Abstract
The abscopal effect is a term that has been used to describe the phenomenon of tumour regression in sites distant from targeted fields of irradiation. It has been reported in multiple malignancies and is thought to be due to a systemic immune response that radiation elicits in the treated individual. We describe the case of a female patient who originally presented with advanced multiple myeloma in 1996 at the age of 50. She failed multiple chemotherapeutic regimens including high-dose melphalan with autologous stem cell transplantation. Subsequently, the patient achieved a sustained complete remission, after receiving palliative radiotherapy to a symptomatic gastric plasmacytoma. She has remained in remission for >15 years. To the best of our knowledge, this case represents the first report of an abscopal effect against multiple myeloma.
Background
Radiation has been used for many years in the treatment of malignancy. Its primary mechanism of action appears to be through direct toxic effects on tumour cells.1 However, increasing attention has focused recently on the potential of radiotherapy to induce systemic host immune responses with potent antitumour activity. The theory of radiation therapy inducing a systemic immune response is based, in part, on a few rare case reports describing tumour regression in sites distant from the irradiated fields, a phenomenon known as ‘the abscopal effect’. The term is derived from Latin; ‘ab’ and ‘scopy’ meaning ‘away from’ and ‘target’, respectively. The abscopal effect was first described in 1953 by Mole2 and has been increasingly reported in clinical scenarios and investigated in laboratory studies.
Case presentation
Currently, a 69-year-old woman was diagnosed with IgG λ multiple myeloma (MM) in 1996. She was initially treated with conventional melphalan and prednisone for four cycles with a minor response. She was subsequently treated with vincristine, BCNU, adriamycin and prednisone (VBAP) for eight cycles but again achieved only a limited response. Salvage therapy with cyclophosphamide, dexamethasone, etoposide, cisplatin (CDEP) achieved a good response with reduction of plasma cells in the bone marrow to 8–9%. She underwent mobilisation chemotherapy with cyclophosphamide (2 g/m2) on 22 December 1997. Consolidative high-dose melphalan and autologous stem cell transplant was performed in February 1998 with subsequent complete remission by March of that year. In November of 1998, a bone marrow biopsy revealed relapse with 20% myeloma cells, on which a course of interferon α and dexamethasone was administered. The patient responded well to the latter treatment and achieved a partial remission with fluctuating serum IgG levels. In May of 1999, she developed bony pain and was found to have new osteolytic lesions involving the left humerus, right scapula and bilateral clavicles. She was managed with escalating doses of thalidomide and pulsed doses of corticosteroids. Palliative radiation to symptomatic bony sites was initiated as well and completed in June of that year. In September of 1999, new nodular lesions were detected by radiographic imaging in the left parietal skull and right thigh. A biopsy of the right thigh lesion was consistent with a plasmacytoma and further palliative radiation was administered to the involved sites in October of that year. In November of 1999, the patient was hospitalised for gastrointestinal (GI) bleeding with melena. She had several palpable nodules over the trunk and extremities. CT of the abdomen demonstrated a new gastric lesion, which proved to be a plasmacytoma per biopsy. Palliative irradiation to the stomach lesion was initiated and completed by the end of November of 1999 with subsidence of GI haemorrhage and early satiety. Table 1 details the radiation therapy given to the patient throughout the course of her disease.
Table 1.
A table of the radiation therapy given to the patient throughout the course of her disease
| Region treated | Radiation energy (MV) | Minimum tumour dose (cGy) | From | To | Total time (days) |
|---|---|---|---|---|---|
| Left humerus | 6 | 2000 | 5/24/1999 | 6/3/1999 | 10 |
| Bilateral clavicles | 9 | 1000 | 5/24/1999 | 5/27/1999 | 3 |
| Right scapula+clavicle | 6 | 1750 | 5/28/1999 | 6/8/1999 | 11 |
| Left clavicle | 9 | 1000 | 5/28/1999 | 6/3/1999 | 10 |
| Left skull | 12 | 2400 | 10/7/1999 | 10/25/1999 | 18 |
| Right anterior thigh | 16 | 2400 | 10/7/1999 | 10/25/1999 | 18 |
| Right posterior thigh | 9 | 2400 | 10/7/1999 | 10/25/1999 | 18 |
| Stomach APPA | 6 | 500 | 11/11/1999 | 11/12/1999 | 1 |
| Stomach RPO/LPO | 9 | 1600 | 11/15/1999 | 11/24/1999 | 13 |
APPA, anteroposterior-posteroanterior; LPO, left posterior-anterior oblique; RPO, right posterior-anterior oblique.
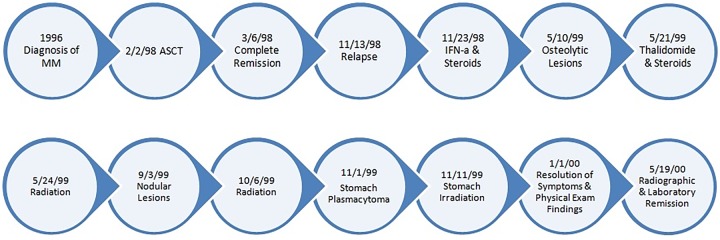
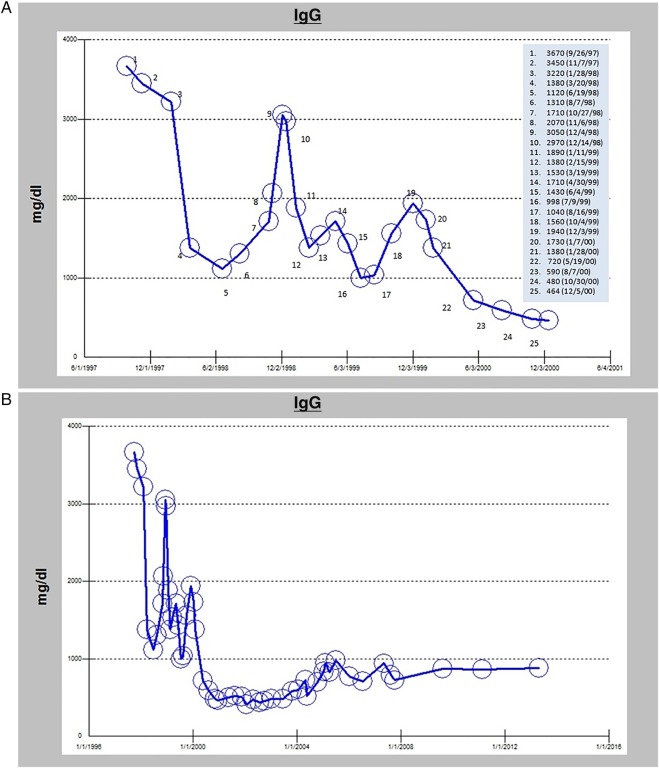
However, the patient had persistent generalised aches and pains. In December, she noted the development of a new nodule near the head of the left triceps. When evaluated in clinic in January 2000, the lesion was approximately 4×5 cm in size. However, it had stopped growing as per the patient’s report. The patient also noticed decreasing bony pains. Throughout January of 2000, the patient experienced reduction of all palpable nodules and symptomatic relief of her bony pains. A timeline of the highlights in the course of the disease from diagnosis to remission is depicted in figure 1. At her follow-up examination in March, all nodular lesions outside the radiation field were no longer detectable on physical examination. Serum IgG levels also decreased significantly (figure 2A) and sequential radiographs showed stable bony changes without further progression.
Figure 1.
A timeline of the highlights in the course of the disease from diagnosis to remission. ASCT, autologous stem cell transplant; IFN-α, interferon α; MM, multiple myeloma.
Figure 2.
A histogram of the serum IgG levels (A) from the time of diagnosis until remission and (B) from the time of diagnosis, over the course of the disease, to date.
Outcome and follow-up
She continued to be evaluated at the oncology clinic over the next 15 years to the present time. Follow-up IgG levels fluctuated minimally, but remained within normal range (figure 2B). Serial bone surveys showed chronic stable sequelae at the sites of old osteolytic lesions, without any progression or new findings. The patient has experienced arthritic pains of the bilateral knees and left shoulder but otherwise has enjoyed excellent quality of life in the years subsequent to her remission.
Discussion
The effect of radiation
The direct impact of ionising radiation is cellular death. It is thought to be mediated through DNA damage, which leads to mitotic catastrophe, necrosis and apoptosis.1 In this model, tumours regress due to impaired damage repair capacity while normal tissues recover through five presumed steps: repair, reassortment, repopulation, reoxygenation and radiosensitivity.3 Recent studies, however, suggest that the response to radiation also involves the immune system as a whole. The immunomodulatory characteristics of radiation can be summarised in four points: (1) exposing the tumour by altering its microenvironment and facilitating the presentation of its antigens to the immune system; (2) activating the different immune cells lines, including the innate and adaptive effector cells through various pathways; (3) enhancing the proinflammatory process by induction of various cytokines and inflammatory markers and (4) by attenuating inhibitory immunoregulatory mechanisms.4 The desired outcome is to mount an immune response capable of reducing tumour burden to the point of complete elimination.
Reliably achieving antitumour immunogenic effects of radiation remains a challenge, as many factors are implicated in the process. Demaria and Formenti5 looked at the predictors of an abscopal effect. They suggested a gene signature based on individual tumour characteristics, the genetic susceptibility of the host, and the nature of the radiation regimen each contributes to the phenomenon. This was derived from their review of the literature which revealed significant variation in the appropriate radiation dose and fractionation between different studies—in vitro, in preclinical and in clinical cases. Furthermore, radiation therapy may be non-beneficial, but it could also have deleterious consequences, such as secondary carcinogenesis. The correlation between radiation exposure and development of malignancy is well documented in the literature, including sites that are distant from the irradiated areas.6 The broad immunomodulatory effects of radiation, which could be therapeutic in some circumstances, have the potential of becoming carcinogenic due to chronic inflammation, oxidative stress and genetic instability.7
The role of immunotherapy
One approach to reinforce the favourable outcomes of radiotherapy has been the incorporation of concurrent immunotherapy. This was first highlighted in a case of malignant melanoma presented by Postow et al8 where the benefit of immune checkpoint blockade by cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibody was enhanced by the use of radiation. The aforementioned report opened the door to additional reports9 and research in the field. Various other immune potentiators have been described to date, such as a blockade of transforming growth factor β.10
The abscopal effect in haematological malignancy
The abscopal effect in a haematological malignancy was first reported by Li11 who described the leucocytopenic effect of splenic irradiation in patients with leukaemia in 1963. In 1969, Nobler12 noted a similar phenomenon in a patient with lymphoma and multiple case reports followed thereafter.13 14 The suggested mechanism of the abscopal effect in lymphoma is thought to be largely due to the irradiation of the spleen;15 as cancerous lymphocytes circulate through the irradiated spleen, they become damaged, which results in the overall regression of malignancy. This is different from the postulated mechanism in solid malignancies, which is thought to be due to the release of various cytokines and inflammatory markers such as tumour necrosis factor.16 Other aspects of the immune system have been implicated as well in the abscopal effect, for example, the enhanced response of natural killer cells in some cases.17
Our case
On review of the disease timeline in our case, we noted a correlation between the stomach irradiation and the remission that occurred shortly thereafter. We suggest two explanations to this phenomenon: the proximity of the irradiated stomach plasmacytoma to the spleen, and the overall cumulative dose of radiation. Splenic irradiation has been suggested to explain the abscopal effect in haematological malignancies, as detailed above. This remains a possibility in our case, although it is less likely considering the targeted radiation to the stomach. Additionally, MM differs from leukaemia and lymphoma in the less circulating nature of cancerous cells evidenced by the low levels of malignant cells in peripheral blood. The disease course does not favour a delayed response to prior medical treatments, considering the elapsed time between those therapies and the noted response. Additionally, the transient adenopathy following stomach irradiation may be due to the initial inflammatory response,18 and a similar occurrence has been reported in a case of natural killer cell lymphoma.19 The constellation of our findings points towards an abscopal effect due to a global immunogenic effect of radiation, similar to that seen in solid tumours. This makes our case the first reported in the literature of the abscopal effect in MM and one of the very few, to the best of our knowledge, of long- term survival (20 years to date) and possible cure.
Learning points.
The adaptive survival of malignancies is a fascinating phenomenon based on very complex interactions between neoplastic cells and the normal host milieu. The mechanisms by which tumour cells evade host immune defences remain unclear.
The abscopal effect is a term that has been used to describe the phenomenon of tumour regression in sites distant from targeted fields of irradiation. It is thought that perturbation of the tumour environment by appropriately targeted radiotherapy can bolster strong host defence mechanisms with long-lasting benefit.
Our case highlights the distal effects local radiation therapy can have on disseminated haematological malignancies such as multiple myeloma and implicates a role for radiation in the induction of systemic host immune responses capable of mediating enduring tumour regression, even in the absence of active immunotherapy.
We encourage clinicians to report cases of the abscopal effect to increase the level of awareness of this promising phenomenon in the battle against malignancy.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ross GM. Induction of cell death by radiotherapy. Endocr Relat Cancer 1999;6:41–4. 10.1677/erc.0.0060041 [DOI] [PubMed] [Google Scholar]
- 2.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953;26:234–41. 10.1259/0007-1285-26-305-234 [DOI] [PubMed] [Google Scholar]
- 3.Steel GG, McMillan TJ, Peacock JH. The 5Rs of radiobiology. Int J Radiat Biol 1989;56:1045–8. 10.1080/09553008914552491 [DOI] [PubMed] [Google Scholar]
- 4.de la Cruz-Merino L, Illescas-Vacas A, Grueso-López A.et al. , Cancer Immunotherapies Spanish Group (GETICA). Radiation for awakening the dormant immune system, a promising challenge to be explored. Front Immunol 2014;5:102 10.3389/fimmu.2014.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol 2012;2:153 10.3389/fonc.2012.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hei TK, Zhou H, Chai Y et al. Radiation induced non-targeted response: mechanism and potential clinical implications. Curr Mol Pharmacol 2011;4:96–105. 10.2174/1874467211104020096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 2003;3:276–85. 10.1038/nrc1046 [DOI] [PubMed] [Google Scholar]
- 8.Postow MA, Callahan MK, Barker CA et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925–31. 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiniker SM, Chen DS, Knox SJ. Abscopal effect in a patient with melanoma. N Engl J Med 2012;366:2035; author reply 2035–6 10.1056/NEJMc1203984#SA1 [DOI] [PubMed] [Google Scholar]
- 10.Vanpouille-Box C, Diamond JM, Pilones KA et al. TGFβ is a master regulator of radiation therapy-induced anti-tumor immunity. Cancer Res 2015;75:2232–42. 10.1158/0008-5472.CAN-14-3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li JG. The leukocytopenic effect of focal splenic x-irradiation in leukemic patients. Radiology 1963;80:471–6. 10.1148/80.3.471 [DOI] [PubMed] [Google Scholar]
- 12.Nobler MP. The abscopal effect in malignant lymphoma and its relationship to lymphocyte circulation. Radiology 1969;93:410–12. 10.1148/93.2.410 [DOI] [PubMed] [Google Scholar]
- 13.Rees GJ. Abscopal regression in lymphoma: a mechanism in common with total body irradiation? Clin Radiol 1981;32:475–80. 10.1016/S0009-9260(81)80310-8 [DOI] [PubMed] [Google Scholar]
- 14.Antoniades J, Brady LW, Lightfoot DA. Lymphangiographic demonstration of the abscopal effect in patients with malignant lymphomas. Int J Radiat Oncol Biol Phys 1977;2:141–7. 10.1016/0360-3016(77)90020-7 [DOI] [PubMed] [Google Scholar]
- 15.Byhardt RW, Brace KC, Wiernik PH. The role of splenic irradiation in chronic lymphocytic leukemia. Cancer 1975;35:1621–5. [DOI] [PubMed] [Google Scholar]
- 16.Ohba K, Omagari K, Nakamura T et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut 1998;43:575–7. 10.1136/gut.43.4.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchida A, Mizutani Y, Nagamuta M et al. Effects of X-ray irradiation on natural killer (NK) cell system. I. Elevation of sensitivity of tumor cells and lytic function of NK cells. Immunopharmacol Immunotoxicol 1989;11:507–19. 10.3109/08923978909005381 [DOI] [PubMed] [Google Scholar]
- 18.Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers. Int J Mol Sci 2014;15:927–43. 10.3390/ijms15010927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isobe Y, Aritaka N, Sasaki M et al. Spontaneous regression of natural killer cell lymphoma. J Clin Pathol 2009;62:647–50. 10.1136/jcp.2008.062976 [DOI] [PubMed] [Google Scholar]