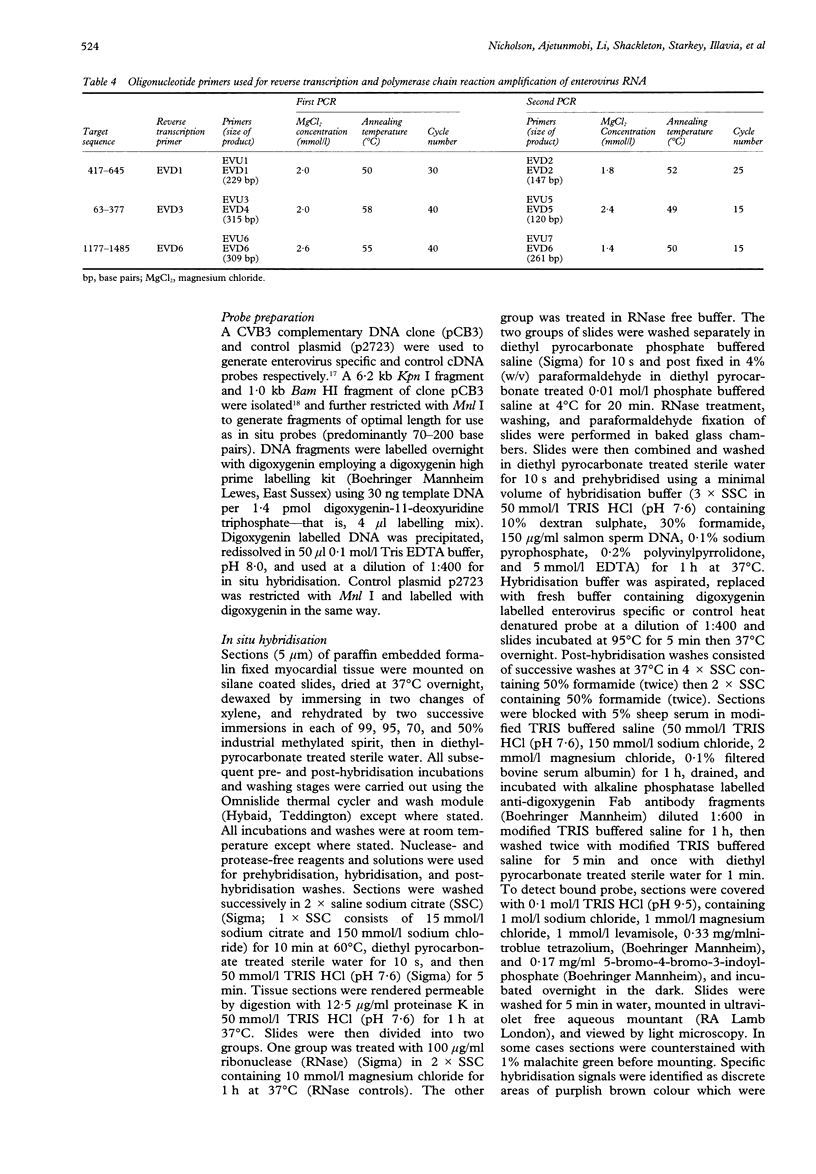
Abstract
OBJECTIVE--To determine whether enterovirus RNA can be demonstrated in archival necropsy material in acute myocarditis. DESIGN--Analysis of paraffin embedded myocardial tissue from cases of acute myocarditis. SETTING--University virology department. METHODS--Extraction of RNA from tissue followed by polymerase chain reaction (PCR) and DNA sequence analysis. PATIENTS--Six patients with histologically proven myocarditis and eight controls. RESULTS--Enterovirus RNA was identified in 5 of 6 patients with myocarditis and in none of the controls. The nucleotide sequences of the PCR products showed greatest similarity to group B coxsackieviruses, particularly coxsackievirus B3. CONCLUSION--This study indicates that archival tissue samples, even histologically stained tissue sections, can be used to study the role of enteroviruses in myocardial disease using molecular detection techniques. If a predominant role for coxsackievirus B3 in myocarditis is confirmed by further study, this may have implications for the development of a specific vaccine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aretz H. T., Billingham M. E., Edwards W. D., Factor S. M., Fallon J. T., Fenoglio J. J., Jr, Olsen E. G., Schoen F. J. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987 Jan;1(1):3–14. [PubMed] [Google Scholar]
- Bresters D., Schipper M. E., Reesink H. W., Boeser-Nunnink B. D., Cuypers H. T. The duration of fixation influences the yield of HCV cDNA-PCR products from formalin-fixed, paraffin-embedded liver tissue. J Virol Methods. 1994 Jul;48(2-3):267–272. doi: 10.1016/0166-0934(94)90125-2. [DOI] [PubMed] [Google Scholar]
- El-Hagrassy M. M., Banatvala J. E., Coltart D. J. Coxsackie-B-virus-specific IgM responses in patients with cardiac and other diseases. Lancet. 1980 Nov 29;2(8205):1160–1162. doi: 10.1016/s0140-6736(80)92595-7. [DOI] [PubMed] [Google Scholar]
- Frisk G., Torfason E. G., Diderholm H. Reverse radioimmunoassays of IgM and IgG antibodies to Coxsackie B viruses in patients with acute myopericarditis. J Med Virol. 1984;14(3):191–200. doi: 10.1002/jmv.1890140302. [DOI] [PubMed] [Google Scholar]
- Hilton D. A., Variend S., Pringle J. H. Demonstration of Coxsackie virus RNA in formalin-fixed tissue sections from childhood myocarditis cases by in situ hybridization and the polymerase chain reaction. J Pathol. 1993 May;170(1):45–51. doi: 10.1002/path.1711700108. [DOI] [PubMed] [Google Scholar]
- Iizuka N., Kuge S., Nomoto A. Complete nucleotide sequence of the genome of coxsackievirus B1. Virology. 1987 Jan;156(1):64–73. doi: 10.1016/0042-6822(87)90436-3. [DOI] [PubMed] [Google Scholar]
- Jackson D. P., Quirke P., Lewis F., Boylston A. W., Sloan J. M., Robertson D., Taylor G. R. Detection of measles virus RNA in paraffin-embedded tissue. Lancet. 1989 Jun 17;1(8651):1391–1391. doi: 10.1016/s0140-6736(89)92837-7. [DOI] [PubMed] [Google Scholar]
- Jenkins O., Booth J. D., Minor P. D., Almond J. W. The complete nucleotide sequence of coxsackievirus B4 and its comparison to other members of the Picornaviridae. J Gen Virol. 1987 Jul;68(Pt 7):1835–1848. doi: 10.1099/0022-1317-68-7-1835. [DOI] [PubMed] [Google Scholar]
- Jin O., Sole M. J., Butany J. W., Chia W. K., McLaughlin P. R., Liu P., Liew C. C. Detection of enterovirus RNA in myocardial biopsies from patients with myocarditis and cardiomyopathy using gene amplification by polymerase chain reaction. Circulation. 1990 Jul;82(1):8–16. doi: 10.1161/01.cir.82.1.8. [DOI] [PubMed] [Google Scholar]
- Kandolf R., Ameis D., Kirschner P., Canu A., Hofschneider P. H. In situ detection of enteroviral genomes in myocardial cells by nucleic acid hybridization: an approach to the diagnosis of viral heart disease. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6272–6276. doi: 10.1073/pnas.84.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandolf R., Hofschneider P. H. Molecular cloning of the genome of a cardiotropic Coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4818–4822. doi: 10.1073/pnas.82.14.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Why H., Richardson P., Archard L. Nucleotide sequencing of PCR products shows the presence of Coxsackie-B3 virus in endomyocardial biopsies from patients with myocarditis or dilated cardiomyopathy. Biochem Soc Trans. 1994 May;22(2):176S–176S. doi: 10.1042/bst022176s. [DOI] [PubMed] [Google Scholar]
- Klump W. M., Bergmann I., Müller B. C., Ameis D., Kandolf R. Complete nucleotide sequence of infectious Coxsackievirus B3 cDNA: two initial 5' uridine residues are regained during plus-strand RNA synthesis. J Virol. 1990 Apr;64(4):1573–1583. doi: 10.1128/jvi.64.4.1573-1583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney R. A., Banatvala J. E., Bell E. J. Routine use of mu-antibody-capture ELISA for the serological diagnosis of Coxsackie B virus infections. J Med Virol. 1986 Jul;19(3):205–212. doi: 10.1002/jmv.1890190302. [DOI] [PubMed] [Google Scholar]
- Miller N. A., Carmichael H. A., Calder B. D., Behan P. O., Bell E. J., McCartney R. A., Hall F. C. Antibody to Coxsackie B virus in diagnosing postviral fatigue syndrome. BMJ. 1991 Jan 19;302(6769):140–143. doi: 10.1136/bmj.302.6769.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir P., Banatvala J. E. Reactivity of enterovirus-specific IgM with infective and defective coxsackie B virions in patients with monotypic and multitypic IgM responses. J Virol Methods. 1990 Aug;29(2):209–224. doi: 10.1016/0166-0934(90)90114-u. [DOI] [PubMed] [Google Scholar]
- Muir P., Nicholson F., Jhetam M., Neogi S., Banatvala J. E. Rapid diagnosis of enterovirus infection by magnetic bead extraction and polymerase chain reaction detection of enterovirus RNA in clinical specimens. J Clin Microbiol. 1993 Jan;31(1):31–38. doi: 10.1128/jcm.31.1.31-38.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson F., Meetoo G., Aiyar S., Banatvala J. E., Muir P. Detection of enterovirus RNA in clinical samples by nested polymerase chain reaction for rapid diagnosis of enterovirus infection. J Virol Methods. 1994 Jul;48(2-3):155–166. doi: 10.1016/0166-0934(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Severini G. M., Mestroni L., Falaschi A., Camerini F., Giacca M. Nested polymerase chain reaction for high-sensitivity detection of enteroviral RNA in biological samples. J Clin Microbiol. 1993 May;31(5):1345–1349. doi: 10.1128/jcm.31.5.1345-1349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenburg H. C., Dekker G. A., Makovitz J. W., Rotmans P. Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensin-sensitive primigravidae. Lancet. 1986 Jan 4;1(8471):1–3. doi: 10.1016/s0140-6736(86)91891-x. [DOI] [PubMed] [Google Scholar]
- Woodall C. J., Watt N. J., Clements G. B. Simple technique for detecting RNA viruses by PCR in single sections of wax embedded tissue. J Clin Pathol. 1993 Mar;46(3):276–277. doi: 10.1136/jcp.46.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Wilsden G., Knowles N. J., McCauley J. W. Complete nucleotide sequence of a coxsackie B5 virus and its relationship to swine vesicular disease virus. J Gen Virol. 1993 May;74(Pt 5):845–853. doi: 10.1099/0022-1317-74-5-845. [DOI] [PubMed] [Google Scholar]