Abstract
A 69-year-old man who presented with abdominal discomfort was, on examination, found to have a palpable abdominal mass. Contrast-enhanced CT showed a mass arising from the inferior vena cava, which biopsy confirmed to be a leiomyosarcoma. One month after chemoradiotherapy, CT demonstrated a new 15 mm solitary central right liver metastasis. Microwave ablation (MWA) of the metastasis was performed using an Acculis Sulis V system (Angiodynamics, USA) at a power of 140 Watts for 4 min, with no immediate complications. After 1 month, MRI with gadolinium was performed to assess the liver ablation zone. The MRI demonstrated thrombosis of a right inferior hepatic vein branch leading to the ablation zone and extension of the ablation zone 1 cm into the tissue around the thrombosed vessel.
Background
To the best of our knowledge, this is the first clinical case of such a complication reported in the literature. Microwave ablation (MWA) is a relatively new technology used to ablate liver tumours; it has become more popular after having been reviewed by the National Institute of Care Excellence (NICE). With new techniques, it is important that clinicians, including Interventional Radiologists, Oncologists and Hepatologists, are aware of unexpected procedure-related complications. This case raises some interesting questions because the pathogenesis of the complication is unclear.
Case presentation
A 69-year-old man presented to his general practitioner, with a 3-week history of progressive abdominal pain. He had been previously fit and well with no comorbidities.
Abdominal examination revealed a midline abdominal mass.
Investigations
The patient proceeded to have an oesophagogastroduodenoscopy (OGD) and CT of the abdomen and pelvis. The OGD was normal but the CT revealed a large, lobulated retroperitoneal mass arising from the inferior vena cava (figure 1). The 12.7×9.2 cm mass was ill defined and enhanced in a heterogeneous manner. CT-guided biopsy was performed and histology revealed the mass to be a spindle cell tumour, with mild to focally moderate nuclear pleomorphism. On immunostaining, the sample was strongly positive for desmin, and weak for smooth muscle actin and CD99. The immunoprofile and histological appearances were in keeping with a grade 1 leiomyosarcoma.
Figure 1.
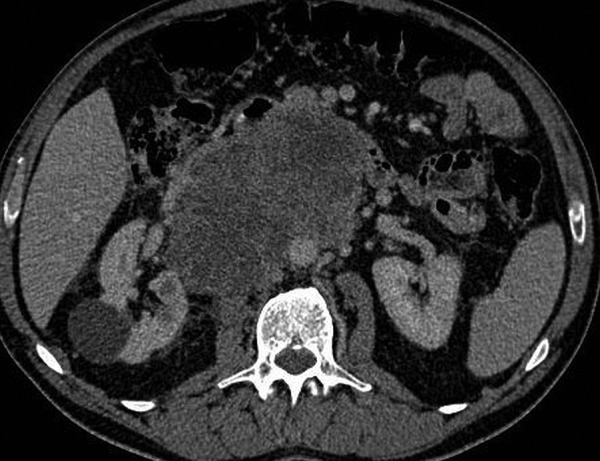
Contrast-enhanced CT shows a large lobulated retroperitoneal mass with heterogeneous enhancement in axial and coronal planes.
Differential diagnosis
The differential diagnoses considered for an upper abdominal midline mass were: a retroperitoneal mass (lymphoma or teratoma), abdominal aortic aneurysm (the mass was not pulsatile), gastric mass (gastroscopy was normal) and pancreatic pseudocyst (no history or risk factors for pancreatitis). Therefore imaging was performed to establish the aetiology.
Treatment
The case was discussed at a sarcoma multidisciplinary team meeting, which concluded that the mass was inoperable due to encasement of the renal arteries and aorta. The patient proceeded to chemotherapy consisting of three cycles of gemcitabine and doxetaxel, and a radiotherapy regime including intensity-modulated radiotherapy and concurrent ifosfamide and doxorubicin. Follow-up imaging showed initial slight reduction in the volume of retroperitoneal tumour—considered stable by Response Evaluation Criteria in Solid Tumours (RECIST) criteria. Subsequent imaging with CT demonstrated a new solitary 15 mm central segment VIII liver lesion, which was not well-defined and hypoenhancing. This lesion was biopsied under CT guidance and reported as a spindle cell neoplasm compatible with a metastasis from the known leiomyosarcoma.
The patient elected for this to be treated by percutaneous MWA.
MWA was performed under general anaesthesia with ultrasound and CT guidance, using an Acculis Sulis V (Angiodynamics, Denmead, USA) system at a power time setting of 140 W for 4 min. There were no immediate complications.
Outcome and follow-up
MRI was performed after 1 month to assess the ablation zone. The MRI demonstrated a T1 hypointense, hypoenhancing ellipsoid ablation zone that measured 4.6×2.7×2.2 cm and, on diffusion-weighted imaging (DWI), had higher apparent diffusion coefficient values compared to background liver. A right inferior hepatic vein branch leading to the ablation zone was expanded and did not enhance, indicating thrombosis (figure 2), which was not present on the pre-treatment MRI. At its inferior margin, the ablation zone extended 1 cm into the perivascular liver parenchyma along the thrombosed inferior hepatic vein (figure 3). There were no clinical sequelae of these complications based on outpatient follow-up 3 months after the procedure.
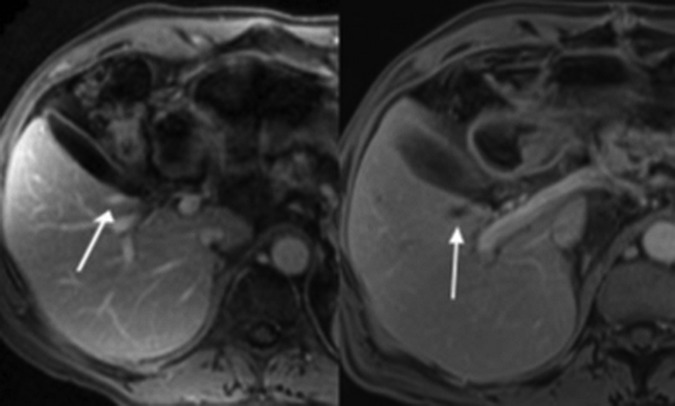
Figure 2.
(A) Preablation MRI axial T1-weighted volumetric interpolated breath-hold examination (VIBE) postgadolinium portal venous phase images show normal enhancement of the right inferior hepatic vein branch. (B) Postablation the vessel is expanded and lacks enhancement, consistent with thrombosis.
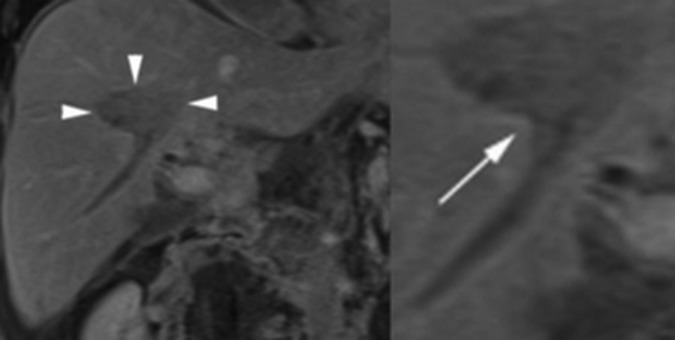
Figure 3.
(A) MRI coronal T1-weighted volumetric interpolated breath-hold examination (VIBE) postgadolinium portal venous phase images. The ablation zone (arrow heads) is seen as a hypoenhancing ellipsoid region within the central right lobe of the liver. (B) Magnified view of the ablation zone showing a cone shaped area of extension (white arrow) along a thrombosed right inferior hepatic vein branch.
Discussion
MWA is a more recently developed technology than the widely used radiofrequency ablation (RFA) and results are therefore less reported in the literature. MWA has several theoretical advantages over RFA, including more rapid and large ablation zone formation, and less variability of ablation zone size in different tissues.1 However, data on the benefits and potential harms related to clinical use of MWA are limited.2 There have been no reported cases in the existing literature describing extension of the ablation zone into the perivascular tissues. This phenomenon has, however, been reported in two in vivo porcine studies, both describing ablation zone extension along thrombosed vessels. In the first study, the authors postulate that the perivascular extension may have been due to water vapour tracking alongside vessels, thus causing extension of the thermal tissue injury.3 In the second study, the authors postulate thrombosis of the vessel as the primary event, with thrombus allowing conduction of heat away from the primary treatment area.4
In the case reported here, imaging at the time of ablation showed no evidence of hepatic vein thrombosis. A causal association between extension of the ablation zone and the venous thrombosis is yet to be made, but two likely possibilities exist. Either thrombosis of the vein was the primary event facilitating thermal conduction and extension of the ablation zone during the ablation, or, alternatively, the ablation zone may have extended along the perivascular tissues leading to vascular endothelial damage, precipitating thrombosis. Further research is required to distinguish between these two explanations. Perivascular ablation extension may have potential clinical implications. Owing to the difficulty posed by intraprocedural monitoring of the MW ablation zone,5 6 it is important that a predictable volume of tissue destruction is produced. Unexpected extension of the ablation zone along major intrahepatic vessels (figure 2) may lead to non-target ablation, hence a comprehensive understanding of factors influencing the ablation zone is vital for safe planning and delivery of treatment. MRI offers better soft tissue resolution than CT and techniques such as DWI have an increasing role in the follow-up of liver thermal ablation.7 The increased use of these techniques may help detect unexpected complications.
This case report highlights an unexpected but important complication of liver ablation, which was detected on follow-up MRI. Further research is needed to establish why the ablation zone extended along a vessel and its possible clinical effects.
Learning points.
Microwave ablation is an increasingly popular method of treatment for tumours in the liver.
Thrombosis of vessels in the ablation zone is a recognised complication of microwave ablation.
Extension of the ablation zone along vessels is now reported.
Further research is needed to establish the mechanism.
Footnotes
Contributors: SS, PNS, EWJ, SB, BRD and RI contributed to conception and design, acquisition of data or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content and final approval of the version published.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 2014;14:199–208. 10.1038/nrc3672 [DOI] [PubMed] [Google Scholar]
- 2.Li D, Kang J, Madoff DC. Locally ablative therapies for primary and metastatic liver cancer. Expert Rev Anticancer Ther 2014;14:931–45. 10.1586/14737140.2014.911091 [DOI] [PubMed] [Google Scholar]
- 3.Wright AS, Lee FT Jr, Mahvi DM. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol 2003;10:275–83. 10.1245/ASO.2003.03.045 [DOI] [PubMed] [Google Scholar]
- 4.Yu NC, Raman SS, Kim YJ et al. . Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol 2008;19:1087–92. 10.1016/j.jvir.2008.03.023 [DOI] [PubMed] [Google Scholar]
- 5.Correa-Gallego C, Karkar AM, Monette S et al. . Intraoperative ultrasound and tissue elastography measurements do not predict the size of hepatic microwave ablations. Acad Radiol 2014;21:72–8. 10.1016/j.acra.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 6.Ringe KI, Wacker F, Raatschen HJ. Is there a need for MRI within 24 hours after CT-guided percutaneous thermoablation of the liver? Acta radiol 2015;56:10–17. 10.1177/0284185114520858 [DOI] [PubMed] [Google Scholar]
- 7.Schraml C, Schwenzer NF, Clasen S et al. . Navigator respiratory-triggered diffusion-weighted imaging in the follow-up after hepatic radiofrequency ablation-initial results. J Magn Reson Imaging 2009;29:1308–16. 10.1002/jmri.21770 [DOI] [PubMed] [Google Scholar]