.78±0.12b |
Data is presented as the mean + standard deviation (n=3).
P<0.05 vs. the SKOV3-PM4 group
P<0.05 vs. the HLEC group. SKOV3-PM4, human ovarian carcinoma cells with directional high lymphatic metastasis; HLEC, human lymphatic endothelial cells.
Figure 3.
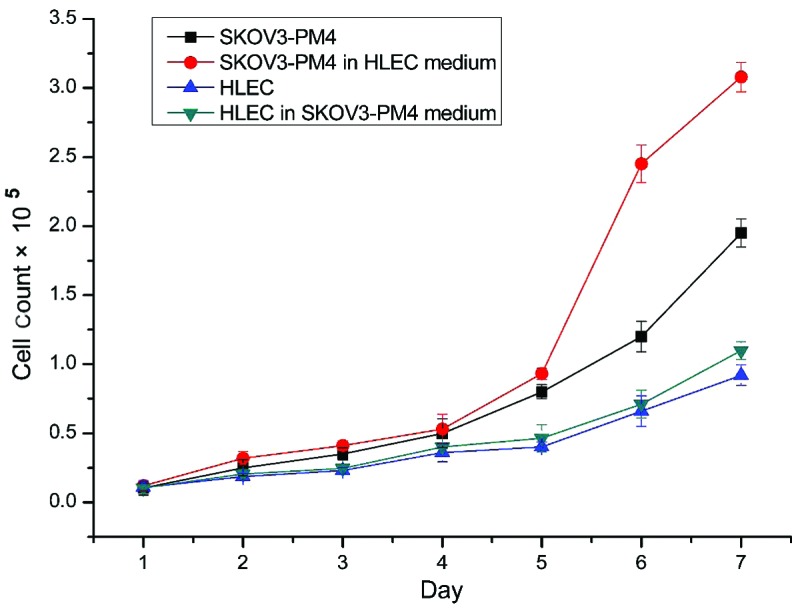
Cell growth curves for the four groups. The growth rate of SKOV3-PM4s cultured in HLEC medium was significantly increased, as compared with the growth rate of SKOV3-PM4s cultured in normal medium. Data are presented as the mean ± standard deviation (n=3). SKOV3-PM4, human ovarian carcinoma cells with directional high lymphatic metastasis; HLEC, human lymphatic endothelial cells.
HLECs promote the invasion and migration of ovarian cancer cells
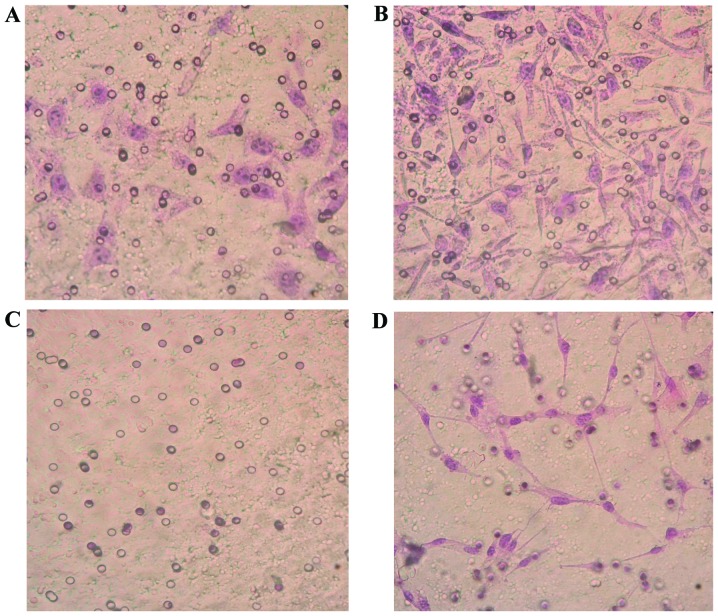
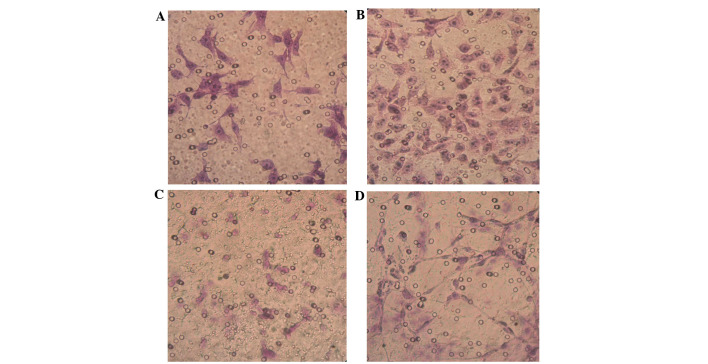
SKOV3-PM4s were highly invasive and metastatic when co-cultured with the HLECs for 3 days; thus suggesting that the highly invasive HLEC cells are able to induce the activation of SKOV3-PM4s. The invasion ability of the SKOV3-PM4s cultured in HLECs medium was significantly increased, as compared with the SKOV3-PM4s cultured in normal medium (P<0.05). The HLECs cultured in normal medium were unable to cross the Matrigel matrix membrane to any significant degree; however, the invasion ability of the HLECs cultured in SKOV3-PM4s medium was significantly increased (P<0.05; Fig. 4 and Table II). The migration assay demonstrated that the transmembrane cell count of the SKOV3-PM4s cultured in HLECs medium and the HLECs cultured in SKOV3-PM4s medium were significantly increased, as compared with the cells cultured in normal medium (P<0.05; Fig. 5 and Table III).
Figure 4.
Alterations in the invasion abilities of the human ovarian carcinoma cells with directional high lymphatic metastasis (SKOV3-PM4) and the human lymphatic endothelial cells (HLECs; hematoxylin-eosin staining; magnification, ×400). SKOV4-PM4s were cultured in (A) normal medium and (B) the supernatant of the HLECs. The invasion ability of the SKOV4-PM4s cultured in the supernatant of the HLECs were markedly increased, as compared with the SKOV4-PM4s cultured in normal medium. HLECs were cultured in (C) normal medium and (D) the supernatant of the SKOV3-PM4s. The invasion abilities of the HLECs cultured in the supernatant of the SKOV4-PM4s were markedly increased, as compared with the HLECs cultured in the normal medium.
Table II.
Invasion ability.
| Group | Transmembrane cell count |
|---|---|
| SKOV3-PM4 | 63.93±13.62 |
| SKOV3-PM4 in HLEC medium | 106.13±10.34a |
| HLEC | 0 |
| HLEC in SKOV3-PM4 medium | 32.33±4.76b |
Data is presented as the mean ± standard devation (n=3).
P<0.05 vs. the SKOV3-PM4 group
P<0.05 vs. the HLEC group. SKOV3-PM4, human ovarian carcinoma cells with directional high lymphatic metastasis; HLEC, human lymphatic endothelial cells.
Figure 5.
Alterations in the migratory abilities of the human ovarian carcinoma cells with directional high lymphatic metastasis (SKOV3-PM4) and the human lymphatic endothelial cells (HLECs; hematoxylin-eosin staining; magnification, ×400) ability. SKOV4-PM4s were cultured in (A) normal medium and (B) the supernatant of the HLECs. The migratory ability of the SKOV4-PM4s cultured in the supernatant of the HLECs were markedly increased, as compared with the SKOV4-PM4s cultured in normal medium. HLECs were cultured in (C) normal medium and (D) the supernatant of the SKOV3-PM4s. The invasion abilities of the HLECs cultured in the supernatant of the SKOV4-PM4s were markedly increased, as compared with the HLECs cultured in the normal medium.
Table III.
Migration ability.
| Group | Transmembrane cell count |
|---|---|
| SKOV3-PM4 | 78.07±6.08 |
| SKOV3-PM4 in HLEC medium | 110.27±10.92a |
| HLEC | 21.00±3.46 |
| HLEC in SKOV3-PM4 medium | 44.47±5.49b |
Data is presented as the mean ± standard deviation (n=3).
P<0.05 vs. the SKOV3-PM4 group
P<0.05 vs. the HLEC group. SKOV3-PM4, human ovarian carcinoma cells with directional high lymphatic metastasis; HLEC, human lymphatic endothelial cells.
Tube formation assay of the HLECs cultured in SKOV3-PM4s medium
The HLECs cultured in SKOV3-PM4s medium exhibited significantly enhanced tube formation abilities, as compared with the HLECs grown in the normal medium. In addition, after 4 days of culture, tubular networks were detected in the cultures containing HLECs and SKOV3-PM4 medium (Fig. 6A). Conversely, these tubular structures were not detected in the cultures containing HLECs and normal mediums (Fig. 6B).
Figure 6.
Tube forming abilities of human lymphatic endothelial cells cultured with (A) the supernatant of human ovarian carcinoma cells with directional high lymphatic metastasis (SKOV3-PM4) or (B) normal medium for 4 days. Tubular networks were formed by HLECs cultured with the supernatant of SKOV3-PM4s, whereas tubular structures were not obvious for HLECs cultured in normal medium.
Interactions of the co-culture cells
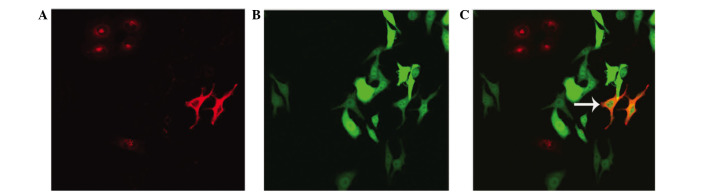
The co-cultured cells were observed by laser confocal microscopy over 48 h. The fluorescent-labelled SKOV3-PM4 and HLECs were co-cultured, and fusion phenomena were observed after 48 h. The fused cells are indicated by the arrows (Fig. 7).
Figure 7.
Fluorescent-labeled human ovarian carcinoma cells with directional high lymphatic metastasis (SKOV3-PM4) and human lymphatic endothelial cells (HLECs) were co-cultured for 48 h (magnification, ×400). (A) DiI-stained HLECs and (B) green fluorescent protein-labeled SKOV3-PM4. (C) Fused cells (white arrow) were observed after 48 h.
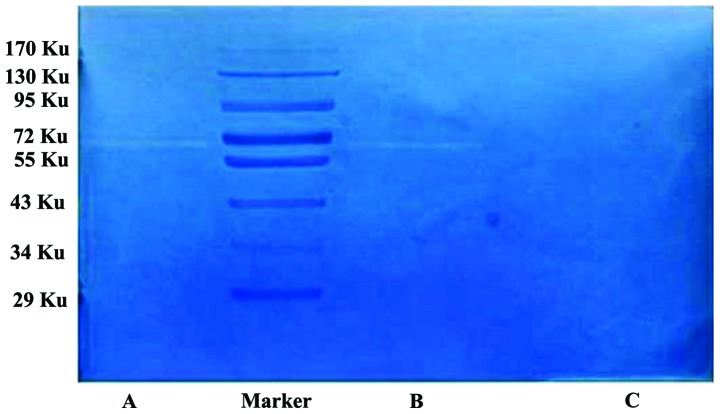
Gelatin is a substrate of MMPs. In the gelatin zymography assays, gelatin is hydrolysed by MMP-2 and MMP-9, and negative staining bands appear in the corresponding positions. Typically, MMP-2 appears at 72 KU, and MMP-9 appears at 92 KU. In the present study, the HLECs produced no significant bands at 72 KU, whereas the SKOV3-PM4s exhibited a clear band at 72 KU, and the co-cultured SKOV3-PM4s and HLECs produced a significant band at 72 KU, which was brighter and wider, as compared with that of the SKOV3-PM4s (Fig. 8).
Figure 8.
Enzymatic activity of matrix metalloproteinase-2 in each group. (A) The co-cultured human ovarian carcinoma cells with directional high lymphatic metastasis (SKOV3-PM4)-human lymphatic endothelial cells (HLECs) produced a brighter and wider band at 72 KU, as compared with. (B) the SKOV3-PM4 and (C) HLEC groups.
Discussion
Tumour metastasis, which is responsible for the majority of cancer-associated mortalities, is a continuous biological process involving numerous stages and factors, and the regulation of multiple genes (18,19). The spread of cancer cells to lymph nodes is an early event in metastasis, and is regularly used to predict disease outcome and to guide therapeutic strategies (20,21). Tumour-associated lymphatic vessels are a key component of metastatic spread (20). Lymphatic metastasis is the primary route for the metastasis of ovarian cancer (22). Previous studies demonstrated that lymphatic vessel neogenesis had a key role in the process of tumour lymph node metastasis (23,24). Lymphatic capillaries are lined by a single layer of endothelial cells, and a previous study reported that the lymphatic endothelium may provide a protective microenvironment for long-term tumour cell survival (23). Therefore, the present study used HLECs to investigate the molecular mechanisms underlying the interaction of tumour cells with the lymphatic endothelium. In the present study, HLECs that were cultured with the supernatants of SKOV3-PM4s exhibited numerous characteristics, including irregular nuclei and pseudopodia, that differed from those of their parent cells. These results suggested that the supernatant produced by cultured tumour cells was able to induce alterations in normal endothelial cells. This phenomenon may be associated with factors in the supernatant that promote lymphatic growth, including VEGF-C, which may combine with the VEGFR-3 receptors on lymphatic endothelial cells to promote their proliferation, differentiation, migration and lumen formation (24–26).
Previous studies reported that ovarian cancer metastasis is a non-random event that is dependent on the tumour microenvironment created by the tumour cells themselves, the specific tissue or organ, and the adaptation of the cells to the microenvironment (21,27). Peritumoural lymphatic vessels are predominantly responsible for promoting lymphatic cancer metastasis (21). The lymph of the tumour is drained and metastasised by lymph vessels or lymphangiogenesis, which is mediated by factors that promote lymphatic growth (28). The new vessels then increase the lymph tissue, which creates an ideal environment for tumour growth. Therefore, lymph vessel formation may be considered a critical aspect of tumour invasion and metastasis (20,21). Lymphatic endothelial cells surrounding the tumour secrete growth factors, adhesion factors and chemokines that promote tumour cellular proliferation, invasion and metastasis (29). In the present study, the SKOV3-PM4s that were cultured with the supernatant of the HLECs exhibited increased numbers of pseudopodia, greater alterations in cell morphology and motility, and enhanced invasion and migratory abilities.
In the present study, SKOV3-PM4s were transfected with a lentiviral pCDH-COPGFP plasmid, such that GFP was integrated into the SKOV3-PM4s, and the HLECs were labelled with DiI, which is a carbocyanine membrane dye that exhibits enhanced fluorescence upon insertion of its lipophilic hydrocarbon chains into the lipid membranes of cells. This enabled the entire cellular profile to be observed under an inverted fluorescence microscope. Under a laser confocal microscope, the spontaneous fusion produced by two types of cells was observed in the SKOV3-PM4 and HLEC co-culture system. This was consistent with a previous study (30) in which the co-culture of breast cancer cells and endothelial cells in vitro resulted in the spontaneous formation of fused cells that expressed parental genes and protein markers, and thus exhibited the biological characteristics of endothelial cells. These are involved in the degradation of the extracellular basement membrane and therefore the promotion of tumour metastasis (31). The present study hypothesized that the cytoplasmic connection between the tumour cells and endothelial cells may be a channel through which signal transduction or substance circulation is established, and that such connections are also the material basis of the changes in cell morphology in both cells.
The MMP family is a group of zinc-dependent proteolytic enzymes, of which MMP-2 is a glycoprotein that participates in the degradation of the extracellular matrix, thereby promoting tumour invasion and metastasis (32). As such, MMP-2 is an important marker of malignant tumour progression (33,34), and has been associated with lymphatic invasion and lymph node metastases (35,36). Previous studies demonstrated that high expression levels of MMP-2 were associated with the growth, invasion and metastasis of ovarian cancer, that the expression of the MMP-2 protein was associated with the progression of ovarian cancer, and that the survival rates of patients with ovarian cancer were correlated with MMP-2 expression (37,38). In the present study, MMP-2 secretion was significantly upregulated in the SKOV3-PM4-HLEC co-culture system, as compared with the individually cultured SKOV3-PM4s and HLECs. These results suggested that various autocrine and paracrine cytokines produced by the HLECs may have induced increases in the expression levels and secretion of MMP-2 by the SKOV3-PM4s. This in turn may have caused the tumour cells to colonize and metastasize.
In conclusion, the results of the present study suggested that lymphatic endothelial cells cultured in the supernatants of tumour cells were altered, as compared with normal lymphatic endothelial cells. In addition, the supernatants of the HLECs enhanced the invasion and migratory abilities of the SKOV3-PM4s. These results suggested that tumour cells exposed to the actions of lymphatic endothelial cells may be more likely to metastasize in vivo. Therefore, by simulating the growth-inducing microenvironment of human ovarian carcinoma cells in vitro by using SKOV3-PM4s and HLECs, the present study was able to employ genomic and proteomic technology to identify the key molecules in the tumour microenvironment. Furthermore, the present study may have elucidated in part the molecular mechanisms underlying ovarian cancer metastasis and the formation of tumour-adjacent lymphatic endothelial cells. The results of the present study may be considered useful for the development of novel therapeutic strategies that block cancer metastasis.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81060218 and 81360502) and the Guangxi Natural Science Foundation (grant nos. 2012GXNSFAA053157 and 2014GXNSFAA118161).
Glossary
Abbreviations
- HLEC
lymphatic endothelial cells
- SKOV3-PM4
human ovarian carcinoma cells with directional high lymphatic metastasis
References
- 1.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood SL, Pernemalm M, Crosbie PA, Whetton AD. The role of the tumour-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev. 2014;40:558–566. doi: 10.1016/j.ctrv.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. doi: 10.1016/S0140-6736(00)49915-0. [DOI] [PubMed] [Google Scholar]
- 4.Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 5.Whiteside TL. The tumour microenvironment and its role in promoting tumour growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorusso G, Rüegg C. The tumour microenvironment and its contribution to tumour evolution toward metastasis. Histochem Cell Biol. 2008;130:1091–1103. doi: 10.1007/s00418-008-0530-8. [DOI] [PubMed] [Google Scholar]
- 7.Joyce JA. Therapeutic targeting of the tumour microenvironment. Cancer Cell. 2005;7:513–520. doi: 10.1016/j.ccr.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Berns A, Pandolfi PP. Tumour microenvironment revisited. EMBO Rep. 2014;15:458–459. doi: 10.1002/embr.201438794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumour microenvironment. J Clin Invest. 2011;121:3804–3809. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brauer HA, Makowski L, Hoadley KA, Casbas-Hernandez P, Lang LJ, Romàn-Pèrez E, D'arcy M, Freemerman AJ, Perou CM, Troester MA. Impact of tumor microenvironment and epithelial phenotypes on metabolism in breast cancer. Clin Cancer Res. 2013;19:571–585. doi: 10.1158/1078-0432.CCR-12-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lunt SJ, Chaudary N, Hill RP. The tumour microenvironment and metastatic disease. Clin Exp Metastas. 2009;26:19–34. doi: 10.1007/s10585-008-9182-2. [DOI] [PubMed] [Google Scholar]
- 12.Salo T, Vered M, Bello IO, Nyberg P, Bitu CC, Hurvitz Zlotogorski A, Dayan D. Insights into the role of components of the tumour microenvironment in oral carcinoma call for new therapeutic approaches. Exp cell Res. 2014;325:58–64. doi: 10.1016/j.yexcr.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. doi: 10.1016/S1535-6108(02)00051-X. [DOI] [PubMed] [Google Scholar]
- 14.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 15.Schoppmann SF, Jesch B, Zacherl J, Riegler MF, Friedrich J, Birner P. Lymphangiogenesis and lymphovascular invasion diminishes prognosis in esophageal cancer. Surgery. 2013;153:526–534. doi: 10.1016/j.surg.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Dutta S, Going J, Crumley A, Mohammed Z, Orange C, Edwards J, Fullarton G, Horgan P, McMillan D. The relationship between tumour necrosis, tumour proliferation, local and systemic inflammation, microvessel density and survival in patients undergoing potentially curative resection of oesophageal adenocarcinoma. Br J Cancer. 2012;106:702–710. doi: 10.1038/bjc.2011.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzur A, Kafri R, LeBleu VS, Lahav G, Kirschner MW. Cell growth and size homeostasis in proliferating animal cells. Science. 2009;325:167–171. doi: 10.1126/science.1174294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steeg PS. Tumour metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 19.Valastyan S, Weinberg RA. Tumour metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pepper MS. Lymphangiogenesis and tumour metastasis: Myth or reality? Clin Cancer Res. 2001;7:462–468. [PubMed] [Google Scholar]
- 21.Tobler NE, Detmar M. Tumour and lymph node lymphangiogenesis-impact on cancer metastasis. J Leukocyte Biol. 2006;80:691–696. doi: 10.1189/jlb.1105653. [DOI] [PubMed] [Google Scholar]
- 22.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest. 2014;124:922–928. doi: 10.1172/JCI71606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pepper MS, Tille JC, Nisato R, Skobe M. Lymphangiogenesis and tumour metastasis. Cell Tissue Res. 2003;314:167–177. doi: 10.1007/s00441-003-0748-7. [DOI] [PubMed] [Google Scholar]
- 25.He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumour lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 26.He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T, Alitalo K. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumour cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–4746. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- 27.White EA, Kenny HA, Lengyel E. Three-dimensional modeling of ovarian cancer. Adv Drug Deliv Rev. 2014;79:184–192. doi: 10.1016/j.addr.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest. 2014;124:878–887. doi: 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomes FG, Nedel F, Alves AM, Nör JE, Tarquinio SBC. Tumor angiogenesis and lymphangiogenesis: tumor/endothelial crosstalk and cellular/microenvironmental signaling mechanisms. Life Sci. 2013;92:101–107. doi: 10.1016/j.lfs.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortensen K, Lichtenberg J, Thomsen PD, Larsson L. Spontaneous fusion between cancer cells and endothelial cells. Cell Mol Life Sci. 2004;61:2125–2131. doi: 10.1007/s00018-004-4200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han R, Clark C, Black A, French A, Culshaw G, Kempson S, Corcoran B. Morphological changes to endothelial and interstitial cells and to the extra-cellular matrix in canine myxomatous mitral valve disease (endocardiosis) Vet J. 2013;197:388–394. doi: 10.1016/j.tvjl.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Moss LAS, Jensen-Taubman S, Stetler-Stevenson WG. Matrix metalloproteinases: Changing roles in tumor progression and metastasis. Am J Pathol. 2012;181:1895–1899. doi: 10.1016/j.ajpath.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koyama S. Enhanced cell surface expression of matrix metalloproteinases and their inhibitors and tumour-induced host response in progression of human gastric carcinoma. Digest Dis Sci. 2004;49:1621–1630. doi: 10.1023/B:DDAS.0000043375.35611.dd. [DOI] [PubMed] [Google Scholar]
- 34.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Regulators of the tumour microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langenskiöld M, Holmdahl L, Falk P, Ivarsson M. Increased plasma MMP-2 protein expression in lymph node-positive patients with colorectal cancer. Int J Colorectal Dis. 2005;20:245–252. doi: 10.1007/s00384-004-0667-4. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura ES, Koizumi K, Kobayashi M, Saiki I. Inhibition of lymphangiogenesis-related properties of murine lymphatic endothelial cells and lymph node metastasis of lung cancer by the matrix metalloproteinase inhibitor MMI270. Cancer Sci. 2004;95:25–31. doi: 10.1111/j.1349-7006.2004.tb03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuecheng Y, Xiaoyan X. Stromal-cell derived factor-1 regulates epithelial ovarian cancer cell invasion by activating matrix metalloproteinase-9 and matrix metalloproteinase-2. Eur J Cancer Prev. 2007;16:430–435. doi: 10.1097/01.cej.0000236259.88146.a4. [DOI] [PubMed] [Google Scholar]
- 38.Huang KJ, Sui LH. The relevance and role of vascular endothelial growth factor C, matrix metalloproteinase-2 and E-cadherin in epithelial ovarian cancer. Med Oncol. 2012;29:318–323. doi: 10.1007/s12032-010-9817-4. [DOI] [PubMed] [Google Scholar]