Abstract
The aim of the present study was to investigate the effect of penehyclidine (PHC) on endotoxin-induced acute lung injury (ALI), as well as to examine the mechanism underlying this effect. A total of 60 rats were randomly divided into five groups, including the control (saline), LPS and three LPS + PHC groups. ALI was induced in the rats by injection of 8 mg lipopolysaccharide (LPS)/kg body weight. The rats were then treated with or without PHC at 0.3, 1 or 3 mg/kg body weight 1 min following LPS injection. After 6 h, serum levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 were determined by ELISA. In addition, the mRNA expression levels of toll-like receptor (TLR)2 and TLR4 were examined by reverse transcription-quantitative polymerase chain reaction in the lung tissue samples, and nuclear factor (NF)-κB p65 protein expression levels were examined by western blot analysis. The results demonstrated that lung injury was ameliorated by treatment with PHC (1 and 3 mg/kg body weight) as compared with treatment with LPS alone. Injection of LPS significantly increased the mRNA expression levels of TLR2 and TLR4, as well as the protein expression levels of NF-κB p65 in the lung tissue samples. Serum levels of TNF-α and IL-6 were also upregulated by LPS injection. Treatment of the rats with PHC following LPS injection suppressed the LPS-induced increase in TLR2/4 mRNA and NF-κB p65 protein expression levels. PHC also inhibited the increase in TNF-α and IL-6 serum levels. In addition, PHC reduced LPS-induced ALI and decreased the serum levels of TNF-α and IL-6, possibly by downregulating TLR2/4 mRNA expression and inhibiting NF-κB activity, and consequently alleviating the inflammatory response.
Keywords: penehyclidine, endotoxin, toll-like receptor 2/4, nuclear factor-κB, inflammatory response
Introduction
Acute lung injury (ALI) is characterized by damage to the alveolar epithelial and capillary endothelial cells (1). ALI can be induced by various factors, such as pulmonary alveolar hemorrhage, shock and transfusion, resulting in diffuse pulmonary interstitial and alveolar edema, refractory hypoxemia and respiratory distress (2–4). Although the pathogenesis of ALI remains unclear, Toll-like receptor (TLR)-mediated systemic inflammatory response may be involved (5–8).
TLRs are a class of cellular receptors that recognize pathogen-associated molecular patterns in innate immunity. TLRs associate the innate and adaptive immunity (9). The activation of TLR2 and TLR4 signaling cascades leads to the production of cytokines and co-stimulatory factors (9,10). The release of inflammatory cytokines by various effector cells is an important factor contributing to ALI (11). Ligands bind TLR4, which in turn activates nuclear factor (NF)-κB through a myeloid differentiation protein 88 (MyD88)-dependent signaling pathway. This ultimately stimulates the expression of pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-α, which leads to pathological changes in ALI (12).
Anticholinergic drugs are an effective and widely accepted treatment for ALI (13,14). They decrease pro-inflammatory effects by resisting M cholinergic function, inhibiting choline and preventing the release of 5-hydroxytryptamine from epithelial cells and of neutrophilic chemotactic factor from lung alveolus macrophages (15). Anisodamine treatment of lipopolysaccharide (LPS)-induced ALI in rats has been found to inhibit the expression of pro-inflammatory cytokines, including TNF-α and IL-8 (16). Penehyclidine (PHC) is a novel anticholinergic agent that selectively acts on receptors of M1, M3, ganglion and central N1 cells to decrease the TNF-α expression levels and NF-κB activity, inhibiting the inflammatory response (17). Furthermore, PHC treatment decreased TNF-α and IL-6 expression levels and suppressed the inflammatory response following extracorporeal circulation (18). However, the mechanism underlying the effects of PHC treatment on the inflammatory response remains unclear. To the best of our knowledge, few studies have investigated the effect of PHC on endotoxin-induced ALI (19,20). Therefore, the aim of the present study was to investigate whether PHC treatment was able to alleviate ALI in rats. The expression levels of TLR2/4 and NF-κB activation were also examined in the lung tissue.
Materials and methods
Animals and reagents
A total of 60 Sprague-Dawley rats (age, 8–10 weeks; weight, 200–220 g) were used in the present study (Identification Institute of Pharmaceutical and Biological Products of Beijing, Beijing, China). Rats were maintained in the animal laboratory at the Tuberculosis and Thoracic Tumor Research Institute (Beijing, China) at room temperature (16–26°C) and relative humidity (60%)with a 12:12 h light/dark cycle. A total of 12 100% fresh air changes were made per hour. Rats had ad libitum access to a standard diet and water. All experimental procedures in the present study adhered to the Principles of Experimental Animal Care and Use in accordance with the guidelines of the Helsinki Declaration and was approved by the Ethics Committee of the Beijing Chest Hospital, Capital Medical University. Ethical approval was obtained from the Institutional Review Board and Ethics Committee of the Tuberculosis and Thoracic Tumor Research Institute. LPS was purchased from Sigma-Aldrich (St. Louis, MO, USA; cat. no. L2880). PHC injection solution was purchased from Chengdu Lisite Pharmaceutical, Co., Ltd. (Chengdu, China; batch, H20020606). Rat TNF-α and IL-6 ELISA kits were purchased from Sigma-Aldrich.
Animal models and grouping
Rats were randomly divided into five equal groups (n=12) and intraperitoneally injected with 2 ml 0.9% saline (control group), 2 ml 0.9% saline containing LPS (8 mg/kg body weight; LPS-alone group), or three groups injected with 2 ml 0.9% saline containing LPS (8 mg/kg body weight) and different PHC concentrations (0.3, 1, or 3 mg/kg body weight). PHC was administered 1 min following the LPS injection. At 6 h after drug administration, the rats were sacrificed via cervical dislocation following anesthetization via intraperitoneal injection of sodium pentobarbital (35 mg/kg body weight; Tianjin Jinyao Amino Acid Co., Ltd., Beijing, China). Whole blood (1 ml) was harvested from the ventricles and placed into disposable vacuum vessels to measure the serum levels of TNF-α and IL-6. Left lung tissue samples were obtained, homogenized, snap-frozen and stored in liquid nitrogen until further use. The remaining lung tissue samples were fixed in 10% formaldehyde solution.
ELISA
The levels of TNF-α and IL-6 in the blood samples were detected using ELISA kits according to the manufacturer's protocol. Briefly, 100 µl serum was added to a 96-well plate pre-coated with TNF-α or IL-6 antibody and incubated at 4°C overnight. Following washing three times with washing buffer, 100 µl 1x biotinylated detection antibody was added to each well and incubated at room temperature for 1 h with gentle shaking, following with incubation with 100 µl horseradish peroxidase (HRP) conjugated-streptavidin solution for 45 min at room temperature. Subsequently, the plate was treated with 100 µl 3,3′,5,5′-tetramethylbenzidine substrate, incubated for 30 min at room temperature and then the reaction was terminated with 50 µl Stop solution per well. Immediately, the plate was read at 450 nm using a microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Western blot analysis of NF-κB p65 expression
Nuclear extracts of the left lung tissue samples (0.2 g) were extracted using buffer A [10 mM HEPES pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol (DTT), 0.4 mM phenylmethanesulfonyl fluoride (PMSF)] and buffer B (20 mM HEPES pH 7.9, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM DTT, 0.4 mM PMSF, 10 µg/ml leupeptin, 1 µg/ml aprotinin, 25% glycerol). Nucleoprotein concentrations were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and adjusted to 0.2 µg/μl. Equal amounts (20 µg) of protein samples were resolved by 4–12% SDS-PAGE, and transferred to nitrocellulose membranes (Beijing Dingguo Biological Technology Co., Ltd., Beijing, China). Following blocking with 5% milk/Tris-buffered saline with Tween 20 (TBST; Sigma-Aldrich) at room temperature for 1 h, the membranes were incubated at 4°C overnight with rat anti-NF-κB p65 polyclonal antibody (1:300; BA0610) in 5% milk/TBST, then incubated with HRP-conjugated goat anti-rabbit immunoglobulin G (1:4,000; BA1054; both Wuhan Boster Biological Technology, Ltd., Wuhan, China) in 5% milk/TBST. Blots were analyzed using a gel imager (Gel Doc XR; Bio-Rad Laboratories, Inc., Hercules, CA, USA). Band Leader 3.0 software (Magnitec, Ltd., Tel Aviv, Israel) was used for semi-quantitative analysis of protein expression.
Reverse transcription-quantitative polymerase chain reaction (PCR) analysis of TLR2/4 expression in the lung tissue samples
A total of 100 mg left lung tissue sample was lysed with TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.,) and RNA was extracted according to the manufacturer's protocol. An equal amount of total RNA (1 µg) was used for each sample to synthesize cDNA, prior to PCR analysis. The following primers were designed using Primer 3 software and synthesized by SBS Genetech Co., Ltd., (Shanghai, China): TLR4, forward 5′-GGCATGGCATGGCTTAAACC-3′ and reverse 5′-AATTCTCCCAAGATCAACCGATG-3′; TLR2, forward 5′-GGGCTGACCTCTCTCAACGA-3′ and reverse 5′-CAGTTCCAAGATGTAACGCAACA-3′; and β-actin, forward 5′-ACTGCCGCATCCTCTTCCTC-3′ and reverse 5′-AAGCATTTGCGGTGCACGA-3′. Thermal cycling was performed using SYBR Green PCR Master Mix on a MyCycler (both Bio-Rad Laboratories, Inc.) as follows: TLR4, 95°C for 2 min, followed by 30 cycles of 95°C for 1 min, 59°C for 1 min and 72°C for 45 sec, with a final hold at 72°C for 10 min; TLR2, 95°C for 2 min, followed by 30 cycles of 95°C for 1 min, 60°C for 1 min and 72°C for 45 sec, with a final hold at 72°C for 10 min; and β-actin: 95°C for 2 min, followed by 28 cycles of 95°C for 1 min, 59°C for 1 min and 72°C for 45 sec, with a final hold at 72°C for 7 min. Amplification products were resolved by 2% agarose gel electrophoresis, and imaged using a Gel Doc EZ imaging system, and analyzed using GIS gel analysis software (Gel Doc XR; Bio-Rad Laboratories, Inc.). TLR2 and TLR4 mRNA expression levels were normalized to those of β-actin.
Examination of lung tissue pathology
Left lung tissue samples were obtained and embedded in paraffin wax, sectioned (0.5×0.5 cm), and then stained with hematoxylin and eosin (Beijing Dingguo Biological Technology Co., Ltd.). The tissue was analyzed using a light microscope (DM4; Leica Microsystems GmbH, Wetzlar, Germany) at ×400 magnification. Smith-scoring (21) was performed on the tissue samples. Lung pathology was assessed for edema and alveolar and interstitial inflammation and hemorrhage scored on a 0- to 4-point scale: 0, no injury; 1, injury in 25% of the field; 2, injury in 50% of the field; 3, injury in 75% of the field; and 4, injury throughout the field (>75%) (21).
Statistical analysis
All values are expressed as the mean ± standard deviation. One-way analysis of variance was performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant result.
Results
LPS-induced endotoxemia in rats
LPS-induced endotoxemia rat models are recognized as valid experimental models for clinical and experimental applications (22). In the ALI model established in the present study, rats injected with LPS exhibited mental fatigue, curling-up behavior, a delayed response, ocular discharge, and steady breathing compared with the control rats. Pathological analysis demonstrated that the lung tissue samples in the LPS group contained lesions, vascular engorgements and alveolar epithelial cells with edema (Fig. 1). Lymphocyte and neutrophil infiltration was observed in the alveolar wall, peribronchiolar and interstitial lungs, as well as serous effusion and extravasated red cells in the alveolar cavities. These data indicate that a rat model of endotoxemia was successfully established.
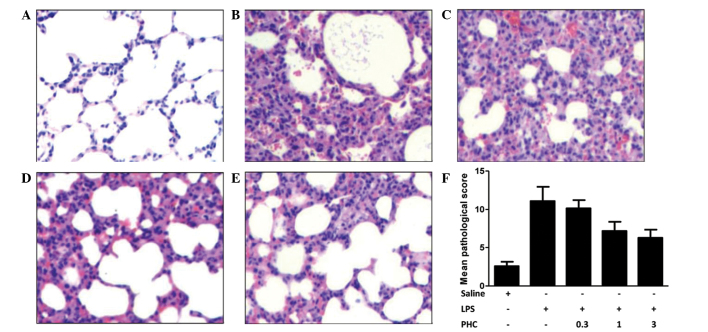
Figure 1.
Histology and pathological scoring of the left lung tissue samples in LPS-treated rats with or without PHC treatment. Rats were treated with 8 mg LPS/kg body weight for 1 min prior to treatment with the indicated concentrations of PHC (mg/kg body weight). After 6 h of treatment, the left lung tissue samples were harvested and stained with hematoxylin and eosin. The tissue sections were analyzed by light microscopy (magnification, ×400). (A) Control group; (B) LPS-only group; (C) 0.3, (D) 1 and (E) 3 mg PHC/kg body weight + LPS groups. (F) Pathology of the left lung tissue samples was scored using Smith-scoring. Data are presented as the mean ± standard deviation. *P<0.05 vs. the saline control; #P<0.05 vs. the LPS-treated groups. TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; LPS, lipopolysaccharide; PHC, penehyclidine.
Smith-scoring and examination of lung tissue pathology
Tissue analysis was performed using a light microscope (magnification, ×400). The pulmonary morphology was normal in the control group (Fig. 1A). By contrast, pulmonary consolidations, vascular engorgements, alveolar epithelial cells with edema, lymphocyte and neutrophil infiltration in the alveolar wall, peribronchiolar and interstitial lungs, serous effusion and extravasated red cells in the alveolar cavities were observed in the LPS-treated group (Fig. 1B). Lung tissue inflammatory changes, inflammatory cell infiltration, widening of the alveolar septum and hyperemia were observed in the rats treated with high doses of PHC (1 and 3 mg/kg body weight); however, there was no serous effusion or extravasated red cells in the alveolar space (Fig. 1C–E). Low dose PHC (0.3 mg/kg body weight) had no significant effect. The Smith-scores in all of the LPS-induced groups were increased compared with the control group (P<0.05; Fig. 1F), which indicated edema of lung epithelial cells and infiltration of lymphocytes and neutrophils into the lung parenchyma (1,21). Scores in the PHC-treated groups (1 and 3 mg/kg body weight) were decreased compared with the LPS group (P<0.05).
Effects of PHC treatment on TNF-α and IL-6 expression levels in the endotoxemia rat model
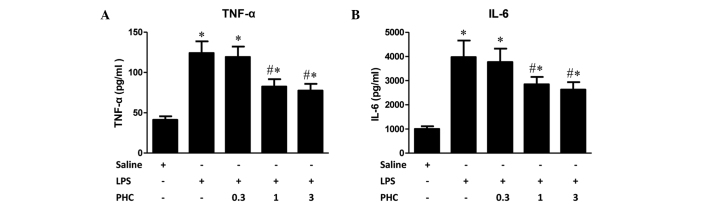
LPS markedly increased the expression levels of TNF-α, which is primarily produced by monocytes and macrophages. Following the intraperitoneal injection of LPS, TNF-α is the first cytokine to increase to detectable levels (11). IL-6, which is primarily generated by T cells, endothelial cells and monocytes, mediates acute responses contributing to lung injury during systemic inflammatory response syndrome (SIRS). Trauma, shock, infection and surgical procedures have been shown to increase IL-6 expression levels (18). Increased expression levels of IL-6 activate complement activity and C-reactive protein, causing cellular damage, adhesion factor production, and activation of glial cells, vascular endothelial cells and lymphocytes, leading to an aggravated inflammatory response (23). In the present study, serum levels of TNF-α and IL-6 significantly increased following intraperitoneal injection of LPS compared with the levels in the control rats. PHC treatment at doses of 1 and 3 mg/kg body weight following LPS injection resulted in decreased serum levels of TNF-α and IL-6 compared with the group treated with LPS alone (P<0.05; Fig. 2); whereas 0.3 mg/kg PHC had no significant effect.
Figure 2.
Effect of PHC on LPS-induced expression of inflammatory cytokines. Rats were treated with 8 mg LPS/kg body weight for 1 min prior to treatment with the indicated concentrations of PHC (mg/kg body weight). After 6 h of treatment, the blood was collected and the serum levels of (A) TNF-α and (B) IL-6 were measured by ELISA. Data were expressed as the mean ± standard deviation (n=12). *P<0.05 vs. the saline control; #P<0.05 vs. the LPS-treated groups. TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; LPS, lipopolysaccharide; PHC, penehyclidine.
TLR2/4 mRNA expression levels in the lung tissue of LPS-induced rats
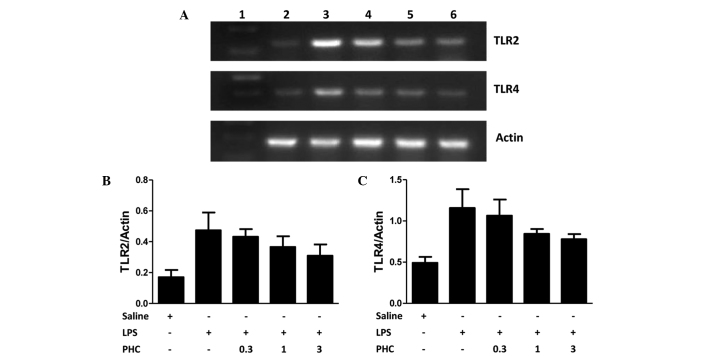
TLR2 and TLR4 mRNA expression levels in the lung tissue samples were increased in the LPS-induced rats compared with the levels in the control group (P<0.05; Fig. 3). PHC treatment significantly decreased TLR2 and TLR4 mRNA expression levels in lung tissue samples at a dose of 1 and 3 mg PHC/kg body weight, although no statistically significant difference was observed between the rats treated with 0.3 mg PHC/kg body weight and those treated with LPS alone (Fig. 3).
Figure 3.
(A) TLR2 and TLR4 mRNA expression in the left lung tissue samples of LPS-treated rats, as determined using reverse transcription-quantitative polymerase chain reaction and agarose gel electrophoresis. β-actin was used as the loading control. (B) TLR2 and (C) TLR4 mRNA expression levels, quantified from the gels based on the average absorbance values of the bands. TLR mRNA expression levels are presented as the mean ± standard deviation of TLR/β-actin for each sample. Groups: Lane 1, marker; lane 2, control (saline-treated); lane 3, LPS only; lane 4, 0.3 mg PHC/kg body weight + LPS; lane 5, 1 mg PHC/kg body weight + LPS; lane 6, 3 mg PHC/kg body weight + LPS. *P<0.05 vs. the saline control; #P<0.05 vs. the LPS-treated groups. TLR2/4, toll-like receptor 2/4; LPS, lipopolysaccharide; PHC, penehyclidine.
Effects of PHC treatment on nuclear NF-κB p65 expression levels in endotoxemic rats
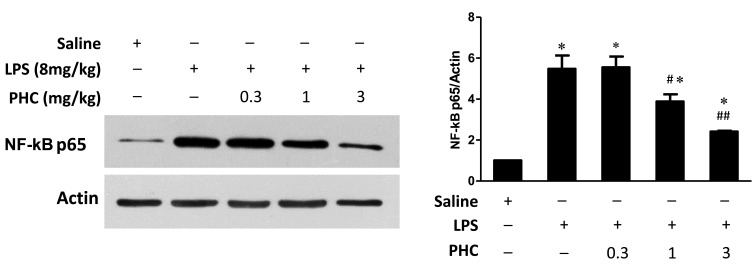
Cellular inflammatory signaling pathways may be regulated by activated nuclear factors. In particular, NF-κB is an important nuclear transcription factor during this process. NF-κB mediates various inflammatory cellular events, including immune cell activation, T and B lymphocyte development, stress response and apoptosis (24). In the current study, NF-κB p65 protein expression levels were increased in the lung tissue samples in the LPS group, compared with those in the control group (Fig. 4). However, PHC treatment resulted in a dose-dependent decrease in NF-κB p65 expression levels in lung tissue samples, as compared with the LPS group (1 and 3 mg/kg groups, P<0.05).
Figure 4.
NF-κB p65 expression in the left lung tissue samples of LPS-treated rats. Rats were treated with 8 mg/kg body weight LPS for 1 min prior to treatment with the indicated concentrations of PHC (mg/kg body weight) for 6 h. Western blot analysis was performed using NF-κB p65 and β-actin, which was used as the internal control. Treatment with 1 and 3 mg/kg LPS significantly attenuated the LPS-induced upregulation of NF-κB p65 expression. *P<0.05 vs. the saline control #P<0.05 and ##P<0.01 vs. the LPS-treated groups. NF-κB, nuclear factor-κB; PHC, penehyclidine; TLR2/4, toll-like receptor 2/4.
Discussion
Cytokine release in SIRS is involved in the pathogenesis of ALI (25). The response to intravenous or intraperitoneal injection of LPS is similar to the response to endotoxin release in severe gram-negative bacterial infection, which triggers the inflammatory response (26). In the present study, intraperitoneal injection of LPS was used to establish a rat model of endotoxemia.
TLRs mediate the innate immunity and play important roles in inflammation, signal transduction, apoptosis and tumorigenesis (27). As a specific receptor of LPS, TLR4 transmits the inflammatory signal induced by LPS (28). The LPS-binding protein (LBP) is a plasma protein that is synthesized and secreted by the liver. When LBP binds lipid A in LPS, a CD14-LPS complex forms. LBP is involved in phospholipid transfer, and disassociates LPS from its oligomeric state into monomers (29). Depolymerized LPS binds to TLR4, leading to polymerization and activation of TLR4.
MD-2 is a secretory protein lacking a transmembrane region that assists in the binding of the cytoplasmic tail of TRL4 to the TLR4 receptor-binding domain of the MyD88 adaptor protein (30). MyD88 binds IL-1 receptor-associated kinase (IRAK) via its death domain. IRAK is autophosphorylated, and TNF-α receptor-associated factor-6 (TRAF-6) is activated. Subsequent activation of the mitogen-binding protein kinase family leads to the activation of NF-κB-inducing kinase (NIK) (31). IκB kinase is activated and phosphorylated by NIK, leading to its degradation and NF-κB release. NF-κB then translocates to the nucleus to initiate transcription (32). TRAF-6 also activates mitogen-activated protein kinase kinase kinase (MAPKKK), MAPKK, extracellular signal-regulated kinase, p38 and c-Jun N-terminal kinase/stress-activated protein kinase signaling, leading to the activation of transcription factor-activating protein-1 (AP-1) (33). Activation of NF-κB and AP-1 leads to increased expression levels of inflammatory factors, such as TNF-α, IL-1, IL-6 and nitric oxide, resulting in ALI/acute respiratory distress syndrome.
TLR2 predominantly mediates the activation of cells by gram-positive bacteria. LPS and cytokines induced by LPS upregulate TLR2 mRNA expression in macrophages (34). Therefore, although TLR2 does not bind LPS directly, it may play an important role in enhancing stress-induced lung injury by functioning as a secondary receptor. TLR2 expression is known to be regulated by NF-κB (35). In addition, LPS-induced activation of TLR4 leads to NF-κB activation, and has been shown to induce the synthesis of large quantities of inflammatory factors and to upregulate TLR2 mRNA expression, increasing the inflammatory response (31,36,37). The results demonstrated that PHC treatment may alleviate ALI caused by hemorrhagic shock by decreasing TLR4 mRNA expression levels in the lung, inhibiting NF-κB activity and reducing TNF-α expression.
In the present study, the results suggested that PHC treatment of LPS-induced rats resulted in decreased TLR4 and TLR2 mRNA expression levels and NF-κB activity levels in the lung tissue samples, as well as reduced serum levels of TNF-α and IL-6. The extent of lung tissue damage was reduced, consistent with the molecular results, suggesting that PHC may inhibit rat inflammatory responses and alleviate ALI by acting on the inflammatory response signaling pathways in a dose-dependent manner.
There were several limitations to the present study. Firstly, a limited number of animals were used, and therefore additional studies should be performed to further examine the results. Secondly, clinical data demonstrating the results presented here would be beneficial. Thirdly, additional experiments such as investigations of the exact mechanism underlying TLR ligand recognition and of the transcriptional regulation of TLR4 and TLR activation signaling pathway that links the innate and acquired immunity should be performed to provide further evidence for the results presented in the current study.
In conclusion, the present study demonstrated that treatment with 1 and 3 mg/kg PHC significantly attenuated LPS-induced upregulation of lung TLR2 and TLR4 mRNA and decreased NF-κB activation and serum TNF-α and IL-6 levels, resulting in the alleviation of inflammation. Therefore, PHC may have a protective role in LPS-induced ALI in rats and may be a novel candidate for the treatment of inflammation-related lung injury.
Acknowledgements
The authors of the present study would like to thank Dr Ting-Ming Cao and Dr Yang Liu (both Capital Medical University) for their technical assistance.
References
- 1.Luh SP, Chiang CH. Acute lung injury/acute respiratory distress syndrome (ALI/ARDS): The mechanism, present strategies and future perspectives of therapies. J Zhejiang Uni Sci B. 2007;8:60–69. doi: 10.1631/jzus.2007.B0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, Zhang L, Jin Z, Jin E, Fujiwara M, Ghazizadeh M, Asoh S, Ohta S, Kawanami O. Anti-apoptotic PTD-FNK protein suppresses lipopolysaccharide-induced acute lung injury in rats. Exp Mol Pathol. 2007;83:377–384. doi: 10.1016/j.yexmp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Liu YY, Li LF, Yang CT, Lu KH, Huang CC, Kao KC, Chiou SH. Suppressing NF-κB and NKRF pathways by induced pluripotent stem cell therapy in mice with ventilator-induced lung injury. PLoS One. 2013;8:e66760. doi: 10.1371/journal.pone.0066760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas R, Verin AD, Black SM, Catravas JD. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol. 2009;77:1763–1772. doi: 10.1016/j.bcp.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andonegui G, Bonder CS, Green F, Mullaly SC, Zbytnuik L, Raharjo E, Kubes P. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J Clin Invest. 2003;111:1011–1020. doi: 10.1172/JCI16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andonegui G, Goyert SM, Kubes P. Lipopolysaccharide-induced leukocyte-endothelial cell interactions: A role for CD14 versus toll-like receptor 4 within microvessels. J Immunol. 2002;169:2111–2119. doi: 10.4049/jimmunol.169.4.2111. [DOI] [PubMed] [Google Scholar]
- 7.Ayala A, Chung CS, Lomas JL, Song GY, Doughty LA, Gregory SH, Cioffi WG, LeBlanc BW, Reichner J, Simms HH, Grutkoski PS. Shock-induced neutrophil mediated priming for acute lung injury in mice: Divergent effects of TLR-4 and TLR-4/FasL deficiency. Am J Pathol. 2002;161:2283–2294. doi: 10.1016/S0002-9440(10)64504-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basu S, Fenton MJ. Toll-like receptors: Function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol. 2004;286:L887–L892. doi: 10.1152/ajplung.00323.2003. [DOI] [PubMed] [Google Scholar]
- 9.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 10.Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM, Vogel SN. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001;69:1477–1482. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takaoka Y, Goto S, Nakano T, Tseng HP, Yang SM, Kawamoto S, Ono K, Chen CL. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) prevents lipopolysaccharide (LPS)-induced, sepsis-related severe acute lung injury in mice. Sci Rep. 2014;4:5204. doi: 10.1038/srep05204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perros F, Lambrecht BN, Hammad H. TLR4 signalling in pulmonary stromal cells is critical for inflammation and immunity in the airways. Respir Res. 2011;12:125. doi: 10.1186/1465-9921-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiu RJ, Hammerschmidt DE, Coppo PA, Jacob HS. Anisodamine inhibits thromboxane synthesis, granulocyte aggregation and platelet aggregation. A possible mechanism for its efficacy in bacteremic shock. JAMA. 1982;247:1458–1460. doi: 10.1001/jama.247.10.1458. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Chen Q, Liu S, Huang Y, Liu L, Wu X, Chen G, Jin J, Teng G, Qiu H. Effects of recruitment maneuvers with PEEP on lung volume distribution in canine models of direct and indirect lung injury. Mol Biol Rep. 2014;41:1325–1333. doi: 10.1007/s11033-013-2978-4. [DOI] [PubMed] [Google Scholar]
- 15.Disse B. Antimuscarinic treatment for lung diseases from research to clinical practice. Life Sci. 2001;68:2557–2564. doi: 10.1016/S0024-3205(01)01052-9. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Wang C, Tao J, Xiong J, Wan C, Zhou F. Effect of early hemofiltration on pro- and anti-inflammatory responses and multiple organ failure in severe acute pancreatitis. J Huazhong Univ Sci Technol Med Sci. 2004;24:456–459. doi: 10.1007/BF02831107. [DOI] [PubMed] [Google Scholar]
- 17.Feng S, Ge Y, Yang J, Duan M, Xu J. Effects of penehyclidine hydrochloride on lung inflammatory response in septic rats. Zhong Hua Ma Zui Xue Za Zhi Bian Ji Bu. 2008;28:63–67. (In Chinese) [Google Scholar]
- 18.Huang C, He J, Chen Y, Zhang Y, Chen C. Penehyclidine hydrochloride inhibits the LPS-induced inflammatory response in microglia. J Surg Res. 2014;188:260–267. doi: 10.1016/j.jss.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Lei L, Wang Y, Jia B, Zhan J, Wang C. Protective effect of penehyclidine hydrochloride on lung injury in mice with sepsis and its mechanism. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007;19:623–625. (In Chinese) [PubMed] [Google Scholar]
- 20.Li BQ, Sun HC, Nie SN, Shao DB, Liu HM, Qian XM. Effect of penehyclidine hydrochloride on patients with acute lung injury and its mechanisms. Chin J Traumatol. 2010;13:329–335. [PubMed] [Google Scholar]
- 21.Smith KM, Mrozek JD, Simonton SC, Bing DR, Meyers PA, Connett JE, Mammel MC. Prolonged partial liquid ventilation using conventional high-frequency ventilatory techniques: Gas exchange and lung pathology in animal model of respiratory distress syndrome. Crit Care Med. 1997;25:1888–1897. doi: 10.1097/00003246-199711000-00030. [DOI] [PubMed] [Google Scholar]
- 22.Rojas M, Woods CR, Mora AL, Xu J, Brigham KL. Endotoxin-induced lung injury in mice:structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005;288:L333–L341. doi: 10.1152/ajplung.00334.2004. [DOI] [PubMed] [Google Scholar]
- 23.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 24.Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aikawa N, Ishizaka A, Hirasawa H, Shimazaki S, Yamamoto Y, Sugimoto H, Shinozaki M, Taenaka N, Endo S, Ikeda T, Kawasaki Y. Reevaluation of the efficacy and safety of the neutrophil elastase inhibitor, Sivelestat, for the treatment of acute lung injury associated with systemic inflammatory response syndroma phase IV study. Pulm Pharmacol Ther. 2011;24:549–554. doi: 10.1016/j.pupt.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Haddad JJ. Nuclear factor (NF)-kappa B blockade attenuates but does not abrogate LPS-mediated interleukin (IL)-1 beta biosynthesis in alveolar epithelial cells. Biochem Biophys Res Commun. 2002;293:252–257. doi: 10.1016/S0006-291X(02)00213-9. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Addi A, Mambole-Dema A, Brender C, Martin SR, Janzen J, Kjaer S, Smerdon SJ, Ley SC. IκB kinase-induced interaction of TPL-2 kinase with 14-3-3 is essential for Toll-like receptor activation of ERK-1 and −2 MAP kinases. Proc Natl Acad Sci USA. 2014;111:E2394–E2403. doi: 10.1073/pnas.1320440111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 29.Erridge C, Kennedy S, Spickett CM, Webb DJ. Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: Roles for CD14, LPS-binding protein and MD2 as targets for specificity of inhibition. J Biol Chem. 2008;283:24748–24759. doi: 10.1074/jbc.M800352200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimazu RA, Kashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confer 1ipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akira S. Toll-like receptor signaling. J Biol Chem. 2003;278:38105–38108. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- 32.Brikos C, Wait R, Begum S, O'Neill LA, Saklatvala J. Mass spectrometric analysis of the endogenous type I interleukin-1 (IL-1) receptor signaling complex formed after IL-1 binding identifies IL-1RAcP, MyD88 and IRAK-4 as the stable components. Mol Cell Proteomics. 2007;6:1551–1559. doi: 10.1074/mcp.M600455-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Kopp EB, Medzhitov R. The Toll-receptor family and control innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/S0952-7915(99)80003-X. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Wang Y, Yamakuchi M, Isowaki S, Nagata E, Kanmura Y, Kitajima I, Maruyama I. Upregulation of toll-like receptor 2 gene expression in macrophage response to peptidoglycan and high concentration of lipopolysaccharide is involved in NF-κB activation. Infect Immun. 2001;69:2788–2796. doi: 10.1128/IAI.69.5.2788-2796.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musikacharoen T, Matsuguchi T, Kikuchi T, Yoshikai Y. NF-kappa B and STAT5 play important roles in the regulation of mouse Toll-like receptor 2 gene expression. J Immunol. 2001;166:4516–4524. doi: 10.4049/jimmunol.166.7.4516. [DOI] [PubMed] [Google Scholar]
- 36.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 37.Gilmore TD. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]