Abstract
Neurodegenerative disorders such as Alzheimers disease (AD) are multifaceted and there are currently a limited number of therapeutic strategies available to treat them. Aspirin is known to act on multiple therapeutic targets and is a successful anti-inflammatory agent in various tissues. The present study aimed to ascertain the performance of aspirin when employed as a therapeutic agent to treat neurodegeneration on novel targets, including opioid system genes, in an AlCl3-induced neurotoxicity mouse model. The effects of two doses of aspirin (5 and 20 mg/kg aspirin for 12 days) were investigated in an AlCl3-induced neurotoxicity mouse model (150 mg/kg AlCl3 for 12 days). Neurological improvements were assessed through different behavioral tests and the effects of aspirin on opioid system gene expression levels were assessed by reverse transcription-polymerase chain reaction. Both doses resulted in improvements in cognitive behavior. A 5 mg/kg dose of aspirin was revealed to be effective for spatial memory improvement (7.14±0.84 sec), whilst a 20 mg/kg dose was superior for improving extinction learning (7.63±4.04%). Aspirin (5 mg/kg) also significantly improved contextual memory (48.05±10.6%) when compared with the AlCl3-treated group (1.49±0.62%; P<0.001). Aspirin was also observed to significantly decrease δ-opioid receptor expression in the cortex (1.09±0.08 and 1.27±0.08, respectively) at both doses (5 and 20 mg/kg) when compared with the AlCl3-treated group (3.69±1.43; P<0.05). Furthermore, aspirin at 5 mg/kg significantly reduced expression of prodynorphin in the cortex (0.57±0.20) when compared with the AlCl3-treated group (1.95±0.84; P<0.05). Notably, the effect of aspirin was significant in the cortex but not in the hippocampus. In summary, aspirin was effective in ameliorating the AD-like symptoms via the modulation of opioid systems. However, additional studies are required to determine the long term effects of aspirin on such conditions.
Keywords: hippocampus, amygdala, δ opioid receptors, κ opioid receptors, social preference, fear memory
Introduction
The worldwide prevalence of dementia was 35.6 million in 2010, with 58% of patients with dementia residing in countries of low or middle income (1). There are multiple causes associated with neurodegenerative diseases. Certain chemicals, including trace elements and metals, such as aluminium, copper, arsenic, mercury, lead and manganese, are neurotoxicant in high amount (2). In particular, aluminium is known for its neurotoxicant properties (3). Aluminium exposure through the oral route results in an accumulation within the body, particularly the brain (4,5), causing a number of neurological disorders, including Alzheimer's disease (AD) (6). An epidemiological study supported the hypothesis of an association between chronic exposure to aluminium and the incidence of AD (7); however, this has yet to be elucidated. Additionally it has been revealed that levels of aluminium are elevated in the brains of patients with AD (8).
Nonsteroidal anti-inflammatory drugs (NSAIDs) are used to treat inflammation (9). NSAIDs primarily inhibit cyclooxygenases (COXs), and their role in the modulation of ion channels and nociceptors is well established (9). NSAIDs have been shown to decrease the risk of AD by 50% (10,11). Amongst the NSAIDs, aspirin (acetylsalicylic acid) is one of the most prominently used in medical practice. Aspirin is widely utilized for the treatment of vascular dementia and possesses potential as a therapeutic agent in neurodegenerative disorders (12,13). Aspirin has been revealed to target pathological pathways in neurodegenerative disorders (14,15), however, the exact mechanisms of action of aspirin have yet to be elucidated. Furthermore, there is limited data concerning the therapeutic efficacy of aspirin on the opioid system in AD.
Endogenous opioid neuropeptides (including dynorphins and enkephalins) and their receptors (κ and δ) are important in modulating sensory, motivational, emotional, and cognitive function and addictive behaviors (16–18). Different types of opioid receptor (including µ, δ and κ) have unique patterns of distribution within the human brain, typically displaying a high density in gray matter, and are preferentially found in limbic and limbic-associated brain structures (19,20). The distribution of opioid receptors in these areas have a critical role in higher cognitive and emotional behaviors. These effects are hypothesized to be mediated by modulating neuronal firing, and the release of neurotransmitters and hormones (21,22).
Dynorphins, for example dynorphin A (Dyn A), Dyn B and α-neoendorphin, are derived from the long precursor prodynorphin (Pdyn), and modulate cognition through κ-opioid receptors (KOR) (23–25). Enkephalins, namely met- and leu-enkephalins, are produced by the proteolytic cleavage of pre-proenkephalin (Penk), which is responsible for the modulation of learning and memory (26), synaptic plasticity (27), and emotional behaviors (28).
Peptide (including somatostatin, corticotrophin releasing factor and opioid peptide) neurotransmission is vital for normal cognitive functions; however, it is impaired in neurodegeneration (29). There is strong evidence to suggest that alterations in the level of neuropeptides may be associated with neuropathology (30,31); however, the exact roles of the majority of peptides involved in neurodegeneration remains unclear.
The opioid system is hypothesized to have an association with neuropathology, making it an attractive therapeutic target in neurodegeneration. Thus, the present study aimed to assess the performance of a frequently used multi-targeting therapeutic agent, aspirin, known for its effectiveness against such novel targets, for the treatment of neurodegeneration induced by AlCl3. The results observed were promising in favour of the use of aspirin.
Materials and methods
Drugs and chemicals
AlCl3 hexahydrate (cat no. AL0770) was purchased from Scharlab, S.L. (Barcelona, Spain), aspirin (batch no. 3083) was purchased from the Reckitt Benckiser Group plc, (Karachi, Pakistan) and TRIzol was purchased from Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA). Chemicals used for polymerase chain reaction (PCR), including MgCl2, dNTPs and Taq polymerase (EP0402), were purchased from Fermentas (Thermo Fisher Scientific, Inc.).
Animals
All experiments performed complied with the Guide for the Care and Use of Laboratory Animals (32). The protocol was approved by the Internal Review Board, Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology (Islamabad, Pakistan). Male BALB/c mice (age, 3–4 months; weight range, 27–45 g) were purchased from the National Institute of Health (Islamabad, Pakistan). Mice were housed in a controlled environment (temperature, 25±2°C; natural light and dark cycle), and were provided with tap water and a standard diet. A total of 40 mice were used in this study (n=7–12 per group) and each mouse was assigned a unique identity.
Animal model for AlCl3-induced neurotoxicity, study design and aspirin treatment plan
An AlCl3-induced neurotoxicity mouse model was developed as previously reported by our laboratory (33). Animals were divided into four groups, termed: i) Control group, received normal saline through intraperitoneal (IP) injection for 12 days; ii) AlCl3-treated group, received 150 mg/kg AlCl3 through IP injection according to the weight of mice every day for 12 days; iii) aspirin 40 ppm group, received a 5 mg/kg dose of aspirin (40 ppm mixed in feed for 12 days; and iv) aspirin 160 ppm group, received a 20 mg/kg dose of aspirin (160 ppm mixed in feed for 12 days). The required dose/kg of aspirin was delivered by adding aspirin into the feed. The ppms of aspirin were calculated based on the feed consumption of mice prior to the commencement of the study.
Behavior testing
Behavior tests were performed on day 12 of treatment between 12:00 and 6:00 p.m. with minimal human interference. All tests were recorded using a video camera (Sony Corporation, Tokyo, Japan)and analyzed offline. The mice were transferred to the testing room 30 min prior to the first test.
Spatial reference memory
The Morris water maze test protocol was performed as previously described (33,34), with slight modifications. In this test, a 90-cm diameter pool containing a hidden platform submerged in the south western quadrant 1 cm below the surface of water was included. The water pool was made opaque and the temperature of the water was maintained at 20±2°C. The room contained high contrast spatial cues, including black geometric shapes against a white wall background.
Procedure
The test commenced on the seventh day of treatment and trials were conducted each day for the next 6 days. Mice underwent five trials each day with an inter-trial interval of 10 min. The platform position remained consistent throughout the trials while mouse starting direction differed in each trial. Maximum trial duration was 60 sec, and the time taken by the mouse to reach the platform was recorded and plotted as escape latency. In the event of a mouse failing to locate the platform within the duration, the mouse was guided to the platform and escape latency (sec) for that trial was taken as 60 sec.
Test trial
On the sixth day of the test, animals were assessed for their memory by performing a test trial. During test trial, the platform was removed, and the time spent by the mouse within the platform quadrant was recorded and plotted as platform exploration time (sec).
Social interaction behavior
The procedure was performed as described previously (33). The apparatus consisted of an iron box (40×40×40 cm) and two identical small cages placed within the box. The treated mice were termed test subject, and two further mice were termed mouse A and B, respectively. Mice A and B were of the same background with respect to age, gender and weight, and had not had any prior contact with the test subject mice.
Session I: Social affiliation test
Following a habituation period of 5 min inside the box, the cage containing mouse A was placed on one side of the box and an empty cage was placed on the other side. The test subject was allowed to explore both cages for 10 min and the time spent with the empty cage (defined as the test subject facing the cage, with a distance of <1 cm between the test subject and the cage) and mouse A were recorded. The percent interaction time (%) was calculated by the dividing the time spent exploring by the total time for mouse A and the empty cage.
Session II: Social novelty preference test
In this session, the empty cage was replaced with mouse B and mouse A remained unchanged. The test subject was allowed to explore for 10 min and the interaction time (%) was calculated by dividing the percent of time spent exploring with mouse A or mouse B by the total time.
Discrimination index (DI)
DIs for sessions I and II were calculated using the following formulas:
Session I
Time spent with mouse A + time spent with empty cage
Session II
Nesting behavior
The organizational behavior of the mice was assessed through the nesting test, following a previously described protocol (35). The test mice were kept in individual cages, containing a nestlet (a compressed cotton batting of weight 2 g), 1 h before the dark phase started. The following morning, the mice were scored between 1 and 5 dependent on the utilization of cotton and the quality of the nest, as follows: 1, cotton not noticeably touched; 2, cotton partially torn up; 3, cotton mostly shredded with no identifiable location; 4, identifiable nest but flat; 5, perfect or nearly perfect nest.
Fear learning and memory testing
Fear conditioning
Fear conditioning protocol was performed as previously described (36), with slight modifications. The system was loaded with an audio speaker to provide a tone [conditioned stimulus (CS)] of 3,000 Hz, 80 dB intensity and 30 sec duration. A foot shock [US (unconditioned stimulus)] was delivered at the end of the CS for 1 sec with 0.5 mA intensity. The test trial consisted of 2 min habituation followed by five CS-US pairings, each with 2 min intervals. Freezing was assessed as an index of fear response during the 30 sec of CS.
Contextual fear
Context-dependent fear memory was measured 24 h after fear conditioning in the same box in the absence of CS or US. The freezing response to the context was measured for 5 min.
Fear extinction
Fear extinction was performed in a different context to that of the fear conditioning chamber. The box utilized for this experiment was made of plastic. Following 7 min of habituation in the chamber, animals were exposed to 20 CS trials with each trial lasting for 30 sec and followed by a 30 sec interval. Freezing response was measured during CS trials and plotted.
Gene expression analysis
Gene expression analysis was performed in accordance with the previously described protocol (37). After completion of the behavior assessments on day 12, animals were anesthetized with diethyl ether (676845; Sigma-Aldrich, St. Louis, MO, USA) and sacrificed by decapitation in order to extract the cortex and hippocampus. RNA was extracted using TRIzol reagent, according to the manufacturer's protocol. From each sample, 1 µg extracted RNA was selected and reverse transcribed by reverse transcriptase (Fermentas; Thermo Fisher Scientific, Inc.) to produce cDNA (40 µl). cDNA was used for targeted gene amplification by PCR using gene-specific primers (Eurofins Genomics GmbH, Ebersberg, Germany) with different annealing temperatures, as indicated in Table I. The thermal cycling conditions were as follows: Initial denaturation at 95°C for 5 min followed by denaturation at 94°C for 30 sec, annealing for 30 sec and an initial extension at 72°C for 30 sec. This cycle was repeated for a specific number of times at specific annealing temperatures, as detailed in Table I. The final extension was performed at 72°C for 10 min. Actin was used as a housekeeping gene and to normalize gene expression. PCR amplified products were run on 2% agarose gel (Bio Basic, Inc., Markham, ON, Canada) and visualized by ethidium bromide staining (E7637; Sigma-Aldrich). The gel images of PCR product bands were captured and saved using a Dolphin-Doc Plus gel imaging system (1141004; Weltec Corporation, Sparks, NV, USA). Each PCR product band was quantified for densitometry using ImageJ software (National Institutes of Health, Bethesda, MO, USA; http://rsbweb.nih.gov/ij/).
Table I.
List of primers.
| Serial No. | Gene | Primer sequence (5′ to 3′) | Ta | Cycles, n |
|---|---|---|---|---|
| 1 | Pdyn | F, CAACCCCCTGATTTGCTCCC | 56 | 35 |
| R, GCTGGTAAGGAGTCGGCTTT | ||||
| 2 | Oprk1 | F, CCAATCAGCGATCTGGAGCTG | 58 | 35 |
| R, GATAACAGGGATGGCCGGAG | ||||
| 3 | Penk | F, AAGCTCTCATTGAGGCACCC | 56 | 35 |
| R, TGCACGCCAGGAAATTGATG | ||||
| 4 | Oprd1 | F, TCGTCCGGTACACCAAATTGA | 54 | 35 |
| R, GGAAGTCCAGGGCTTTGACA | ||||
| 5 | Actin | F, GCCTTCCTTCTTGGGTATGG | 55 | 32 |
| R, CAGCTCAGTAACAGTCCGC |
Ta, annealing temperature; Pdyn, prodynorphin; F, forward; R, reverse; Oprk1, opioid receptor, κ 1; Penk, pre-proenkephalin; Oprd1, opioid receptor, δ 1.
Statistical analysis
Results were analyzed statistically using GraphPad Prism software, version 5.0 (GraphPad Software, Inc., San Diego, CA, USA). For statistical analysis Bonferroni multiple comparison test was applied. P<0.05 was considered to indicate a statistically significant difference. Data are expressed as the mean ± standard error of the mean, where n indicates the number of animals/observations.
Results
Aspirin enhances spatial reference learning and memory in AlCl3-treated mice
Aspirin was investigated in order to determine its involvement in spatial reference memory through the use of the Morris water maze task. The AlCl3-treated group displayed impairments in the spatial reference memory test and learnt at a reduced pace (Fig. 1A), as demonstrated by the increased escape latency in the final 3 days (16.22±1.49 sec) when compared with the control group (7.53±1.21 sec; P<0.05; Fig. 1B). Aspirin administered at a dose of 40 ppm produced a memory enhancing effect (7.14±0.84 sec) from days 3–5; indicating its effectiveness compared with the AlCl3-treated group (P<0.05), whilst doses of 160 ppm were not as effective, compared with the AlCl3-treated group (Fig. 1B). During the test trial, which was performed on day 6 and involved the removal of the platform and recording of the time spent by the mouse within the platform quadrant, aspirin displayed a marked effect on spatial reference memory at doses of 40 and 160 ppm (38.83±5.03 and 36.82±5.51 sec, respectively), when compared with the AlCl3-treated group (13.43±2.03 sec; P<0.001; Fig. 1C).
Figure 1.
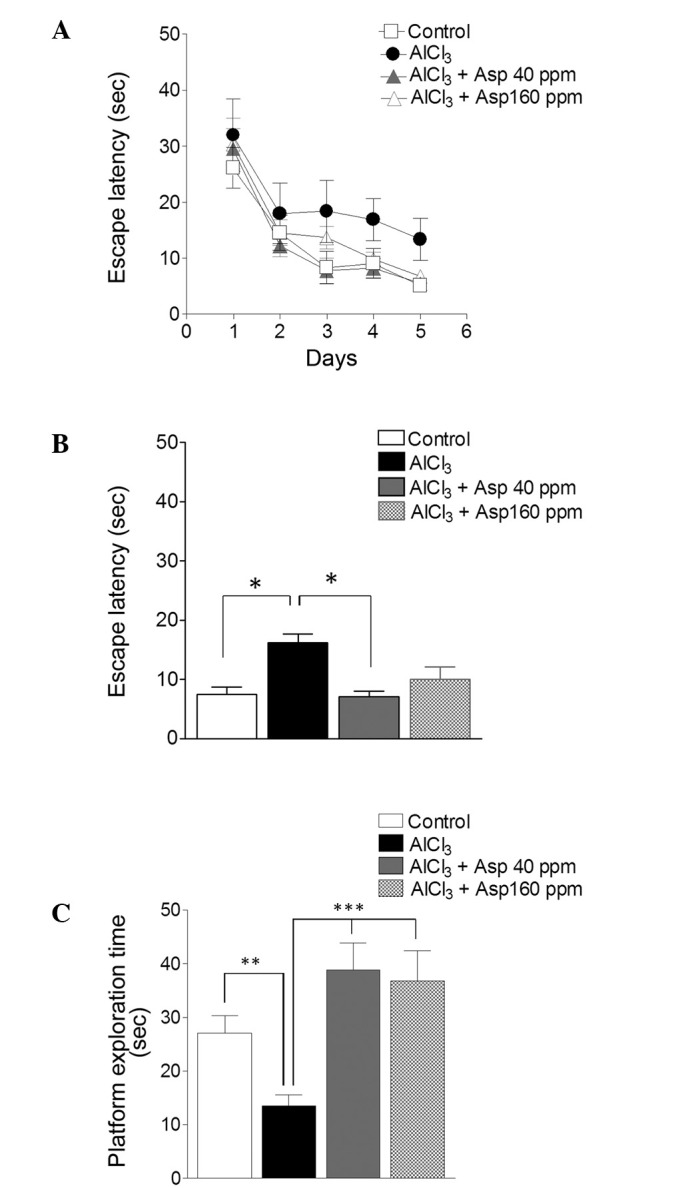
Spatial reference memory. Effects of Asp on memory as assessed by the Morris water maze test. (A) Spatial reference memory over 5 testing days. (B) Spatial reference memory on final 3 days. (C) Test trial on day 6. Data are presented as mean ± standard error of the mean (n=7–12). *P<0.05, **P<0.01, ***P<0.001 (analysis of variance followed by Bonferroni's comparison test). Asp, aspirin.
Aspirin improves social affiliation and social novelty preference in mice
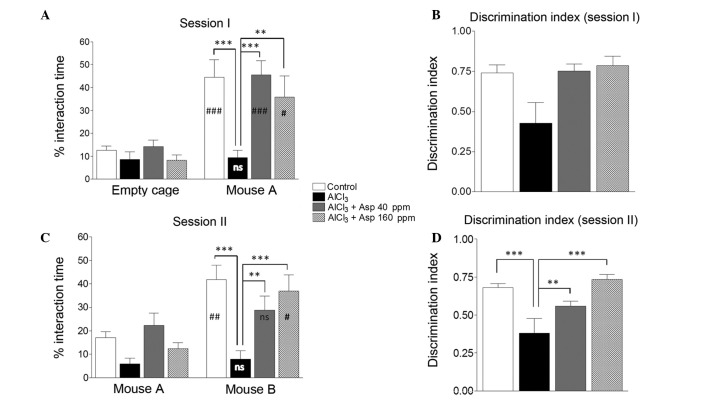
Aspirin was investigated for its effects on the sociability of mice by utilizing social interaction behavior. AlCl3-treated mice displayed significantly reduced levels of interaction with mouse A (9.38±3.33%) compared with the control group (44.52±7.68%; P<0.001). Following treatment with aspirin (40 and 160 ppm), there was a significant improvement in the sociability levels of the mice (45.6±6.09 and 35.72±9.38%, respectively; P<0.001 and P<0.01, respectively; Fig. 2A) compared with the AlCl3-treated group.
Figure 2.
Effect of Asp on social interaction behavior. (A) Interaction time and (B) discrimination index during session I (social affiliation). (C) Interaction time and (D) discrimination index for session II (social novelty preference). Data are presented as mean ± standard error of the mean (n=5–9). **P<0.01, ***P<0.001 (analysis of variance followed by Bonferroni's comparison test). #P<0.05, ##P<0.01, ###P<0.001 vs. empty cage (session I) or mouse A (session II) (unpaired t-test). n.s., no significance; Asp, aspirin.
The AlCl3-induced toxicity group failed to demonstrate social affiliation during session I, as is evident from the similar amount of time spent with mouse A (9.38±3.33%) as with the empty cage (8.65±3.19 %). Conversely, the control group displayed significantly greater interaction levels with mouse A, compared with the empty cage (44.52±7.68 vs. 12.64±1.81% respectively; P<0.001). Mice treated with aspirin at doses of 40 and 160 ppm displayed significantly increased (P<0.001) interaction times with mouse A (45.60±6.08 and 35.72±9.38%, respectively) compared with the empty cage [14.17±2.78% (P<0.001) and 8.26±2.35% (P<0.05), respectively; Fig. 2A).
DI in the AlCl3-treated group was significantly reduced in session I, compared with the control group (0.46±0.13 vs. 0.74±0.05, respectively). At doses of 40 and 160 ppm aspirin, an improvement in DI was observed (0.75±0.04 and 0.79±0.06, respectively; Fig. 2B), when compared with the AlCl3-treated group (0.46±0.13).
In session II, the empty cage was replaced with a new unfamiliar mouse (mouse B), and the mice were tested for social memory and social novelty preference. The AlCl3-treated group displayed significantly reduced interaction levels with mouse B, compared with the control group (7.94±3.48 vs. 41.73±6.27%, respectively; P<0.001). Following treatment with aspirin (40 and 160 ppm), social novelty preference was improved as compared with the AlCl3-treated group (28.69±6.12 and 37.01±6.89%, respectively; P<0.01 and P<0.001; Fig. 2C).
AlCl3-treated mice failed to demonstrate a preference for social novelty and spent similar amounts of time with mouse A (5.86±2.59%) and mouse B (7.94±3.48%). This was in contrast to the control group, which displayed a significantly greater percent interaction time with mouse B (41.73±6.27%) than with mouse A (17.08±2.55%; P<0.01). Mice treated with a 40 ppm dose of aspirin did not display significantly different interaction levels with mouse B and mouse A (28.69±6.12 vs. 22.29±5.26%, respectively; Fig. 2C). However, when treated with a 160 ppm dose of aspirin, mice showed significantly greater interaction levels with mouse B compared with mouse A (37.01±6.89 vs. 12.42±2.63, respectively; P<0.05; Fig. 2C), revealing the therapeutic effect of aspirin on social novelty preference at this dose.
The DI of the AlCl3-treated group was significantly reduced in session II, compared with the control group (0.38±0.09 vs. 0.68±0.02, respectively; P<0.001). However, mice treated with aspirin doses of 40 and 160 ppm displayed a significant improvement in DI when compared with AlCl3-treated mice [0.56±0.03 (P<0.01) and 0.73±0.03 (P<0.001), respectively; Fig. 2D].
Aspirin improves nesting behavior in mice
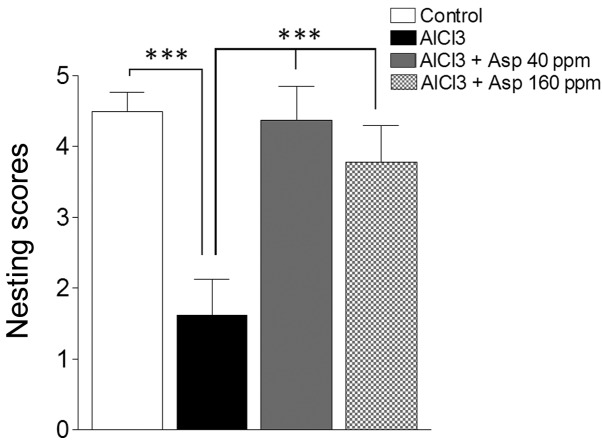
Aspirin was investigated for its role on nesting behavior. The AlCl3-treated group displayed a significant impairment in nest building behavior when compared with the control group (1.63±0.49 vs. 4.50±0.27, respectively; P<0.001). However, treatment with aspirin (40 and 160 ppm) resulted in significant improvements in nesting scores (4.37±0.49 and 3.79±0.52, respectively; P<0.001; Fig. 3) when compared with the AlCl3-treated group.
Figure 3.
Effect of Asp on nesting behavior. Data are presented as mean ± standard error of the mean (n=7–8). ***P<0.001 (analysis of variance followed by Bonferronis comparison test). Asp, aspirin.
Aspirin elevates contextual fear memory and enhances fear extinction learning in mice
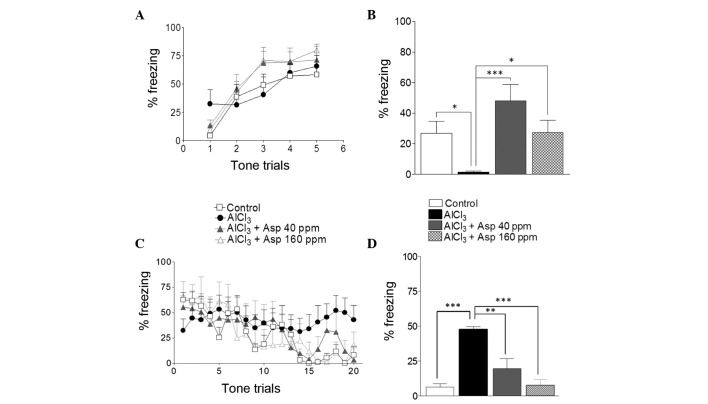
Aspirin was investigated for its role on fear memory and fear extinction learning through fear conditioning, and contextual memory and fear extinction tests. During conditioning, all groups of mice displayed similar levels of freezing response, which was employed to measure levels of fear (P>0.05; Fig. 4A).
Figure 4.
Effect of Asp on fear conditioning, contextual fear memory and fear extinction learning. (A) Fear conditioning test, with fear response plotted as % freezing during the conditioned stimulus trial. (B) Context-dependent fear memory, plotted as % freezing response. (C) Fear extinction learning over 20 trials. (D) Fear extinction learning in the final 4 CS trials. Data are presented as mean ± standard error of the mean (n=5–13). *P<0.05, **P<0.01 and ***P<0.001 (analysis of variance followed by Bonferronis comparison test). Asp, aspirin.
After 24 h of fear conditioning, mice were assessed for context-dependent fear memory. The AlCl3-treated group displayed significantly decreased levels of freezing (1.49±0.62%) in the context compared with the control group (26.81±7.92%; P<0.05). Mice treated with aspirin displayed an elevated freezing response at doses of 40 ppm (48.05±10.6; P<0.001) and 160 ppm (27.25±8.06; Fig. 4B) compared with the AlCl3-treated group.
Mice were then assessed for fear extinction learning. The control group and the aspirin-treated groups (40 and 160 ppm) demonstrated reduced levels of freezing response after 20 CS trials. By contrast, the AlCl3-treated group maintained high freezing response until the completion of the 20 CS trials (Fig. 4C). In the final four tone trials, the AlCl3-treated group displayed a high percentage freezing level, indicating impaired fear extinction learning compared with the control group (47.57±2.07 vs. 6.383±2.47%, respectively; P<0.001). The two doses of aspirin led to a reduction of the percent freezing [19.59±7.16% (P<0.01) and 7.63±4.04% (P<0.001), respectively; Fig. 4D) in the final four tones trials, indicating enhanced fear extinction learning in aspirin-treated groups compared with the AlCl3-treated group.
Aspirin does not affect Penk expression
Within the cortex, increased mRNA expression levels of Penk were observed in the AlCl3-treated group compared with the control group; however, the difference was not significant (1.71±1.10 vs. 0.42±0.05, respectively; P>0.05). The levels of Penk in mice treated with 40 and 160 ppm aspirin were not significantly different when compared with the AlCl3-treated group (0.79±0.09 and 0.62±0.14, respectively; P>0.05; Fig. 5A).
Figure 5.
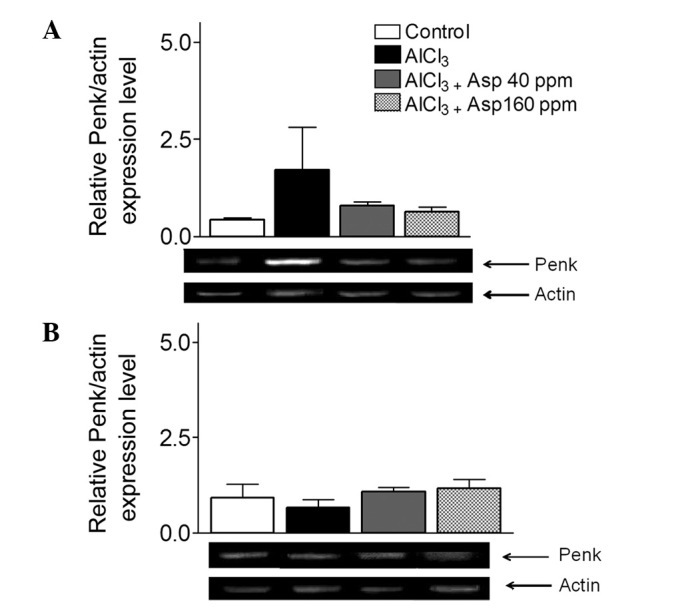
Effect of aspirin on Penk mRNA expression levels in the (A) cortex and (B) hippocampus, normalized with actin. Representative reverse transcription-polymerase chain reaction gels are displayed, and arrows indicate desired product and actin bands from cortex and hippocampus. Data are presented as mean ± standard error of the mean (n=4–8). Analysis of variance followed by Bonferronis comparison test. Penk, proenkephalin; Asp, aspirin.
Within the hippocampus, no significant differences in the levels of Penk were observed in AlCl3-treated mice compared with the control group. Following treatment with aspirin at both doses, expression levels remained unchanged (Fig. 5B).
Aspirin decreases Pdyn expression in the cortex
Within the cortex, Pdyn expression levels were significantly elevated in the AlCl3-treated group compared with the control group (1.95±0.84 vs. 0.49±0.08, respectively; P<0.05). Treatment with aspirin resulted in a decrease in Pdyn expression levels at the 40 ppm dose compared with the AlCl3-treated group (0.57±0.20 vs. 1.95±0.84; P<0.05); however, at the 160 ppm dose, no significant effect was observed (Fig. 6A).
Figure 6.
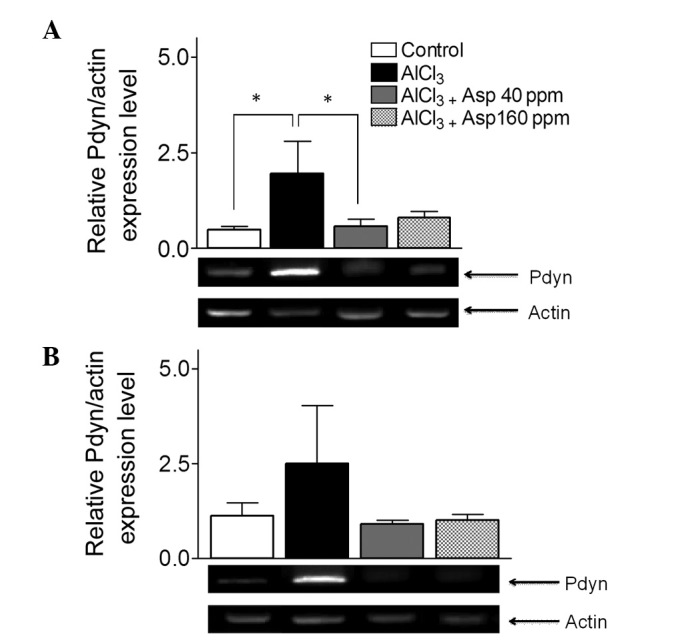
Effect of aspirin on Pdyn mRNA expression levels in the (A) cortex and (B) hippocampus normalized with actin. Representative reverse transcription-polymerase chain reaction gels are displayed and arrows indicate the desired product and actin bands from the cortex and hippocampus. Data are presented as mean ± standard error of the mean (n=4–5). *P<0.05 (analysis of variance followed by Bonferronis comparison test). Pdyn, prodynorphins; Asp, aspirin.
In the hippocampus, despite a general trend depicting an increase in Pdyn expression levels, there was no significant difference in Pdyn expression levels in the AlCl3-treated group compared with the control group (2.50±1.53 vs. 1.13±0.34, respectively; Fig. 6B). Furthermore, Pdyn expression levels in mice treated with aspirin doses of 40 and 160 ppm were not significantly different (0.90±0.11 and 1.01±0.16, respectively) when compared with the AlCl3-treated group (2.50±1.53; Fig. 6B).
Aspirin decreases δ opioid receptor (DOR) expression in the cortex
Within the cortex, DOR expression levels were significantly elevated in the AlCl3-treated group compared with the control group (3.69±1.43 vs. 0.86±0.10; Fig. 7A). Treatment with aspirin resulted in a significant decrease in DOR expression levels at doses of 40 (1.09±0.08) and (1.27±0.08) 160 ppm compared with the AlCl3-treated group (3.69±1.43; P<0.05; Fig. 7A).
Figure 7.
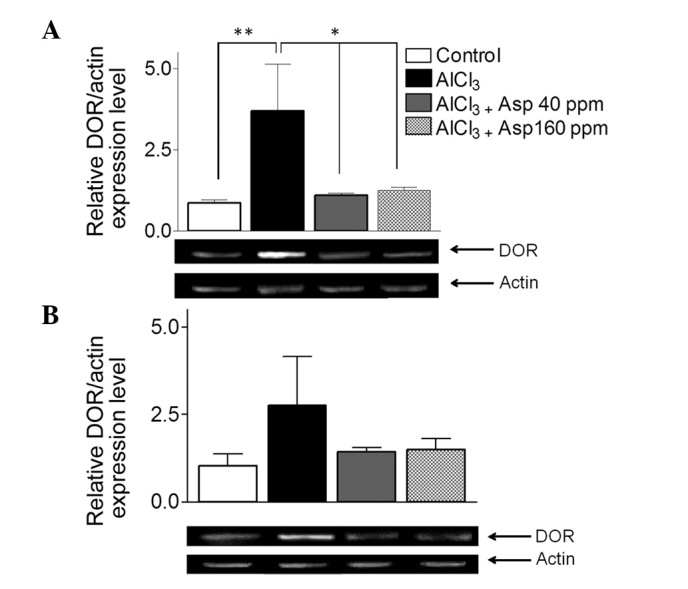
Effect of aspirin on DOR mRNA expression levels in the (A) cortex and (B) hippocampus, normalized with actin. Representative reverse transcription-polymerase chain reaction gels are displayed with arrows indicating desired product and actin bands from cortex and hippocampus. Data are presented as mean ± standard error of the mean (n=4–8). *P<0.05, **P<0.01 (analysis of variance followed by Bonferronis comparison test). DOR, δ opioid receptor; Asp, aspirin.
Within the hippocampus, no significant differences in DOR expression levels were observable in the AlCl3-treated group compared with the control group (2.76±1.41 vs. 1.04±0.33; Fig. 7B). Furthermore, DOR expression levels in the mice treated with aspirin doses of 40 (1.41±0.13) and 160 ppm (1.49±0.32) were not significantly different when compared with the AlCl3-treated group (3.69±1.43; P<0.05; Fig. 7B).
Aspirin does not affect KOR expression
Within the cortex, no significant differences in KOR expression levels were observed in the AlCl3-treated group compared with the control and aspirin-treated groups at both doses (P>0.05; Fig. 8A).
Figure 8.
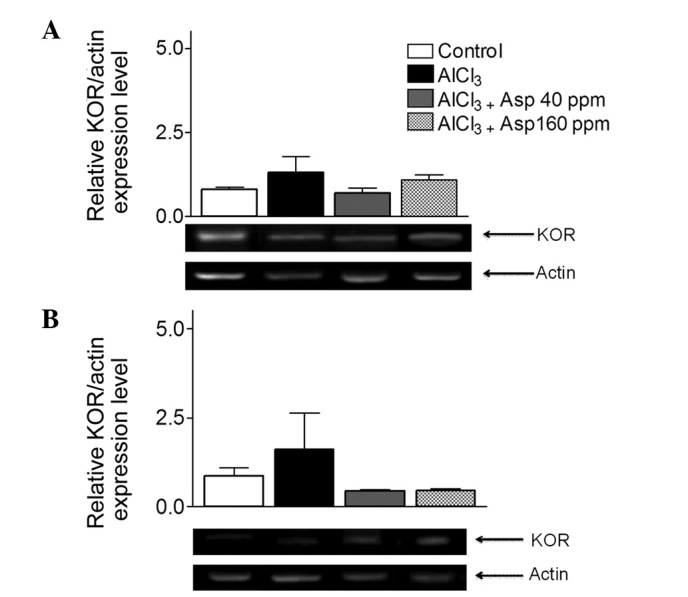
Effect of aspirin on KOR mRNA expression levels in (A) the cortex and (B) hippocampus normalized with actin. Analysis of variance followed by Bonferronis comparison test were performed. Representative reverse transcription-polymerase chain reaction gels are displayed with arrows indicate desired product and actin bands from cortex and hippocampus. Data are presented as mean ± standard error of the mean (n=4–5). Analysis of variance followed by Bonferronis comparison test. KOR, κ opioid receptor; Asp, aspirin.
Similarly, within the hippocampus, no significant difference in KOR expression levels were observed in the AlCl3-treated group (1.59±1.02) compared with the control group (0.86±0.23) or 40 and 160 ppm aspirin-treated groups (0.44±0.03 and 0.45±0.06; P>0.05; Fig. 8B).
Discussion
Aspirin is an anti-inflammatory drug that is known to reduce the synthesis of prostaglandin through the inhibition of COXs (38). Aspirin has multiple therapeutic targets, including COD enzymes and acid-sensing ion channels (39–41). As a result the present study aimed to assess the performance of aspirin against novel peptide targets, including Pdyn, Penk and their respective receptors, KOR and DOR. The opioid system is known for its role in neurodegeneration, cognitive impairment, behavioral dysfunction, amyloid β production and the hyperphosphorylation of τ proteins (42,43).
The hippocampus and cortex are regions of the brain that are vital for learning and memory, and are highly susceptible to damage in the early stages of neuropathology (44). The Morris water maze is typically employed to asses hippocampus- and cortex-dependent learning (34). Elevated expression levels of dynorphin and enkephalin within the hippocampus interfere with spatial learning and memory, and the effect is mediated by opioid receptors within the dorsal hippocampus (45). Through the results of the Morris water maze experiment, the present study revealed that mice treated with aspirin displayed improvements in learning, which is in accordance with observations made in a prior study (46). In the present study, mice treated with aspirin (40 ppm) displayed enhanced levels of learning during the final 3 days of assessment. A potential mechanism by which aspirin improved cognitive impairment may be a result of the downregulation of Pdyn and DOR expression levels in the cortex. The present study observed that a 40 ppm dose of aspirin was more beneficial than a 160 ppm dose in the Morris water maze test. In accordance with previous studies (46,47), the present study revealed a significant distinction in certain aspects of cognitive performance for mice treated with aspirin, compared with an AlCl3-treated group.
Social interaction behaviors are dependent on the hippocampus and cortex regions of the brain (48). KOR agonists were previously revealed to suppress all measures of social play in juvenile mice (49), confirming the inhibitory effects of elevated expression levels of dynorphin on the social behavior of mice. The present study revealed that aspirin-treated mice presented with significantly greater sociability levels when compared with the AlCl3-treated group. In addition, aspirin-treated mice displayed improved results in social novelty preference at both doses of aspirin administered (40 and 160 ppm) and the underlying mechanism may involve a reduction in dynorphin expression levels. However, further investigation is required to consolidate upon this hypothesis. These data suggest the potential of aspirin in cognition, as well as social behavior.
Mice construct nests for the purpose of thermoregulation and reproduction. Nesting behavior in mice indicates the integrity of the hippocampus and the cortex (35). Mice with lesioned hippocampi did not exceed a score of 3 in the nest scoring system in the present study, whilst scores of 4 or 5 indicated a high quality nest and, hence, a healthy hippocampus. Aspirin-treated mice displayed improved nesting scores with either of the two doses administered. Nest construction is a natural, species-typical behavior in rodents (50) and may be considered analogous to the ‘activities of daily living’. Thus, the present study provides evidence to suggest that aspirin has the ability to improve non-cognitive behaviors or the activities of daily living in neurodegenerative patients.
The parts of the brain involved in fear memory, including fear acquisition, contextual fear and fear extinction, are the amygdala, hippocampus, cortex and prefrontal cortex (51,52). Mice treated with aspirin (40 and 160 ppm) displayed improved fear acquisition levels during fear conditioning sessions, enhanced contextual fear memory levels and improved extinction learning compared with the AlCl3-treated mice. This exemplifies a novel role for aspirin in improving hippocampus and amygdala-dependent learning, and extending the therapeutic potential of aspirin in neurodegenerative diseases.
Endogenous dynorphin is involved in learning and memory through the inhibition of presynaptic glutamate release via KOR and modulation of its long-term potentiation (LTP), as a retrograde inhibitory neurotransmitter (53). Cognitive and behavioral deficits arise as a result of enkephalin upregulation and DOR alteration in neurodegeneration (53). It therefore follows that a cause of cognitive decline in neurodegeneration is elevated levels of dynorphins and enkephalins (45,54). The data presented in the current study concerning the expression levels of opioid system genes (Penk, Pdyn and their respective receptors, DOR and KOR), revealed that aspirin treatment decreased the expression levels of opioid system genes, which may be one of several mechanisms through which aspirin restores features damaged by cognitive impairment. The results of the current study demonstrated that the opioid system is partially affected by aspirin, as was evident by the absence of differences in hippocampal expression levels of opioid system genes (Penk, Pdyn, KOR and DOR) among the three groups (control, AlCl3-treated and aspirin-treated). Within the cortex, aspirin displayed a dose-specific effect, with only the 40 ppm dose of aspirin causing a reduction in the expression levels of Pdyn. The expression levels of DOR were affected at both doses of aspirin; however, no significant change in Penk or KOR expression levels were observed; rather, only a general trend toward decreased expression was observed. The hippocampus and cortex are equally important in neurodegenerative disorders, however, acetylcholine release (which is involved in the pathology of AD) has a greater effect on opiate agonists in the cortex in the brains of rats compared with the hippocampus, where opiate agonists display a dose-dependent effect on acetylcholine release (55). Considering the acetylcholine hypothesis concerning AD (56), we hypothesize that the opioid system will have marked effects on neurodegeneration and AD pathogenesis within the cortex through the reduction of acetylcholine release. Furthermore, the present study revealed that aspirin was more effective within the cortex, although the mechanism regarding its effect here remains unknown. In the cortex, aspirin significantly decreased the expression levels of Pdyn and DOR genes; this was accompanied by improvements in cognitive and non-cognitive behaviors. Another possible association between aspirin and dynorphin may be LTP, as aspirin is hypothesized to reverse LTP impairments (57) that occur as a result of elevated levels of dynorphin (53), thereby improving learning and memory.
Additionally, aspirin is known to improve cognitive decline in neurodegenerative disorders through multiple mechanisms (58–60) and, at higher doses, attenuation of inflammatory processes through the inhibition of COXs. The exact mechanism of action of aspirin within various disorders, including AD, remains relevant and requires further exploration.
In conclusion, aspirin improved learning, memory, social behavior and non-cognitive behavior in mice by decreasing Pdyn and DOR expression, suggesting its therapeutic potential as multitarget drug for AD. Further studies are required to assess long term effects and benefits of aspirin in AD.
Acknowledgements
The present authors thank the Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology (Islamabad, Pakistan) for providing funding, support and research facilities.
References
- 1.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75. doi: 10.1016/j.jalz.2012.11.007. e62. [DOI] [PubMed] [Google Scholar]
- 2.Pohl HR, Roney N, Abadin HG. Metal ions affecting the neurological system. Met Ions Life Sci. 2011;8:247–262. [PubMed] [Google Scholar]
- 3.Suárez-Fernández MB, Soldado AB, Sanz-Medel A, Vega JA, Novelli A, Fernández-Sánchez MT. Aluminum-induced degeneration of astrocytes occurs via apoptosis and results in neuronal death. Brain Res. 1999;835:125–136. doi: 10.1016/S0006-8993(99)01536-X. [DOI] [PubMed] [Google Scholar]
- 4.Platt B, Fiddler G, Riedel G, Henderson Z. Aluminium toxicity in the rat brain: Histochemical and immunocytochemical evidence. Brain Res Bull. 2001;55:257–267. doi: 10.1016/S0361-9230(01)00511-1. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Wang M, Ruan D, She J. Early chronic aluminium exposure impairs long-term potentiation and depression to the rat dentate gyrus in vivo. Neuroscience. 2002;112:879–887. doi: 10.1016/S0306-4522(02)00138-0. [DOI] [PubMed] [Google Scholar]
- 6.Walton JR. Chronic aluminum intake causes Alzheimer's disease: Applying Sir Austin Bradford Hill's causality criteria. Alzheimer's Dis. 2014;40:765–838. doi: 10.3233/JAD-132204. [DOI] [PubMed] [Google Scholar]
- 7.Bondy SC. The neurotoxicity of environmental aluminum is still an issue. Neurotoxicology. 2010;31:575–581. doi: 10.1016/j.neuro.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrási E, Páli N, Molnár Z, Kösel S. Brain aluminum, magnesium and phosphorus contents of control and Alzheimer-diseased patients. J Alzheimers Dis. 2005;7:273–284. doi: 10.3233/jad-2005-7402. [DOI] [PubMed] [Google Scholar]
- 9.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGeer EG, McGeer PL. The importance of inflammatory mechanisms in Alzheimer disease. Exp Gerontol. 1998;33:371–378. doi: 10.1016/S0531-5565(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 11.Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer's disease and duration of NSAID use. Neurology. 1997;48:626–632. doi: 10.1212/WNL.48.3.626. [DOI] [PubMed] [Google Scholar]
- 12.Molnar FJ, Man-Son-Hing M, St John P, Brymer C, Rockwood K, Hachinski V. Subcortical vascular dementia: Survey of treatment patterns and research considerations. Can J Neurol Sci. 1998;25:320–324. doi: 10.1017/S0317167100034351. [DOI] [PubMed] [Google Scholar]
- 13.Dennis M, Boyle A. Management of cognitive impairment of vascular origin. Psychiatri Bull. 1998;22:285–287. doi: 10.1192/pb.22.5.285. [DOI] [Google Scholar]
- 14.Hirohata M, Ono K, Naiki H, Yamada M. Non-steroidal anti-inflammatory drugs have anti-amyloidogenic effects for Alzheimer's beta-amyloid fibrils in vitro. Neuropharmacology. 2005;49:1088–1099. doi: 10.1016/j.neuropharm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Medeiros R, Kitazawa M, Passos GF, Baglietto-Vargas D, Cheng D, Cribbs DH, LaFerla FM. Aspirin-triggered lipoxin A4 stimulates alternative activation of microglia and reduces Alzheimer disease-like pathology in mice. Am J Pathol. 2013;182:1780–1789. doi: 10.1016/j.ajpath.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conason AH, Sher L. Alcohol use in adolescents with eating disorders. Int J Adolesc Med Health. 2006;18:31–36. doi: 10.1515/IJAMH.2006.18.1.31. [DOI] [PubMed] [Google Scholar]
- 17.Melzack R. From the gate to the neuromatrix. Pain. 1999;6(Suppl 6):S121–S126. doi: 10.1016/S0304-3959(99)00145-1. [DOI] [PubMed] [Google Scholar]
- 18.Snyder SH, Childers SR. Opiate receptors and opioid peptides. Annu Rev Neurosci. 1979;2:35–64. doi: 10.1146/annurev.ne.02.030179.000343. [DOI] [PubMed] [Google Scholar]
- 19.Blackburn TP, Cross AJ, Hille C, Slater P. Autoradiographic localization of delta opiate receptors in rat and human brain. Neuroscience. 1988;27:497–506. doi: 10.1016/0306-4522(88)90283-7. [DOI] [PubMed] [Google Scholar]
- 20.Hiller JM, Itzhak Y, Simon EJ. Selective changes in mu, delta and kappa opioid receptor binding in certain limbic regions of the brain in Alzheimer's disease patients. Brain Res. 1987;406:17–23. doi: 10.1016/0006-8993(87)90764-5. [DOI] [PubMed] [Google Scholar]
- 21.Alfaras-Melainis K, Gomes I, Rozenfeld R, Zachariou V, Devi L. Modulation of opioid receptor function by protein-protein interactions. Front Biosci (Landmark Ed) 2009;14:3594–3607. doi: 10.2741/3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simonds WF. The molecular basis of opioid receptor function. Endocr Rev. 1988;9:200–212. doi: 10.1210/edrv-9-2-200. [DOI] [PubMed] [Google Scholar]
- 23.Kuzmin A, Madjid N, Terenius L, Ogren SO, Bakalkin G. Big dynorphin, a prodynorphin-derived peptide produces NMDA receptor-mediated effects on memory, anxiolytic-like and locomotor behavior in mice. Neuropsychopharmacology. 2006;31:1928–1937. doi: 10.1038/sj.npp.1300959. [DOI] [PubMed] [Google Scholar]
- 24.Sandin J, Nylander I, Georgieva J, Schött PA, Ogren SO, Terenius L. Hippocampal dynorphin B injections impair spatial learning in rats: A kappa-opioid receptor-mediated effect. Neuroscience. 1998;85:375–382. doi: 10.1016/S0306-4522(97)00605-2. [DOI] [PubMed] [Google Scholar]
- 25.Ukai M, Itoh J, Kobayashi T, Shinkai N, Kameyama T. Effects of the kappa-opioid dynorphin A(1–13) on learning and memory in mice. Behav Brain Res. 1997;83:169–172. doi: 10.1016/S0166-4328(97)86063-9. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher M, King RA, Young NB. Opiate antagonists improve spatial memory. Science. 1983;221:975–976. doi: 10.1126/science.6879198. [DOI] [PubMed] [Google Scholar]
- 27.Derrick BE, Rodriguez SB, Lieberman DN, Martinez JL., Jr Mu opioid receptors are associated with the induction of hippocampal mossy fiber long-term potentiation. J Pharmacol Exp Ther. 1992;263:725–733. [PubMed] [Google Scholar]
- 28.Nieto MM, Guen SL, Kieffer BL, Roques BP, Noble F. Physiological control of emotion-related behaviors by endogenous enkephalins involves essentially the delta opioid receptors. Neuroscience. 2005;135:305–313. doi: 10.1016/j.neuroscience.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 29.Jiang HK, Owyang VV, Hong JS, Gallagher M. Elevated dynorphin in the hippocampal formation of aged rats: Relation to cognitive impairment on a spatial learning task. Proc Natl Acad Sci USA. 1989;86:2948–2951. doi: 10.1073/pnas.86.8.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis KL, Mohs RC, Marin DB, Purohit DP, Perl DP, Lantz M, Austin G, Haroutunian V. Neuropeptide abnormalities in patients with early Alzheimer disease. Arch Gen Psychiatry. 1999;56:981–987. doi: 10.1001/archpsyc.56.11.981. [DOI] [PubMed] [Google Scholar]
- 31.Saito T, Iwata N, Tsubuki S, Takaki Y, Takano J, Huang SM, Suemoto T, Higuchi M, Saido TC. Somatostatin regulates brain amyloid beta peptide Abeta42 through modulation of proteolytic degradation. Nat Med. 2005;11:434–439. doi: 10.1038/nm1206. [DOI] [PubMed] [Google Scholar]
- 32.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals: Guide for the Care and Use of Laboratory Animals. 8th. National Academic Press; Washington: 2011. [Google Scholar]
- 33.Hashmi AN, Yaqinuddin A, Ahmed T. Pharmacological effects of Ibuprofen on learning and memory, muscarinic receptors gene expression and APP isoforms level in pre-frontal cortex of AlCl3-induced toxicity mouse model. Int J Neurosci. 2015;125:277–287. doi: 10.3109/00207454.2014.922972. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed T, Enam SA, Gilani AH. Curcuminoids enhance memory in an amyloid-infused rat model of Alzheimer's disease. Neuroscience. 2010;169:1296–1306. doi: 10.1016/j.neuroscience.2010.05.078. [DOI] [PubMed] [Google Scholar]
- 35.Deacon R. Assessing burrowing, nest construction, and hoarding in mice. J Vis Exp. 2012:e2607. doi: 10.3791/2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S, Ahmed T, Lee S, Kim H, Choi S, Kim DS, Kim SJ, Cho J, Shin HS. Bidirectional modulation of fear extinction by mediodorsal thalamic firing in mice. Nat Neurosci. 2012;15:308–314. doi: 10.1038/nn.2999. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed T, Gilani AH. A comparative study of curcuminoids to measure their effect on inflammatory and apoptotic gene expression in an Aβ plus ibotenic acid-infused rat model of Alzheimer's disease. Brain Res. 2011;1400:1–18. doi: 10.1016/j.brainres.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Amann R, Peskar BA. Anti-inflammatory effects of aspirin and sodium salicylate. Eur J Pharmacol. 2002;447:1–9. doi: 10.1016/S0014-2999(02)01828-9. [DOI] [PubMed] [Google Scholar]
- 39.Fink M, Irwin P. Central nervous system effects of aspirin. Clin Pharmacol Ther. 1982;32:362–365. doi: 10.1038/clpt.1982.172. [DOI] [PubMed] [Google Scholar]
- 40.Bhatt LK, Addepalli V. Potentiation of aspirin-induced cerebroprotection by minocycline: A therapeutic approach to attenuate exacerbation of transient focal cerebral ischaemia. Diab Vasc Dis Res. 2012;9:25–34. doi: 10.1177/1479164111427753. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, Ye SD, Zhou KQ, Wu LM, Huang YN. High doses of salicylate and aspirin are inhibitory on acid-sensing ion channels and protective against acidosis-induced neuronal injury in the rat cortical neuron. J Neurosci Res. 2012;90:267–277. doi: 10.1002/jnr.22742. [DOI] [PubMed] [Google Scholar]
- 42.Anthony IC, Norrby KE, Dingwall T, Carnie FW, Millar T, Arango JC, Robertson R, Bell JE. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain. 2010;133:3685–3698. doi: 10.1093/brain/awq263. [DOI] [PubMed] [Google Scholar]
- 43.Lengauer E. Drug dependent adolescents have Alzheimer disease-like brains. Kinderkrankenschwester. 2007;26:37. (In German) [PubMed] [Google Scholar]
- 44.Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer's disease. Mol Neurodegener. 2011;6:85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDaniel KL, Mundy WR, Tilson HA. Microinjection of dynorphin into the hippocampus impairs spatial learning in rats. Pharmacol Biochem Behav. 1990;35:429–435. doi: 10.1016/0091-3057(90)90180-P. [DOI] [PubMed] [Google Scholar]
- 46.Smith JW, Al-Khamees O, Costall B, Naylor RJ, Smythe JW. Chronic aspirin ingestion improves spatial learning in adult and aged rats. Pharmacol Biochem Behav. 2002;71:233–238. doi: 10.1016/S0091-3057(01)00675-X. [DOI] [PubMed] [Google Scholar]
- 47.Breitner JC. The role of anti-inflammatory drugs in the prevention and treatment of Alzheimer's disease. Annu Rev Med. 1996;47:401–411. doi: 10.1146/annurev.med.47.1.401. [DOI] [PubMed] [Google Scholar]
- 48.Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: Integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci. 2006;18:871–879. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- 49.Vanderschuren LJ, Niesink RJ, Spruijt BM, Van Ree JM. Mu- and kappa-opioid receptor-mediated opioid effects on social play in juvenile rats. Eur J Pharmacol. 1995;276:257–266. doi: 10.1016/0014-2999(95)00040-R. [DOI] [PubMed] [Google Scholar]
- 50.Deacon RM. Assessing nest building in mice. Nat Protoc. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- 51.Einarsson EO, Nader K. Involvement of the anterior cingulate cortex in formation, consolidation, and reconsolidation of recent and remote contextual fear memory. Learn Mem. 2012;19:449–452. doi: 10.1101/lm.027227.112. [DOI] [PubMed] [Google Scholar]
- 52.Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- 53.Wagner JJ, Terman GW, Chavkin C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature. 1993;363:451–454. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meilandt WJ, Yu GQ, Chin J, Roberson ED, Palop JJ, Wu T, Scearce-Levie K, Mucke L. Enkephalin elevations contribute to neuronal and behavioral impairments in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2008;28:5007–5017. doi: 10.1523/JNEUROSCI.0590-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lapchak PA, Araujo DM, Collier B. Regulation of endogenous acetylcholine release from mammalian brain slices by opiate receptors: Hippocampus, striatum and cerebral cortex of guinea-pig and rat. Neuroscience. 1989;31:313–325. doi: 10.1016/0306-4522(89)90376-X. [DOI] [PubMed] [Google Scholar]
- 56.Muir JL. Acetylcholine, aging, and Alzheimer's disease. Pharmacol Biochem Behav. 1997;56:687–696. doi: 10.1016/S0091-3057(96)00431-5. [DOI] [PubMed] [Google Scholar]
- 57.Hein AM, O'Banion MK. Neuroinflammation and memory: The role of prostaglandins. Mol Neurobiol. 2009;40:15–32. doi: 10.1007/s12035-009-8066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas T, Nadackal TG, Thomas K. Aspirin and non-steroidal anti-inflammatory drugs inhibit amyloid-beta aggregation. Neuroreport. 2001;12:3263–3267. doi: 10.1097/00001756-200110290-00024. [DOI] [PubMed] [Google Scholar]
- 59.Harris JR. In vitro fibrillogenesis of the amyloid beta 1–42 peptide: Cholesterol potentiation and aspirin inhibition. Micron. 2002;33:609–626. doi: 10.1016/S0968-4328(02)00029-X. [DOI] [PubMed] [Google Scholar]
- 60.Tortosa E, Avila J, Pérez M. Acetylsalicylic acid decreases tau phosphorylation at serine 422. Neurosci Lett. 2006;396:77–80. doi: 10.1016/j.neulet.2005.11.066. [DOI] [PubMed] [Google Scholar]