Abstract
The derived neutrophil to lymphocyte ratio (dNLR) has been proposed as an easily determinable prognostic factor for cancer patients, but the prognostic significance of the dNLR in hepatocellular carcinoma (HCC) has not been investigated. The present study aimed to validate the prognostic power of the NLR and dNLR in HCC patients undergoing transarterial chemoembolization (TACE). The data of 279 consecutive patients who underwent TACE for unresectable HBV-associated HCC between September 2009 and November 2011 at the Department of Hepatobiliary Surgery, Sun Yat-sen University Cancer Center (Guangzhou, China) were retrieved from a prospective database. The cut-off values for the NLR and dNLR were determined by receiver operating characteristic (ROC) analysis. The association between the NLR and dNLR and the clinicopathological characteristics and overall survival (OS) rates and times of patients was analyzed. The area under the curve (AUC) was calculated to evaluate the discriminatory ability of the NLR and dNLR. The median follow-up period was 446 days, the 1, 2 and 3-year OS rates were 38.8, 18.5 and 11.1% respectively, and the median OS time was 264 days. The cut-off values were determined as 2.6 and 1.8 for the NLR and dNLR, respectively. The NLR and dNLR were each associated with patient age, presence of vascular invasion, tumor size, AST level and ALP level. Multivariate analysis showed that the NLR, dNLR, ALT level and AFP level were independent prognostic factors for OS. An elevated NLR or dNLR was associated with a poor prognosis (P=0.001 and P=0.002, respectively). The prognostic power of NLR [AUC=0.539; 95% confidence interval (CI), 0.423–0.656] and dNLR (AUC=0.522; 95% CI, 0.406–0.638) was similar. Elevated dNLR predicted poor prognosis for patients with HBV-associated HCC undergoing TACE, with similar prognostic power to NLR. The dNLR may be used as an alternative to the NLR, as it is easily available and inexpensive.
Keywords: neutrophil, lymphocyte, hepatocellular carcinoma, transarterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is the sixth most frequent cancer and the third most common cause of cancer-associated mortality in the world (1). There are numerous risk factors associated with the causes of liver disease, including hepatitis B virus (HBV), hepatitis C virus (HCV), alcohol abuse (≥80 g/day), iron overload and fatty liver, which results in HCC being heterogeneous and complex (2–4). The prognosis of patients with HCC is poor. Resection, liver transplantation and percutaneous treatment may be curative for an early stage of tumor, which accounts for ≤30% of patients. For intermediate to advanced-stage HCC, transarterial chemoembolization (TACE) is considered to be the standard treatment by certain international guidelines (5). A meta-analysis reveals that TACE improves survival in patients with unresectable HCC, who have been selected as they have intermediate stage, multimodular and Performance Status Test (PST) 0 or advanced stage, portal invasion, lymph node 1, metastasis 1 and PST 1–2, and evidence obtained from randomized controlled trials has confirmed the beneficial effect of TACE in improving survival (6,7). However, studies have also shown that not all patients with unresectable HCC benefit from TACE. Therefore, it is crucial to differentiate between patients that are most likely to benefit from TACE and those that are not in a heterogeneous HCC population.
The pathogenesis of HCC is based on inflammation. Particularly in China, the majority of HCC cases develop due to underlying chronic HBV infection. Tumor inflammation and immunology have previously been identified to enable cancer characteristics, and increasing evidence supports the involvement of inflammation and immunology in cancer progression and metastases (8,9). In addition, the combination of hematological components of the systemic inflammatory response have been shown to have prognostic value in patients with a variety of cancers, including the Glasgow prognostic score (GPS) (10–13), modified GPS (mGPS) (10–13), neutrophil to lymphocyte ratio (NLR) (14–17), prognostic nutritional index (PNI) (18), platelet-lymphocyte ratio (PLR) (19) and prognostic index (PI) (20). Of all these scores, the NLR is the most inexpensive and easily obtained. Studies have previously shown that an elevated NLR indicated a poor prognosis for patients with HCC (14–17). However, in clinical trials, only the white blood cell and neutrophil counts of the patients are commonly entered into clinical trial databases. Therefore, Proctor et al (21) recently implemented a derived NLR (dNLR), which is composed of the neutrophil count and the white blood cell count minus neutrophil count. Proctor et al evaluated the prognostic value of dNLR on cancer outcome in different cancer types and demonstrated that the dNLR had a similar prognostic value to the well-established NLR, and dNLR was suggested to be a cheaper and more easily determinable parameter than NLR. However, the application of dNLR in HCC patients was not fully validated. The present study was conducted to investigate the prognostic value of the pre-treatment dNLR on overall survival (OS) in patients with unresectable HCC undergoing TACE.
Patients and methods
Patients
Patients treated with TACE for unresectable HCC between September 2009 and November 2011 at the Department of Hepatobiliary Surgery of Sun Yat-sen University Cancer Canter (Guangzhou, China) were identified using the prospective database of the hospital. The present study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center, and written informed consent was obtained from all patients.
The diagnosis of HCC was based on the diagnostic criteria for HCC used by the American Association for the Study of the Liver guidelines (22). HCC was diagnosed by at least two radiological images showing the characteristic features of HCC, or one radiological image showing characteristic features of HCC associated with elevated serum AFP level (≥400 ng/ml) or histopathological evidence. Patients who met all of the following criteria were included in analysis: i) No previous treatment prior to TACE; ii) HBV-positive; iii) no HCV and HIV expression; iv) liver function Child-Pugh grade A or B; and v) a follow-up period ≥3 months.
All parameters were recorded and evaluated as possible predictors of survival, such as the gender, age, C-reactive protein (CRP) level, white blood cell count (WBC), neutrophil count, lymphocyte count, platelet count (PLT), α-fetoprotein (AFP) level, alkaline phosphatase (ALP) level, total bilirubin level (TBIL), albumin (ALB) level, tumor size and number and vascular invasion status of patients.
TACE procedure
TACE was performed using a previously reported protocol (23). A selective 5-Fr catheter (Terumo Corporation, Tokyo, Japan) was introduced into the hepatic artery and visceral angiography was performed to assess the arterial blood supply to the liver. Depending on the size, location and arterial supply of the tumor, the tip of the catheter was advanced into the right or left hepatic artery; if all the tumors were fed by one enlarged independent hepatic artery branch, the tip of catheter was introduced into this tumor-feeding artery. If the conventional catheter could not enter the hepatic artery due to technical reasons, a 2.9-Fr micro catheter (Terumo Corporation, Tokyo, Japan) was used. Hepatic artery infusion chemotherapy was performed using 300 mg carboplatin (Bristol-Myers Squibb, New York, NY, USA). Subsequently, chemolipiodolization was performed using 50 mg epirubicin (Pfizer, Wuxi, Jiangsu, China), and 6 mg mitomycin C (Zhejiang Hisun Pharmaceutical Co. Ltd., Taizhou, Zhejiang, China) mixed with 5 ml lipiodol (Lipiodol Ultra-Fluide; Guerbet, Villepinte, France). If the chemolipiodolized arterial territory did not show stagnant flow, pure lipiodol was then injected. In certain patients, stasis in a tumor-feeding artery was not achieved, even subsequent to the injection of the maximum amount of iodized oil (25 ml), due to the large size of the tumor. Embolization was then performed in these patients with the injection of absorbable gelatin sponge particles (Gelfoam; Hangzhou ALC Ltd., Hangzhou, China), 1–2 mm in diameter, through the angiographic catheter. This treatment regimen was used consistently in the present study, regardless of tumor type and size.
Follow-up
Patients were followed carefully subsequent to treatment. Patients underwent liver computed tomography (CT) scans 1 month subsequent to TACE, and liver CT scans were performed at 3-month intervals during the first 2 years, then every 6 months thereafter, with physical examination, blood tests for the AFP level and liver function. When metastasis was suspected, CT chest, bone scintigraphy, positron emission tomography (PET) and biopsy, if indicated, were also performed to confirm the presence of metastasis. The end of follow-up was December 2013, which was the time of the last follow-up, or the date of mortality.
Another session of TACE was performed every 4–10 weeks after the original administration of TACE until CT scans and AFP levels indicated stabilization of the tumor, or until TACE was not technically feasible, either due to hepatic artery occlusion or impaired liver function. The OS time was defined as the interval between the date of treatment and the date of mortality or last follow-up of surviving patients. Causes of mortality were determined from death certificates, medical interviews and radiological findings.
Statistical analysis
The receiver operating characteristic (ROC) analysis was used to determine the cut-off values of NLR and dNLR. The OS was calculated using the Kaplan-Meier method and compared by the log-rank test. The prognostic varieties in predicting the OS were assessed by multivariate Cox proportional hazards regression analysis. All covariates that affected survival at the P<0.10 level of significance in univariate analysis were included in the multivariate Cox proportional hazards model. A ROC curve was also generated and the area under the curve (AUC) was calculated to evaluate the discriminatory ability. The association between the NLR and dNLR was assessed by Spearman's rank correlation analysis. Data are expressed as the mean ± standard deviation. All statistical tests were two-sided, and P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using the SPSS 13.0 statistical software (SPSS, Inc., Chicago, IL, USA).
Results
Baseline characteristics
A total of 279 consecutive patients that met the inclusion criteria were included in present study. The baseline characteristics of the patients are summarized in Table I. Overall, 251 patients were male (90%) and 28 patients were female (10%), with a median age of 50 years (range, 23–80 years). The majority of the present patients exhibited a good liver functional reserve, classified as Child-Pugh grade A (92.5%).
Table I.
Baseline characteristics and univariate analysis for overall survival in 279 patients undergoing transarterial chemoembolization for hepatocellular carcinoma.
| Variables | n | Value | Univariate analysis P-value | ||
|---|---|---|---|---|---|
| Median age, years (range) | 50 (23–80) | 0.049 | |||
| <50 | 158 | ||||
| ≥50 | 121 | ||||
| Gender, n | NA | 0.111 | |||
| Male | 251 | ||||
| Female | 28 | ||||
| Mean WBC, n x109/1 (range) | 6.6 (2.1–24.6) | 0.080 | |||
| <9 | 36 | ||||
| ≥9 | 243 | ||||
| Mean neutrophil count, n ×109/1 (range) | 4.2 (0.7–21.5) | 0.019 | |||
| <7 | 28 | ||||
| ≥7 | 251 | ||||
| Mean lymphocyte count, n ×109/1 (range) | 1.5 (0.3–4.8) | 0.166 | |||
| <0.8 | 268 | ||||
| ≥0.8 | 11 | ||||
| Mean PLT count, n ×109/1 (range) | 182 (23–548) | 0.412 | |||
| <100 | 47 | ||||
| ≥100 | 232 | ||||
| Mean ALT, µ/1 (range) | 56.6 (8–304) | 0.003 | |||
| <40 | 169 | ||||
| ≥40 | 110 | ||||
| Mean AST, µ/1 (range) | 75.8 (19.3–472.6) | <0.001 | |||
| <45 | 191 | ||||
| ≥45 | 88 | ||||
| Mean albumin, g/1 (range) | 39 (25–79) | 0.052 | |||
| <35 | 238 | ||||
| ≥35 | 41 | ||||
| Mean total serum bilirubin, µmol/1 (range) | 17.8 (4.8–222.9) | <0.001 | |||
| <20 | 68 | ||||
| ≥20 | 191 | ||||
| Mean ALP, IU/1 (range) | 150 (13–761.5) | <0.001 | |||
| <110 | 171 | ||||
| ≥110 | 126 | ||||
| Mean AFP, ng/ml (range) | 751.2 (1.26–1,210,000) | <0.001 | |||
| <400 | 154 | ||||
| ≥400 | 125 | ||||
| Mean AFU, U/1 (range) | 35.2 (13–992) | 0.022 | |||
| <40 | 105 | ||||
| ≥40 | 174 | ||||
| Mean PT, sec (range) | 12.5 (9.8–36.8) | 0.001 | |||
| ≤13.5 | 242 | ||||
| >13.5 | 37 | ||||
| Mean diameter of largest lesion, cm (range) | 10 (1.4–20.0) | <0.001 | |||
| <10 | 109 | ||||
| ≥10 | 170 | ||||
| Tumor number, n | NA | 0.041 | |||
| Solitary | 83 | ||||
| Multiple | 196 | ||||
| Vascular invasion, n | NA | <0.001 | |||
| Absent | 185 | ||||
| Present | 94 | ||||
| Child-Pugh grade, n | NA | 0.005 | |||
| A | 258 | ||||
| B | 21 | ||||
| NLR, n | NA | 0.001 | |||
| <2.6 | 139 | ||||
| ≥2.6 | 140 | ||||
| dNLR, n | NA | 0.002 | |||
| <1.8 | 153 | ||||
| ≥1.8 | 126 |
NA, not applicable; WBC, white blood cell count; PLT, platelet count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; AFP, α-fetoprotein; AFU, α-L-fucosidase; PT, prothrombin time; NLR, neutrophil to lymphocyte ratio; dNLR, derived NLR.
By applying the aforementioned criteria, a cut-off value of 2.6 for NLR and 1.8 for dNLR was determined by ROC analysis to be best to discriminate between patients' survival in the whole cohort. In total, 140 patients had NLR ≥2.6 (50%) and 126 patients had dNLR ≥1.8 (45.2%). The association between inflammatory scores and clinicopathological features was analyzed (Table II). The NLR and dNLR were each associated with patient age, tumor size, presence of vascular invasion, and aspartate aminotransferase (AST) and ALP levels.
Table II.
Association between NLR or dNLR and clinical variables.
| NLR, n | dNLR, n | |||||
|---|---|---|---|---|---|---|
| Variables | ≤2.6 | >2.6 | P-value | ≤1.8 | >1.8 | P-value |
| Age | 0.03 | 0.016 | ||||
| >50 years | 87 | 71 | 96 | 62 | ||
| ≤50 years | 52 | 69 | 57 | 64 | ||
| Gender | 0.437 | 0.179 | ||||
| Male | 127 | 124 | 141 | 110 | ||
| Female | 12 | 16 | 12 | 16 | ||
| Diameter of largest lesion | <0.001 | <0.001 | ||||
| >10 cm | 29 | 80 | 35 | 74 | ||
| ≤10 cm | 106 | 56 | 114 | 48 | ||
| Number of lesions | 0.096 | 0.235 | ||||
| 1 | 35 | 48 | 41 | 42 | ||
| >1 | 104 | 92 | 112 | 84 | ||
| Vascular invasion | <0.001 | <0.001 | ||||
| Absent | 107 | 78 | 115 | 70 | ||
| Present | 32 | 62 | 38 | 56 | ||
| ALT | 0.063 | 0.024 | ||||
| >40 µ/1 | 76 | 93 | 84 | 85 | ||
| ≤40 µ/1 | 63 | 47 | 69 | 41 | ||
| AST | <0.001 | <0.001 | ||||
| >45 µ/1 | 81 | 110 | 94 | 97 | ||
| ≤45 µ/1 | 58 | 30 | 59 | 29 | ||
| Total serum bilirubin | 0.049 | 0.104 | ||||
| >20 µmol/1 | 25 | 42 | 32 | 35 | ||
| ≤20 µmol/1 | 114 | 98 | 121 | 91 | ||
| ALP | <0.001 | 0.001 | ||||
| >110 IU/1 | 72 | 98 | 82 | 88 | ||
| ≤110 IU/1 | 67 | 42 | 71 | 38 | ||
| AFP | 0.115 | 0.204 | ||||
| >400 ng/ml | 70 | 83 | 79 | 74 | ||
| ≤400 ng/ml | 69 | 57 | 74 | 52 | ||
| PT | 0.012 | 0.128 | ||||
| Normal | 126 | 116 | 137 | 105 | ||
| Abnormal | 13 | 24 | 16 | 21 | ||
| Child-Pugh grade | 0.507 | 0.489 | ||||
| A | 130 | 128 | 143 | 115 | ||
| B | 9 | 12 | 10 | 11 | ||
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; AFP, α-fetoprotein; PT, prothrombin time; NLR, neutrophil to lymphocyte ratio; dNLR, derived NLR.
Survival and prognostic factors
The median follow-up period was 446 days. The 1, 2 and 3-year OS rates were 38.8, 18.5 and 11.1% respectively, and the median OS time was 264 days. The univariate and multivariate analyses of prognostic factors for OS were analyzed. In univariate analysis (Table I), age (P=0.049), CRP (P<0.001), alanine aminotransferase (ALT; P=0.003), AST (P<0.001), ALP (P<0.001), LDH (P<0.001), α-L-fucosidase (AFU; P=0.024), TBIL (P<0.001), AFP (P<0.001), prothrombin time (PT; P=0.001), tumor size (P<0.001), tumor number (P=0.041), vascular invasion (P<0.001), metastasis (P<0.001), Child-Pugh scores (P=0.005), NLR (P=0.001) and dNLR (P=0.002) were prognostic factors for OS.
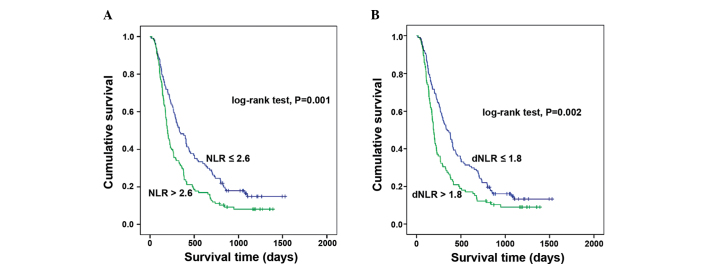
Multivariate analysis (Table III) showed that NLR [hazard ratio (HR), 1.382; 95% confidence interval (CI), 1.037–1.842; P=0.027], ALT (HR, 1.472; 95% CI, 1.099–1.971; P=0.01), and AFP (HR, 1.677; 95% CI, 1.259–2.233; P<0.001) were independent prognostic factors for OS. When NLR was replaced by dNLR, the multivariate analysis also showed that the dNLR (HR, 1.445; 95% CI, 1.086–1.923; P=0.012) was an independent prognostic factor for OS, along with the ALT and AFP levels. An elevated NLR or dNLR is associated with a poor prognosis (P=0.001 and P=0.002 respectively; Fig. 1).
Table III.
Multivariate analyses of prognostic factors for overall survival in 279 patients undergoing TACE for hepatocellular carcinoma.
| Multivariate analysis | |||
|---|---|---|---|
| Variables | Value | Hazard ratio (95% CI) | P-value |
| NLRa | |||
| Mean ALT, µ/1 (range) | 56.6 (8–304) | 1.472 (1.099–1.971) | 0.010 |
| Mean AFP, ng/ml (range) | 751.2 (1.26–1,210,000) | 1.677 (1.259–2.233) | <0.001 |
| NLR, n | |||
| ≤2.6 | 139 | 1.382 (1.037–1.842) | 0.027 |
| >2.6 | 140 | ||
| dNLRa | |||
| Mean ALT, µ/1 (range) | 56.6 (8–304) | 1.469 (1.098–1.966) | 0.010 |
| Mean AFP, ng/ml (range) | 751.2 (1.26–1,210,000) | 1.720 (1.294–2.287) | <0.001 |
| dNLR, n | |||
| ≤1.8 | 153 | 1.445 (1.086–1.923) | 0.012 |
| >1.8 | 126 | ||
NLR and dNLR were used as covariates, adjusted by ALT and AFP. ALT, alanine aminotransferase; ALP, alkaline phosphatase; NLR, neutrophil to lymphocyte ratio; dNLR, derived NLR.
Figure 1.
Kaplan-Meier survival curves for the overall survival of 279 patients undergoing transarterial chemoembolization for hepatocellular carcinoma. (A) NLR, (B) dNLR. NLR, neutrophil to lymphocyte ratio; dNLR, derived NLR.
Association and comparison between the NLR and dNLR
The association between the NLR and dNLR was assessed by Spearman's rank correlation analysis. There was a significant correlation between NLR and dNLR (R=0.875; P<0.001). The prognostic power of NLR and dNLR was compared using AUC analysis. As shown in Fig. 2, the AUC of NLR was 0.539 (95% CI, 0.423–0.656) and the AUC of dNLR was 0.522 (95% CI, 0.406–638), which was similar.
Figure 2.
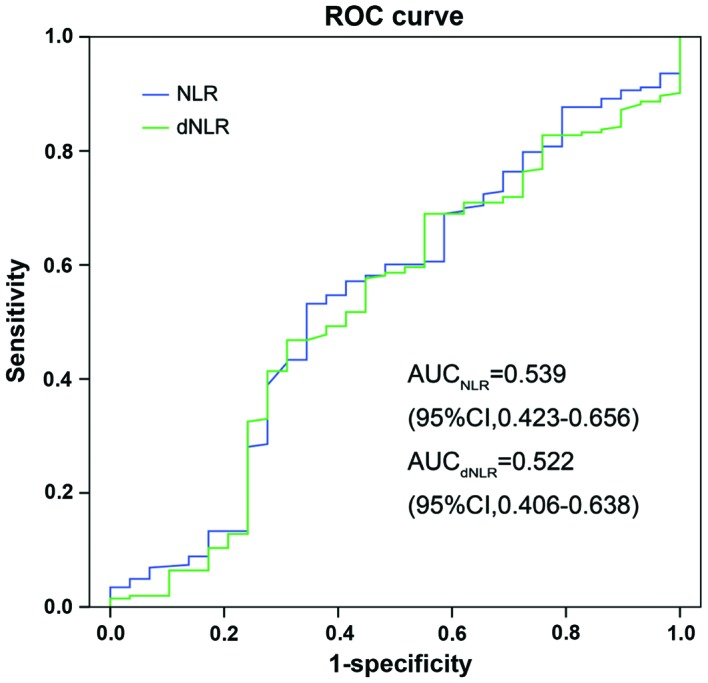
Comparison of AUC for the prediction of the outcome for patients with NLR and dNLR. NLR, neutrophil to lymphocyte ratio; dNLR, derived NLR; ROC, receiver operating characteristic; AUC, area under the curve.
Discussion
In the present study, the prognostic power of the NLR and dNLR was evaluated in patients with HBV-associated HCC undergoing TACE. The present results demonstrated that there was a significant correlation between NLR and dNLR, and NLR and dNLR each predicted the prognosis of patients with a similar prognostic power. Thus, the dNLR may be used as an alternative to NLR.
Previous studies (10–13) have shown that inflammation scores, such as the GPS, mGPS, NLR, PLR, PI and PNI, are associated with the prognosis of patients with HCC undergoing surgical resection, transplantation, TACE and RFA. Among these inflammation-based scores, NLR is inferior to other measures of the systemic inflammatory response, including mGPS, but it is less expensive and more readily available in day-to-day oncological practice (24). It is therefore notable that the NLR has been shown to have prognostic value in patients with a variety of cancers, and dynamic changes in the NLR may predict the prognosis of patients (25). Proctor et al (21) evaluated the prognostic value of the dNLR in a large cohort of 12,118 patients with different cancer types, including hepatopancreaticobiliary cancer (n=721). This study clearly demonstrated that the dNLR has a similar predictive ability for prognosis as the NLR, with patients with an elevated dNLR demonstrating a poor clinical outcome, which can be equally used to predict survival (21). The advantage of the dNLR compared with the NLR is that the dNLR remains available in the absence of the lymphocyte count, and may therefore be widely used on the basis of clinical trial databases.
It is generally accepted that inflammatory processes in the tumor microenvironment play a crucial role in promoting the proliferation, invasion and metastasis of malignant cells (26,27). The infiltrating leucocytes are important factors in this process (26). There are two elements to the dNLR, consisting of the neutrophil count, and the white blood cell count minus the neutrophil count. The latter count is dominated by lymphocytes and monocytes. Neutrophils in the peripheral blood or in the tumor microenvironment have been shown to produce pro-angiogenic factors, including vascular endothelial growth factor, to stimulate tumor development and progression (27). The cytokines involved in cancer-associated inflammation, including interleukin-6 (IL-6) and tumor necrosis factor-α (TNFα), may induce neutrophilia (28,29). The para-neoplastic production of myeloid growth factors by cancer cells may act as an additional cause of neutrophilia (30). Therefore, a high peripheral neutrophil level may indicate a cancer-associated inflammation or tumor progression, and predict poor clinical outcome. In addition, the neutrophils and leucocytes are mostly composed of lymphocytes. Immune cells that infiltrate into or around the tumor engage in dynamic and extensive crosstalk with cancer cells (31). Over the past decade, there has been growing evidence that lymphocytes act as crucial components of the adaptive immune system and are the cellular basis of cancer immuno-surveillance and immuno-editing (32,33). Furthermore, infiltrating lymphocytes have been reported to indicate the generation of an effective anti-tumor cellular immune response (34,35). Therefore, a low lymphocyte count may be responsible for an inadequate immunological reaction to the tumor, and consequently, a weakened defense against cancer, resulting in a poor prognosis (35). The peripheral monocyte count is known to be increased in cancer patients (36–38). Schmidt et al (36) created a prognostic model in metastatic melanoma based on independent prognostic factors in 321 patients receiving IL-2-based immunotherapy. This study showed that an elevated monocyte count may replace an elevated neutrophil count as an independent prognostic factor for poor survival (36). Leitch et al (39) compared the prognostic value of an inflammation-based prognostic score in 149 patients with colorectal cancer and concluded that the monocyte count was independently associated with cancer-specific survival. One possible hypothesis is that macrophages express chemokine (C-X-C motif) (CXC) receptors 1 and 2, corresponding with CXC ligand (CXCL)1, also termed Gro-α, and CXCL8, also termed IL-8, respectively. These chemokines may be involved in tumor invasion and angiogenesis. However, monocytes only account for <8% of leucocytes, with limited effect on the dNLR or NLR.
In the present study the association between the NLR and the dNLR was analyzed by Spearman's rank correlation analysis and it is not notable that a significant correlation was identified between the NLR and the dNLR. The prognostic power of the NLR and the dNLR was also compared, and AUC analysis showed that the prognostic power was similar between the two. In the Cox proportional hazards regression analysis for OS, either NLR or dNLR were considered to be an independent prognostic factor, with a similar hazard ratio. All these results indicated that the dNLR may be used as an alternative to NLR in these patients.
The elevated ALT and AFP levels were also revealed as independent prognostic factors for poor outcome, as has been reported in previous studies (40–42). Notably, in patients with chronic hepatitis and cirrhosis, an increase in the AST/ALT ratio is associated with progressive liver functional impairment (43,44). As a major mammalian embryo-specific and tumor-associated protein, AFP has been used for the diagnosis and screening of HCC worldwide. An increased AFP level is connected with larger tumors and lower hypohepatia, reflecting an aggressive biology (45).
There are potential limitations of the present study, as follows: i) It is a retrospective, small sample, single-institution study; ii) only patients treated with TACE were recruited; and iii) the patient population is biased due to the prevalence of HBV infection, which is unusual in Western countries. Therefore, a large-scale prospective validation study is required to confirm the present results.
The current results revealed that an elevated dNLR predicted poor prognosis with a similar prognostic power to the NLR in patients with HBV-associated HCC undergoing TACE. Due to the dNLR being an easily available and inexpensive marker in clinical studies, the dNLR should be considered as a novel prognostic marker for patients with HBV-associated HCC in routine practice.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N, Horlander JC, Sr, Said A, Hoen H, Kopecky KK, Stockberger SM, Jr, Manam R, Kwo PY, Lumeng L. Screening for hepatocellular carcinoma in patients with advanced cirrhosis. Am J Gastroenterol. 1999;94:2988–2993. doi: 10.1111/j.1572-0241.1999.01448.x. [DOI] [PubMed] [Google Scholar]
- 4.Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797–1801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- 5.Saraswat VA, Pandey G, Shetty S. Treatment algorithms for managing hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:S80–S89. doi: 10.1016/j.jceh.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 8.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dagg K, Scott HR. A prospective longitudinal study of performance status, an inflammation-based score (GPS) and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2005;92:1834–1836. doi: 10.1038/sj.bjc.6602591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto K, Ikeda Y, Korenaga D, Tanoue K, Hamatake M, Kawasaki K, Yamaoka T, Iwatani Y, Akazawa K, Takenaka K. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103:1856–1864. doi: 10.1002/cncr.20976. [DOI] [PubMed] [Google Scholar]
- 12.Ishizuka M, Kubota K, Kita J, Shimoda M, Kato M, Sawada T. Usefulness of a modified inflammation-based prognostic system for predicting postoperative mortality of patients undergoing surgery for primary hepatocellular carcinoma. J Surg Oncol. 2011;103:801–806. doi: 10.1002/jso.21857. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Fushiya N, Koike K, Nishino H, Tajiri H. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer. 2012;107:988–993. doi: 10.1038/bjc.2012.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, Prasad KR. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–1762. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- 15.Halazun KJ, Hardy MA, Rana AA, Woodland DC, IV, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS, Jr, Emond JC. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141–151. doi: 10.1097/SLA.0b013e3181a77e59. [DOI] [PubMed] [Google Scholar]
- 16.Huang ZL, Luo J, Chen MS, Li JQ, Shi M. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol. 2011;22:702–709. doi: 10.1016/j.jvir.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 17.Chen TM, Lin CC, Huang PT, Wen CF. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol. 2012;27:553–561. doi: 10.1111/j.1440-1746.2011.06910.x. [DOI] [PubMed] [Google Scholar]
- 18.Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: The prognostic nutritional index (PNI) Br J Cancer. 2012;106:1439–1445. doi: 10.1038/bjc.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasymjanova G, MacDonald N, Agulnik JS, Cohen V, Pepe C, Kreisman H, Sharma R, Small D. The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr Oncol. 2010;17:52–58. doi: 10.3747/co.v17i4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, Ghaneh P. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466–472. doi: 10.1016/j.amjsurg.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 21.Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. 2012;107:695–699. doi: 10.1038/bjc.2012.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283–287. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 23.Chen MS, Li JQ, Zhang YQ, Lu LX, Zhang WZ, Yuan YF, Guo YP, Lin XJ, Li GH. High-dose iodized oil transcatheter arterial chemoembolization for patients with large hepatocellular carcinoma. World J Gastroenterol. 2002;8:74–78. doi: 10.3748/wjg.v8.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O'Reilly DS, Foulis AK, Horgan PG, McMillan DC. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 25.Dan J, Zhang Y, Peng Z, Huang J, Gao H, Xu L, Chen M. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PloS One. 2013;8:e58184. doi: 10.1371/journal.pone.0058184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 27.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulich TR, del Castillo J, Keys M, Granger GA, Ni RX. Kinetics and mechanisms of recombinant human interleukin 1 and tumor necrosis factor-alpha-induced changes in circulating numbers of neutrophils and lymphocytes. J Immunol. 1987;139:3406–3415. [PubMed] [Google Scholar]
- 29.Ulich TR, del Castillo J, Guo KZ. In vivo hematologic effects of recombinant interleukin-6 on hematopoiesis and circulating numbers of RBCs and WBCs. Blood. 1989;73:108–110. [PubMed] [Google Scholar]
- 30.Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: An analysis of Japan multinational trial organisation LC00-03. Eur J Cancer. 2009;45:1950–1958. doi: 10.1016/j.ejca.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Dunn GP, Ikeda H, Bruce AT, Koebel C, Uppaluri R, Bui J, Chan R, Diamond M, White JM, Sheehan KC, Schreiber RD. Interferon-gamma and cancer immunoediting. Immunol Res. 2005;32:231–245. doi: 10.1385/IR:32:1-3:231. [DOI] [PubMed] [Google Scholar]
- 34.Rabinowich H, Cohen R, Bruderman I, Steiner Z, Klajman A. Functional analysis of mononuclear cells infiltrating into tumors: Lysis of autologous human tumor cells by cultured infiltrating lymphocytes. Cancer Res. 1987;47:173–177. [PubMed] [Google Scholar]
- 35.Hoffmann TK, Dworacki G, Tsukihiro T, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–2562. [PubMed] [Google Scholar]
- 36.Schmidt H, Bastholt L, Geertsen P, Christensen IJ, Larsen S, Gehl J, von der Maase H. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: A prognostic model. Br J Cancer. 2005;93:273–278. doi: 10.1038/sj.bjc.6602702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho H, Kim JH. Multiplication of neutrophil and monocyte counts (MNM) as an easily obtainable tumour marker for cervical cancer. Biomarkers. 2009;14:161–170. doi: 10.1080/13547500902777616. [DOI] [PubMed] [Google Scholar]
- 38.Millrud CR, Kvarnhammar Månsson A, Uddman R, Björnsson S, Riesbeck K, Cardell LO. The activation pattern of blood leukocytes in head and neck squamous cell carcinoma is correlated to survival. PloS One. 2012;7:e51120. doi: 10.1371/journal.pone.0051120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leitch EF, Chakrabarti M, Crozier JE, McKee RF, Anderson JH, Horgan PG, McMillan DC. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer. 2007;97:1266–1270. doi: 10.1038/sj.bjc.6604027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernaez R, Yeh HC, Lazo M, Chung HM, Hamilton JP, Koteish A, Potter JJ, Brancati FL, Clark JM. Elevated ALT and GGT predict all-cause mortality and hepatocellular carcinoma in Taiwanese male: A case-cohort study. Hepatol Int. 2013;7:1040–1049. doi: 10.1007/s12072-013-9476-6. [DOI] [PubMed] [Google Scholar]
- 41.Tarao K, Rino Y, Takemiya S, Tamai S, Ohkawa S, Sugimasa Y, Miyakawa K, Morinaga S, Yoshida M, Shibuya A, et al. Close association between high serum ALT and more rapid recurrence of hepatocellular carcinoma in hepatectomized patients with HCV-associated liver cirrhosis and hepatocellular carcinoma. Intervirology. 2000;43:20–26. doi: 10.1159/000025019. [DOI] [PubMed] [Google Scholar]
- 42.El-Serag HB, Kramer JR, Chen GJ, Duan Z, Richardson PA, Davila JA. Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA. Gut. 2011;60:992–997. doi: 10.1136/gut.2010.230508. [DOI] [PubMed] [Google Scholar]
- 43.Giannini E, Botta F, Fasoli A, Ceppa P, Risso D, Lantieri PB, Celle G, Testa R. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci. 1999;44:1249–1253. doi: 10.1023/A:1026609231094. [DOI] [PubMed] [Google Scholar]
- 44.Giannini E, Risso D, Botta F, Chiarbonello B, Fasoli A, Malfatti F, Romagnoli P, Testa E, Ceppa P, Testa R. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med. 2003;163:218–224. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- 45.Carr BI, Guerra V, Giannini EG, Farinati F, Ciccarese F, Rapaccini GL, Di Marco M, Benvegnù L, Zoli M, Borzio F, et al. Significance of platelet and AFP levels and liver function parameters for HCC size and survival. Int J Biol Markers. 2014;29:e215–e223. doi: 10.5301/jbm.5000064. [DOI] [PubMed] [Google Scholar]