Abstract
Nursing home-associated infections and antibiotic resistant pathogens constitute common and serious problems in the geriatric population. Chryseobacterium indologenes, a non-motile Gram-negative rod, though widely distributed in nature, is an uncommon human pathogen. Typically thought of as an organism of low virulence, it may cause serious infections, particularly among the immunocompromised. The majority of reported cases are nosocomial, often associated with immunosuppression or indwelling catheters. It has been reported as the causative agent in bacteraemia, peritonitis, pneumonia, empyema, pyelonephritis, cystitis, meningitis and central venous catheter-associated infections. We report a rare case of C. indologenes infection affecting a nursing home resident in the USA and we provide a review of similar cases. This report emphasises the importance of individualised treatment and promotes awareness about this organism as one of several emerging pathogens in immunocompromised adults and in the frail elderly who are often nursing home residents, in the Western Hemisphere.
Background
The elderly, including frail nursing home residents, adults with advanced or chronic illnesses regardless of age, immunocompromised patients whether due to systemic illness or medications and patients with indwelling devices or tubes, are especially prone to acquiring healthcare-associated infections. The emergence of new infections and antibiotic-resistant organisms increases this vulnerability even more. Chryseobacterium indologenes appears to be one of the new human pathogens all the more fearsome because of its exceptional antibiotic resistance.1
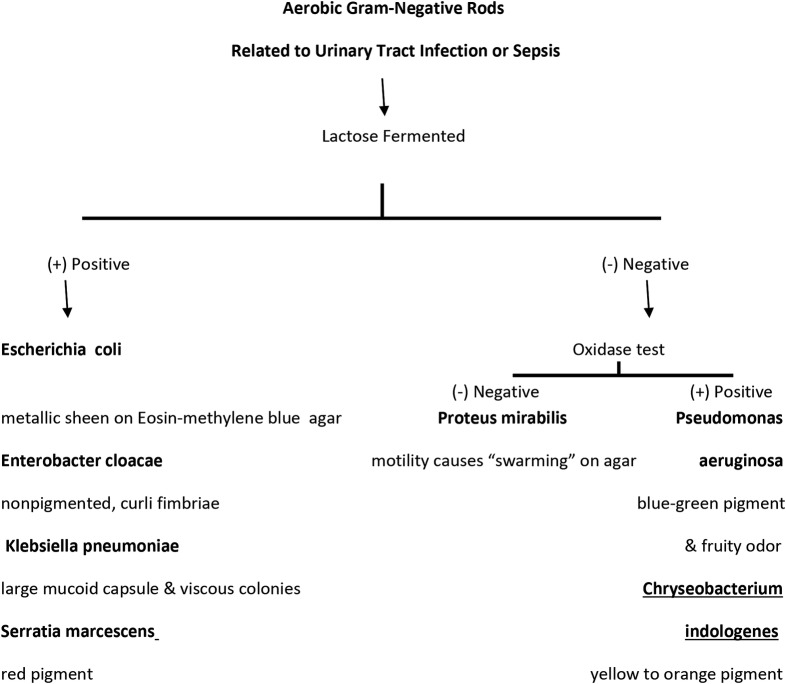
C. indologenes belongs to the Chryseobacterium genus, previously known as Flavobacterium. The genus is composed of six species where Chryseobacterium meningosepticum, in current taxonomy named Elizabethkingia meningosepticum, is reported as the most pathogenic, while C. indologenes was reported to have low virulence.2 C. indologenes is a yellow-pigmented, non-motile, oxidase positive, glucose non-fermentative, Gram-negative rod-shaped bacterium widely distributed in nature. Other clinically significant Gram-negative rod-shaped microorganisms commonly associated with urinary tract infections or sepsis are shown in the flow-diagram (figure 1).
Figure 1.
Aerobic Gram-negative rods associated with urinary tract infection.
Until 1996, C. indologenes had been only rarely implicated in bacteraemia in humans. Since then, the numbers of reported cases of C. indologenes infections are steadily increasing. The majority of reported infections have been from Taiwan3 and only about 10% have been outside of Asia. A few reports have come from Australia, India, Europe and the USA4 5 (table 1). There are many reported cases of C. indologenes in paediatric populations,8 11 as well as in immunocompromised, hospitalised patients12–14 with severe illness and or with indwelling devices.7–9
Table 1.
Chryseobacterium indologenes-related infections reported in the USA
| Author | Year | Place | Age/gender | Predisposing factor | Clinical presentation | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Green et al6 | 2001 | Texas | 77-year-old man | Treatment for squamous cell carcinoma of leg, swam in his pool | Cellulitis and bacteraemia | Levofloxacin | Recovered |
| Cone et al7 | 2007 | California | 57-year-old woman | Breast cancer central catheter | Sepsis due to an infected central catheter | Ciprofloxacin catheter was removed | Recovered |
| Al-Tatari et al8 | 2007 | Michigan | 13-year-old boy | Congenital hydrocephalus and LP shunt | LP shunt infection | Trimethoprim-sulfamethoxazole and rifampin LP shunt was removed | Recovered |
| Shah et al9 | 2012 | New York | 26-year-old woman | Liver transplant on immunosuppressive treatment; subcutaneous port | Worsening ascites, abdominal pain | Levofloxacin and trimethoprim-sulfamethoxazole port was removed | Recovered |
| Yasmin et al5 | 2013 | Georgia | 32-year-old woman | Metastatic breast cancer; on mechanical ventilation | Ventilator-associated pneumonia | Levofloxacin | Patient Died |
| Afshar et al4 | 2013 | District Columbia | 51-year-old man | End stage renal disease; on peritoneal dialysis | Peritonitis | Ceftazidime without catheter removal | Recovered |
| Monteen et al10 | 2013 | Tennessee | 66-year-old man | Critical accident; trapped under water and later intubated | Ventilator-associated pneumonia | Moxifloxacin and cefepime | Recovered |
| This study | 2015 | Michigan | 63-year-old man | Indwelling Foley catheter; nursing home patient | UTI | Imipenem | Recovered |
LP, lumboperitoneal; UTI, urinary tract infection.
We report a C. indologenes infection in a diabetic nursing home adult with an indwelling Foley catheter, in the USA.
Case presentation
A 63-year-old Caucasian man, a resident of an extended care facility, was brought to the hospital, with acute confusion that was preceded by dysuria, fever and diffuse cramping lower abdominal pain. He had a history of spinal stenosis and urinary retention treated with an indwelling Foley catheter of 2 months’ duration. Prior to admission, he had been treated empirically with nitrofurantoin 100 mg orally every 12 h for 5 days. Comorbid conditions included stable chronic obstructive pulmonary disease, coronary artery disease, diabetes mellitus, benign prostatic hypertrophy, atrial fibrillation, bipolar disorder and anaemia with haemoglobin of 7.7 g/dL. On physical examination, he was alert but oriented to neither time nor place; he had stable vital signs and some suprapubic tenderness.
Investigations
On the second hospital day, urine culture showed more than 100 000 colony-forming units of C. indologenes, which was resistant to almost all antimicrobials except imipenem-cilastatin. The patient had no leucocytosis and blood cultures were negative.
Treatment
Ceftriaxone 1 g was administered intravenously, which was later switched to vancomycin 1 g intravenously daily and piperacillin/tazobactam 3.375 g intravenously every 8 h, to provide broader empiric coverage. The source of infection was felt to be the indwelling Foley catheter, which was replaced and antibiotic therapy was changed to imipenem-cilastatin after consulting infectious disease.
Outcome and follow-up
Repeat urine culture was negative and the patient was discharged with resolution of symptoms after 7 days of hospitalisation.
Discussion
C. indologenes is ubiquitous in nature, mainly found in soil and water and may be perceived as a coloniser. However, in some patients, it may cause significant morbidity and mortality. It resists chlorination and can survive in municipal water supplies.2 It is prevalent on wet or humid surfaces in hospitals and also in catheters containing fluids, such as feeding tubes, central venous catheters and tracheostomy tubes.15 The presence of contaminated medical devices in institutionalised and or immunocompromised patients, such as patients with diabetes mellitus, malignancies and neutropaenia and prolonged treatment with antibiotics, may result in serious infections.4 7 16–18 More than half of the reported cases have been among hospitalised, immunocompromised patients with mechanical ventilation or indwelling catheters.5 7 10 15 Although C. indologenes infections are nosocomial, device-related infections and, recently, non-catheter-related community-acquired C. indologenes bacteraemia in immunocompetent patients, have been reported.12 19 20
It has also been reported that C. indologenes infection is more prevalent in the elderly.20–23 However, there are only a few reports in octogenarians and/or nursing home patients. In addition to device-related risk in the elderly, other predisposing factors include immunocompromising conditions such as diabetes and long-term treatment with systemic steroids. Infections such as healthcare-associated pneumonia in an immunocompetent patient and polymicrobial urinary tract infections have been reported in this age group.20 21 24 Outcomes of the hospitalised elderly have been favourable.
The most common clinical presentations of C. indologenes infection are pneumonia, bacteraemia, cellulitis, surgical wound infections, urinary tract infections, ocular infections, meningitis due to central nervous system shunt, peritonitis due to peritoneal catheter dialysis, intra-abdominal and other catheter-related infections.4 6–8 25
C. indologenes associated urinary tract infections have been recently reported worldwide26–28 (table 2).
Table 2.
Urinary tract infections associated with Chryseobacterium indologenes: case reports
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age (years) | 19 | 86 | 42 | 21 |
| Gender | Female | Female | Female | Female |
| Predisposing factor | Urinary catheter for 7 days after pyelolithotomy | Insulin-dependent type 2 diabetes | Chronic myeloid leukaemia | Urinary catheter for 24 h after induced labour for intrauterine fetal death |
| Clinical presentation | High-grade fever, burning micturition on fifth postoperative day | Hospitalised for decompensated congestive heart failure | High-grade fever | Fever spike |
| Treatment | Piperacillin-tazobactam | Levofloxacin | Ceftriaxone | Tigecycline |
| Outcome | Recovered | Recovered | Died with severe sepsis | Recovered |
| Year | 2012 | 2013 | 2014 | 2015 |
| Place | India | Spain | Senegal | India |
| Author | Bhuyar et al26 | Acosta et al21 | Omar et al27 | Solanke et al28 |
The mortality rate of C. indologenes varies with different studies, however, in a 2011 study from Taiwan, which included 10 patients with C. indologenes with sepsis (mean age of 71.1 years), the mortality rate at 14 days was 40%.17 The analysis of 215 other C. indologenes cases, also from Taiwan, revealed that in-hospital mortality rates from bacteraemia were as high as 63.6% and from pneumonia, 35.25%.3
Although C. indologenes exhibits characteristics of low virulence, it may cause life-threatening infections due to its multidrug resistance.22 29 30 Its ability to produce biofilm on foreign materials and produce proteases, can cause several forms of infections and is responsible for its virulent character.31 One study mentioned the production of a metallo-β-lactamase, which allows the bacteria to hydrolyse the β lactam part of some drugs.32 It was sensitive to a limited number of antibiotics that include newer quinolones, in particular, garenoxacin, gatifloxacin and levofloxacin, rifampin, trimethoprim-sulfamethoxazole and piperacillin-tazobactam.22 The antibiotics commonly used to treat Gram-negative organisms, such as cephalosporins, aminoglycosides and imipenem, have—in an in vitro study—been reported to be ineffective against C. indologenes.3 In addition, it is now shown in this study that its resistance is rapidly evolving, with drastically limited antibiotics to which it is susceptible, namely, trimethoprim-sulfamethoxazole and cefoperazone-sulbactam.3
There is controversy regarding whether indwelling catheters should be removed when there is an associated C. indologenes infection. Reports vary on the effectiveness of antibiotic treatment with or without removal of the indwelling device.9 15 18 33–35 In general, when there is failure to respond to appropriate antibiotic treatment, indwelling catheters should be removed.15 If the indwelling catheter-related infection caused by C. indologenes does not cause rapid clinical deterioration, then the device does not require removal.15 18 36 However, in some immunocompromised patients, removal of a port or central catheter may hasten recovery.37
Because of varying susceptibilities, it has been suggested that the treatment of the organism should be based on its sensitivity pattern. In our case, results of susceptibility testing differed from what has been previously reported. Our isolated pathogen was sensitive only to imipenem-cilastatin.
In summary, infection from C. indologenes was initially rarely reported outside Taiwan. It is important to keep C. indologenes in mind as a possible source of infection in patients with the appropriate risk factors. Because of varying susceptibilities to antimicrobials, empiric antibiotic treatment of the patient with possible C. indologenes infection needs to be tailored to its local susceptibilities until a confirmatory culture report is obtained. This may avoid delay in the recovery of the patient. In addition, removing the probable source of infection may also be an important consideration. Moreover, the multidrug resistance makes this organism an ominous emerging pathogen.
Learning points.
In the elderly and people with advanced illness or indwelling catheters and institutionalised or frail nursing home residents, even an organism with low virulence, such as C. indologenes, may become a life-threatening pathogen.
Nosocomial spread is possible, therefore in hospitals and nursing homes, universal precautions need to be observed to avoid spread of the infection.
Because of varying susceptibilities to antimicrobials, empiric antibiotic treatment of the patient with possible C. indologenes infection needs to be tailored to its local susceptibilities until a confirmatory culture report is obtained.
Acknowledgments
The authors would like to acknowledge Dr Grace-Marie Logrono's contribution in patient care and initial data collection.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Asaad AM, Al-Ayed MSZ, Qureshi MA. Emergence of unusual non-fermenting gram-negative nosocomial pathogens in a Saudi hospital. Jpn J Infect Dis 2013;66:507–11. 10.7883/yoken.66.507 [DOI] [PubMed] [Google Scholar]
- 2.Steinberg J. Other Gram-Negative and Gram-variable Bacilli. In: Bennett JE, Dolin R, Blaser MJ et al eds.Anonymous. Mandell, Douglas and Bennett's principles and practice of infectious diseases. Saunders, 2015:2667–83. [Google Scholar]
- 3.Chen FL, Wang GC, Teng SO et al. Clinical and epidemiological features of Chryseobacterium indologenes infections: analysis of 215 cases. J Microbiol Immunol Infect 2013;46:425–32. 10.1016/j.jmii.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 4.Afshar M, Nobakht E, Lew SQ. Chryseobacterium indologenes peritonitis in peritoneal dialysis. BMJ Case Rep 2013;2013:pii: bcr2013009410 10.1136/bcr,2013-009410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasmin S, Garcia G, Sylvester T et al. Chryseobacterium indologenes in a woman with metastatic breast cancer in the United States of America: a case report. J Med Case Rep 2013;7:190 10.1186/1752-1947-7-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green BT, Nolan PE. Cellulitis and bacteraemia due to Chryseobacterium indologenes. J Infect 2001;42:219–20. 10.1053/jinf.2001.0822 [DOI] [PubMed] [Google Scholar]
- 7.Cone LA, Morrow AA, Benson M et al. Chryseobacterium indologenes sepsis due to an infected central catheter in a patient with metastatic breast cancer to the skin. Infect Dis Clin Pract 2007;15:403–5. 10.1097/IPC.0b013e318050d23b [DOI] [Google Scholar]
- 8.Al-Tatari H, Asmar BI, Ang JY. Lumboperitonial shunt infection due to Chryseobacterium indologenes. Pediatr Infect Dis J 2007;26:657–9. 10.1097/INF.0b013e3180616d25 [DOI] [PubMed] [Google Scholar]
- 9.Shah S, Sarwar U, King EA et al. Chryseobacterium indologenes subcutaneous port-related bacteremia in a liver transplant patient. Transpl Infect Dis 2012;14:398–402. 10.1111/j.1399-3062.2011.00711.x [DOI] [PubMed] [Google Scholar]
- 10.Monteen MR, Ponnapula S, Wood GC et al. Treatment of Chryseobacterium indologenes ventilator-associated pneumonia in a critically ill trauma patient. Ann Pharmacother 2013;47:1736–9. 10.1177/1060028013508745 [DOI] [PubMed] [Google Scholar]
- 11.Douvoyiannis M, Kalyoussef S, Philip G et al. Chryseobacterium indologenes bacteremia in an infant. Int J Infect Dis 2010;14:e531–2. 10.1016/j.ijid.2009.06.015 [DOI] [PubMed] [Google Scholar]
- 12.Christakis GB, Perlorentzou SP, Chalkiopoulou I et al. Chryseobacterium indologenes non-catheter-related bacteremia in a patient with a solid tumor. J Clin Microbiol 2005;43:2021–3. 10.1128/JCM.43.4.2021-2023.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayraktar MR, Aktas E, Ersoy Y et al. Postoperative Chryseobacterium indologenes bloodstream infection caused by contamination of distillate water. Infect Control Hosp Epidemiol 2007;28:368–9. 10.1086/508839 [DOI] [PubMed] [Google Scholar]
- 14.Ferreira Rde S, Brandão FFB, Lobo SM. Chryseobacterium indologenes infection: a case report. Rev Bras Ter Intensiva 2010;22:96–8. 10.1590/S0103-507X2010000100016 [DOI] [PubMed] [Google Scholar]
- 15.Hsueh PR, Teng LJ, Ho SW et al. Clinical and microbiological characteristics of Flavobacterium indologenes infections associated with indwelling devices. J Clin Microbiol 1996;34:1908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YT, Jeng YY, Lin ML et al. Clinical and microbiological characteristics of Chryseobacterium indologenes bacteremia. J Microbiol Immunol Infect 2010;43:498–505. 10.1016/S1684-1182(10)60077-1 [DOI] [PubMed] [Google Scholar]
- 17.Chou DW, Wu SL, Lee CT et al. Clinical characteristics, antimicrobial susceptibilities, and outcomes of patients with Chryseobacterium indologenes bacteremia in an intensive care unit. Jpn J Infect Dis 2011;64:520–4. [PubMed] [Google Scholar]
- 18.Wang YC, Yeh KM, Chiu SK et al. Chryseobacterium indologenes peritonitis in a patient with malignant ascites. Int Med Case Rep J 2011;4:13–15. 10.2147/IMCRJ.S16179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunha V, Ferreira M, Fonseca A et al. community-acquired chryseobacterium indologenes in an immunocompetent patient. JMM Case Reports 2014:1.
- 20.Degandt S, Van Hoecke F, Colaert J et al. Bacteremia due to Chryseobacterium indologenes, a naturally carbapenem-resistant Gram-negative pathogen, in a geriatric patient. Eur Geriatr Med 2013;4:345 10.1016/j.eurger.2013.06.003 [DOI] [Google Scholar]
- 21.Acosta-Ochoa MI, Rodrigo-Parra A, Rodriguez-Martin F et al. Urinary infection due to Chryseobacterium indologenes. Nefrologia 2013;33:620 10.3265/Nefrologia.pre2013.Apr.11942 [DOI] [PubMed] [Google Scholar]
- 22.Kirby JT, Sader HS, Walsh TR et al. Antimicrobial susceptibility and epidemiology of a worldwide collection of Chryseobacterium spp: report from the SENTRY Antimicrobial Surveillance Program (1997–2001). J Clin Microbiol 2004;42:445–8. 10.1128/JCM.42.1.445-448.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Hu Z, Fan Y et al. Chryseobacterium indologenes catheter-related meningitis in an elderly patient after intracranial aneurysm clipping surgery. Neurol Sci 2014;35:113–15. 10.1007/s10072-013-1500-z [DOI] [PubMed] [Google Scholar]
- 24.Nemli SA, Demirdal T, Ural S. A case of healthcare associated pneumonia caused by Chryseobacterium indologenes in an immunocompetent patient. Case Rep Infect Dis 2015;2015:483923 10.1155/2015/483923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilvington S, Shovlin J, Nikolic M. Identification and susceptibility to multipurpose disinfectant solutions of bacteria isolated from contact lens storage cases of patients with corneal infiltrative events. Cont Lens Anterior Eye 2013;36:294–8. 10.1016/j.clae.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 26.Bhuyar G, Jain S, Shah H et al. Urinary tract infection by Chryseobacterium indologenes. Indian J Med Microbiol 2012;30:370–2. 10.4103/0255-0857.99511 [DOI] [PubMed] [Google Scholar]
- 27.Omar A, Camara M, Fall S et al. Chryseobacterium indologenes in a woman with acute leukemia in Senegal: a case report. J Med Case Rep 2014;8:138 10.1186/1752-1947-8-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solanke V, Verma S, Nataraj G et al. Chryseobacterium indologenes associated urinary tract infection- a case report. Br Biomed Bull 2015;3:75–80. [Google Scholar]
- 29.Fraser SL, Jorgensen JH. Reappraisal of the antimicrobial susceptibilities of Chryseobacterium and Flavobacterium species and methods for reliable susceptibility testing. Antimicrob Agents Chemother 1997;41:2738–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maravic A, Skocibusic M, Samanic I et al. Profile and multidrug resistance determinants of Chryseobacterium indologenes from seawater and marine fauna. World J Microbiol Biotechnol 2013;29:515–22. 10.1007/s11274-012-1205-0 [DOI] [PubMed] [Google Scholar]
- 31.Chang YC, Lo HH, Hsieh HY et al. Identification, epidemiological relatedness, and biofilm formation of clinical Chryseobacterium indologenes isolates from central Taiwan. J Microbiol Immunol Infect 2015;48:559–64. 10.1016/j.jmii.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 32.Zeba B, De Luca F, Dubus A et al. IND-6, a highly divergent IND-type metallo-beta-lactamase from Chryseobacterium indologenes strain 597 isolated in Burkina Faso. Antimicrob Agents Chemother 2009;53:4320–6. 10.1128/AAC.01607-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nulens E, Bussels B, Bols A et al. Recurrent bacteremia by Chryseobacterium indologenes in an oncology patient with a totally implanted intravascular device. Clin Microbiol Infect 2001;7:391–3. 10.1046/j.1198-743x.2001.00273.x [DOI] [PubMed] [Google Scholar]
- 34.Hsueh PR, Hsiue TR, Wu JJ et al. Flavobacterium indologenes bacteremia: clinical and microbiological characteristics. Clin Infect Dis 1996;23:550–5. 10.1093/clinids/23.3.550 [DOI] [PubMed] [Google Scholar]
- 35.Ozcan N, Dal T, Tekin A et al. Is Chryseobacterium indologenes a shunt-lover bacterium? A case report and review of the literature. Infez Med 2013;21:312–16. [PubMed] [Google Scholar]
- 36.Lin JT, Wang WS, Yen CC et al. Chryseobacterium indologenes bacteremia in a bone marrow transplant recipient with chronic graft-versus-host disease. Scand J Infect Dis 2003;35:882–3. 10.1080/00365540310016637 [DOI] [PubMed] [Google Scholar]
- 37.Mutcali SI, Yemisen M, Soylu H et al. Recurrent port infection due to chryseobacterium indologenes. Eurasian J Med 2013;45:60–1. 10.5152/eajm.2013.11 [DOI] [PMC free article] [PubMed] [Google Scholar]