Abstract
Drug development has been globalized, and multi-regional clinical trial (MRCT) for regulatory submission has widely been conducted by many discovery based global pharmaceutical companies with the objective of reducing the time lag of launch in key markets and improve patient access to new and innovative treatments. Sponsors are facing several challenges while conducting multiregional clinical trials. Challenges under the heads statistics, clinical, regulatory operational, and ethics have been discussed. Regulators in different countries such as USA, EU-Japan, and China have issued guidance documents in respect of MRCT's. Lack of harmonization in the design and planning of MRCT is perceived to create a difficult situation to sponsors adversely affecting progressing MRCT in more and more discoveries. International conference on hormonisation (ICH) has initiated the process for having a harmonized guidance document on MRCT. This document is likely to be issued in early 2017.
Keywords: Clinical trials, international conference on harmonization, multi-regional, United States of America Food and Drug Administration
INTRODUCTION
Safety and efficacy data generated from local patients are a regulatory requirement in many countries including key markets such as USA, EU in the West, and China, India, Japan, Korea in the East.
The drug lag problem in several countries can be attributed to multiple causes; the two major being sluggish or no drug innovation and the suboptimal regulatory environment around the clinical trial and drug approval processes.
Drug development has been globalized and multi-regional clinical trial (MRCT) for regulatory submission has widely been conducted by many discovery based global pharmaceutical companies with the objective of reducing the time lag of launch in key markets and improve patient access to new and innovative treatments. Clinical trials across multiple regions of the world have become common practice, with the ultimate goal to bring good medicinal products to patients around the world, as fast as scientifically possible.
In recent years, data from MRCTs have been submitted to multiple regulatory agencies in international conference on harmonization (ICH) and non-ICH regions. Regulatory agencies are currently facing some challenges in evaluating data from MRCTs for drug approval.
To better understand the nature and extent of industry experience and the approaches to MRCTs, the Pharmaceutical Research and Manufacturers Association of America (PhRMA) MRCT Key issue team work-stream surveyed PhRMA member companies on a number of areas relevant to phase three registration MRCTs.[1] Survey results supported the apparent industry trend that a large number of companies have decided to conduct trials in a new region over the last decade, with a significant majority of those going to new regions to conduct late phase or postmarketing trials. There seems to be an increasing move to Asia, Latin America, the Middle East, and Africa, where the clinical trial environments are not as mature as in North America and Western Europe. The results support the often-heard claim that local medical practice and standards of care can be very different among the regions yet can be successfully navigated. The report elaborates on the impact of conducting MRCTs on sponsors, including changes to regulatory functions and staffing models of those companies expanding their reach.
ICH E5 guideline[2] was adopted in 1998 (updated in 2006) with the purpose to facilitate the registration of medicinal products among different geographic regions by recommending a framework for evaluating the impact of ethnic factors upon the efficacy or safety of a product.
BENEFITS
MRCTs can expedite global clinical development and facilitate registration in all regions across the globe. The ultimate goal of MRCT is to bring new medicines to patients globally as fast as scientifically possible and reduce the drug lag. MRCT also helps in expansion of clinical research into developing countries bringing medical care options to subjects who otherwise may not have access to them. Investment in drug development increases potential benefits to local scientific and medical and paramedical professionals. It provides access to more advanced technologies and helps in the development of technical expertise. MRCT provides the sponsors access to otherwise untapped pools of patients, as well as early patient access to new medications.
REGULATORY GUIDANCE
On one hand, there has been conflicting opinions from several regulatory bodies while reviewing the data from MRCT of New Drug Applications (NDAs). On the other, stringent approval for clinical trials and extended review periods pose big challenges to global pharmaceutical companies in many markets including key countries while conducting MRCT.
Guidance relating to MRCTs from regulatory health authorities is growing, where some bodies position their region in the context of an MRCT, whereas others place an emphasis on their own region, e.g. “basic principles on global clinical trials.”
Pharmaceuticals and Medical Devices Agency[3] in Japan has made concerted efforts to change the situation so that more and more MRCTs are conducted in Japan and drug lag is reduced.
European medicines agency (EMEA)[4] has published a reflection paper on the extrapolation of results from clinical studies conducted outside of the EU to the EU population. This reflection paper highlights examples of mainly extrinsic, but also intrinsic factors that may complicate the extrapolation of results from clinical studies between geographical areas worldwide as well as within the EU population. Concerning medical practice, one of the factors that can complicate the interpretation of the validity of the results in different geographic areas is differences in comedications and invasive procedures. Especially in studies on conditions that require intensive medical care, the standard of care can have an important impact on the outcome parameters. Transferability of the results of these studies might be impaired.
EMEA opined that considering that more clinical trials are performed in new regions in which social and cultural aspects may be different in comparison to the EU population; the influence of extrinsic factors could be of particular interest. However, intrinsic factors are also of high importance, as specified in the ICH E5-guideline.
USA Food and Drug Administration (FDA)[5] has provided its reflection on Regulatory and Scientific Issues Regarding Use of Foreign Data in Support of NDAs in the USA. The key considerations recommended by USFDA includes the potential heterogeneity in treatment effect across regions may need to be considered in the designing and sizing of MRCTs, using a quality-by-design approach, in which quality is built into the scientific and operational design of a trial. Trial quality ultimately rests on having a well-articulated investigational plan with clearly defined objectives and associated outcome measures together with investigators who carry out the study as planned. USFDA has reiterated that the likelihood of a successful trial can be dramatically improved through prospective attention to preventing important errors that could undermine the ability to obtain meaningful information from a trial. USFDA has also suggested improvement in the oversight of MRCT's and improvement in statistical analysis plans that specifically address the features of MRCTs.
The Chinese FDA issued guidance on international multi-center clinical trials of drugs in China on January 30, 2015, which began implementation on March 1, 2015.[6] Now, the Chinese FDA expects to see overseas and multinational pharmaceutical companies conducting international multi-center clinical trials of drugs in China. The Chinese guidance stipulates that for those International multi-center clinical trial data used for application of drug registration in China, must at least involve two countries, including China, and should refer the requirements of this guidance. The guidance includes following additional requirements:
The overseas applicant must conduct a holistic evaluation of the global clinical trial data set and a trending analysis of the data from trial subjects in Asia and China. When analyzing the Chinese clinical trial data, specifically, the overseas applicant should evaluate whether the enrolled Chinese trial subjects are representative of the relevant patient population in Chinese medical practice
The overseas applicant must ascertain whether the Chinese trial subject sample size sufficiently supports the conclusion that the trial drug is safe and effective for Chinese patients, and whether the Chinese trial subject sample size meets the statistical requirements and the relevant laws and regulations' requirements
The overseas applicant must adhere to internationally accepted Good Clinical Practice (GCP) principles and ethical standards. They must also allow the Chinese FDA to inspect the trial sites from time to time, which can be any of the onshore or offshore sites involved in the international multi-center clinical trials.
The impact of different regulatory requirements for Trial endpoints in MRCTs is another issue faced by the sponsors. Harmonized guidance would be the path to more efficient MRCTs.
Lack of harmonization in the design and planning of MRCT is perceived to create a difficult situation to sponsors adversely affecting progressing MRCT in more and more discoveries.
Issues in planning MRCTs include usefulness of MRCTs in drug developments, essential points for conducting MRCTs (GCP, etc.), importance of ethnic factors evaluation on drug efficacy/safety in MRCTs, etc., Issues in designing MRCTs, points to consider in dose determination for MRCT (exploratory and confirmatory),
how to control various concomitant medications in each country, consideration on definition of a population and methods of sample size estimation for a population/region, etc., and encouraging a parallel scientific consultation with multiple regulatory agencies in advance.
To address the challenges faced by Regulatory agencies and promote conducting MRCT by sponsors, a harmonized international guideline especially focusing on scientific issues in planning, designing MRCTs, International conference on harmonization (ICH) has initiated developing ICH E17.[7] This new guideline will complement the guidance on MRCTs provided in ICH E5 (R1)[1] and facilitate MRCT data acceptance by multiple regulatory agencies. Consequently an ICH Expert Working Group (EWG) has been established and mandated to draft an ICH guideline on general principles on planning/designing MRCTs. The EWG consists of two or three members nominated by EU, EFPIA, FDA, PhRMA, MHLW, JPMA, Health Canada, and Swiss medic. One member is also be nominated by the WHO observer and Drug Regulatory Authorities and Department of Health. It is expected that the document will come for public consultation by the end of 2015 or early 2016.
CHALLENGES AND ISSUES
MRCTs have benefits but also come with a set of challenges.
There have been several issues faced by various stakeholders in the conduct of MRCT over the last decade. Concerns were mentioned in a systematic review of controlled trials published in 1998.[8]
Individual companies have come out with position papers, for example, Pfizer has placed a position paper on its website addressing reasons why clinical trials are and should be done globally, including the developing world.[9]
Tsou et al.[10] have described issues related to design and analysis of the study with the objective to satisfy different regional requirements on primary endpoints.
The USA Department of Health and Human Services, the office of the inspector general, issued a report, challenges to FDA's Ability to Monitor and Inspect Foreign Clinical Trials.[11] This report investigated both the extent to which sponsors submitted data from trials outside the USA to support drug and biologic marketing applications approved by FDA in the fiscal year, 2008, and also investigated the extent to which FDA monitors and inspects foreign clinical trials that support marketing applications.
International health authorities and government-sponsored efforts have provided some guidance[2,12,13,14] but there is a lack of harmonized guidance from health authorities.
Issues faced in MRCT's can be classified into five broad categories viz., statistical, clinical, operational, regulatory and ethical.
Statistical issues may include factors such as the importance of predefining the definition of the region, the impact of regional differences on power estimation/sample size, methods for subgroup analysis, randomization issues, how to describe and present data by region and so on.
Clinical factors include extrinsic versus intrinsic factors, lack of quality data showing the comparability of pharmacokinetic/pharmacodynamic relationships among different ethnic/racial groups, or among “regions,” differences in standard of care with markedly varied medical practice, including disease definitions, differences in access to the regional healthcare system, differences in criteria for hospitalization and treatment, concomitant medications, differences in diet, smoking, alcohol, placebo responses, cultural differences, adverse event reporting, and evaluation and endpoints.
Operational issues could include challenges when moving from phase II to phase III to postmarketing (e.g., expanding number of sites, regions, standard operating procedures (SOPs) and manuals, including global clinical SOPs, data handling SOPs, operational SOPs (translations, training), technological standards/telecommunication bandwidth, multi-regional trial versus multiple regional trials, enrollment, access to the appropriate patient population, drug supply, IVRS, randomization, trial quality and integrity, investigator training, quality assurance (QA), data management, and data quality, plan in the protocol for sources of heterogeneity adjust the sample size as needed, how do we measure quality and integrity? Is QA separate from quality control (QC), e.g., the QA audits of the QC activities to ensure compliance to GCP, company policies, etc.,
Regulatory matters include dealing with differing (and possibly opposing) regulatory requirements, including differing primary and secondary endpoint requirements, divergence in the requirements for the control arm, in clinical studies EU verses US verses Asia, obtaining agreement from differing health authorities. The level of evidence needed, based on the studies and based on the health authority resources (reliance on other health authorities). Regulatory issues also include handling different regulatory review and approval times when trying to orchestrate a simultaneous global submission, managing, and responding to requests across multiple agencies, determining the acceptability of MRCT data.
Ethical issues include adequacy of protection of research subjects, informed consent, integrity of research conduct, ease of access, transparency of the research, quality of the review that permits the conduct of the research, local Ethics Committees, Institutional Review Board's, etc. data collection/privacy, need to conduct an MRCT according to GCP standards, relevant country and local statutes regarding Ethical Committee reviews, informed consent, withdrawn consent, protection of human patients participating in biomedical research.
CONCLUSION
A high level of country-independent harmonization is needed to facilitate sponsors in the conduct MRCTs. This harmonized guidance should ensure a common understanding and approach to clinical trial procedures, patient management, assessment, and reporting. The minimum acceptable criterion for running a clinical trial anywhere in the world needs to be established.
Regulatory agencies in Japan have taken measures to encourage MRCT to improve patient access and reduce drug lag. China has also released a guidance document early this year on MRCT. There is a need to define clearly to restrict when protocol amendment may be allowed to be carried out especially if it requested from one country or several regions.
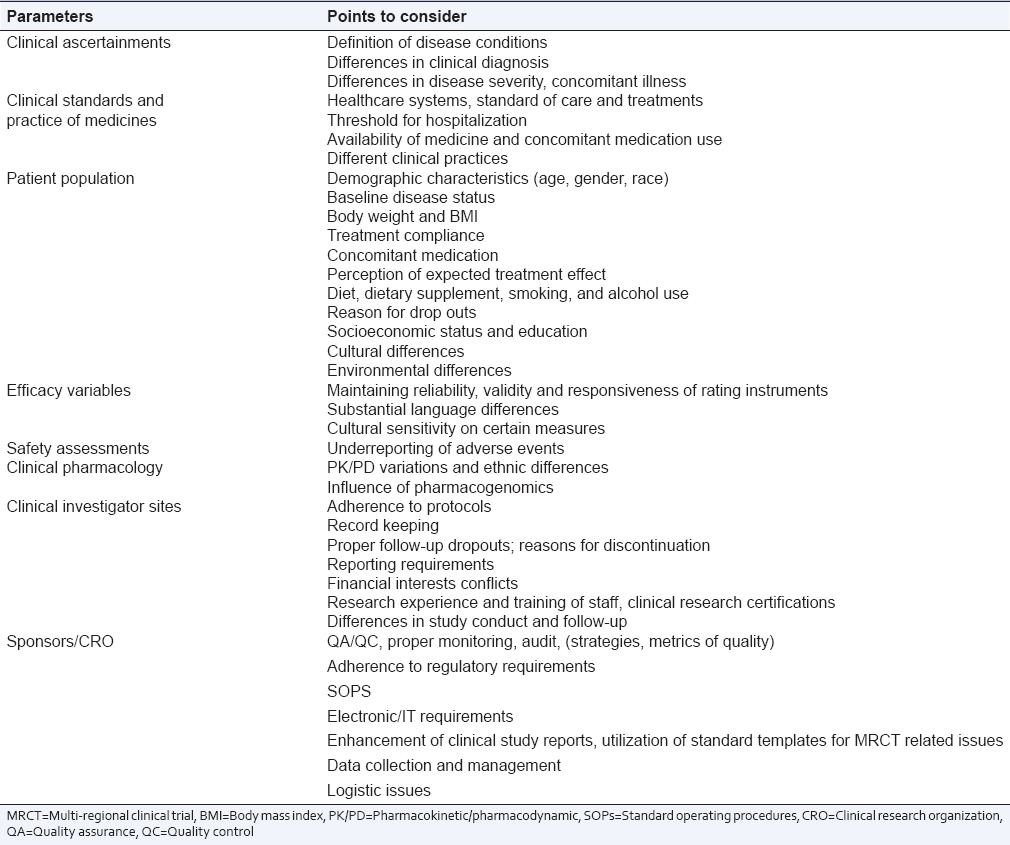
Some of the key points to be considered for the successful conduct of MRCT and deal with various challenges and issues are given in Table 1.
Table 1.
Points to be considered in multi-regional clinical trial's
If the regulatory environment in China and India becomes more conducive in terms of timelines of approval, one large phase III MRCT study could suffice to submit NDA in most of the leading markets in the world and reduce the drug lag substantially.
India needs to have a comprehensive guidance document for MRCT and steer clear of upper age restriction of patients recruited to ensure uniformity in the global study protocol and inclusion/exclusion criteria uniformity and in line with sponsors original proposal and as approved in key countries such as USA and EU.
It may be presumed that substantive research work and future experience on critical data QA and important ethical considerations would help shape successful MRCTs for the continued globalization of drug development.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Binkowitz B, Ibia E. Multiregional clinical trials: An introduction from an industry perspective. Ther Innov Regul Sci. 2011;45:569–73. [Google Scholar]
- 2.International Conference on Harmonisation; guidance on ethnic factors in the acceptability of foreign clinical data; availability – FDA. Notice. Fed Regist. 1998;63:31790–6. [PubMed] [Google Scholar]
- 3.Basic Principles on Global Clinical Trials, Notification No. 0928010, September 28th, 2007, JPMA (This Document is an Informal Translation by PMDA of the Final Notification Published in Japanese on September 28th, 2007 and is Intended to Use as a Reference for Considering Global Clinical Trials. 2007. [Last accessed on 2015 Aug 13]. Available from: https://www.pmda.go.jp/files/000157900.pdf .
- 4.Reflection Paper on the Extrapolation of Results from Clinical Studies Conducted Outside of the EU to the EU Population, EMEA/CHMP/EWP/692702/2008. 2009. Oct 22nd, [Last accessed on 2015 Aug 13]. Available from: http://www.emea.europa.eu .
- 5.Khin NA, Yang P, Hung HM, Maung KU, Chen YF, O'Connell M, et al. Regulatory and scientific issues regarding use of foreign data in support of new drug applications in the United States: An FDA perspective. Clin Pharmacol Ther Genomic Med. 2013;94:230–42. doi: 10.1038/clpt.2013.70. [DOI] [PubMed] [Google Scholar]
- 6.Latest Regulations on Pharmaceutical International Multi-Center Clinical Trials in China Access China Management Consulting Ltd. SKU: ACCH5499504. 2015. Mar 19, [Last accessed on 2015 Aug 16]. Available from: http://www.marketresearch.com .
- 7.Final Concept Paper E17: General Principle on Planning/Designing Multi-Regional Clinical Trials Dated. 2014. May 21, [Last accessed on 2015 Aug 08]. Available from: http://www.ich.org .
- 8.Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials. 1998;19:159–66. doi: 10.1016/s0197-2456(97)00150-5. [DOI] [PubMed] [Google Scholar]
- 9.The Globalization of Clinical Trials. 2009. Mar, [Last accessed on 2015 August 18]. Available from: http://www.pfizer.com/files/research/nejmpaper_globalization_of_trials.pdf .
- 10.Tsou HH, Tsong Y, Chang WJ, Dong X, Hsiao CF. Design and analysis issues of multiregional clinical trials with different regional primary endpoints. J Biopharm Stat. 2012;22:1051–9. doi: 10.1080/10543406.2012.701586. [DOI] [PubMed] [Google Scholar]
- 11.Department of Health and Human Services, Office of Inspector General. Challenges to FDA’s Ability to Monitor and Inspect Foreign Clinical Trials. 2010. Jun, [Last accessed on 2015 Aug 18]. Available from: http://www.oig.hhs.gov/oei/reports/oei-01-08-00510.pdf .
- 12.European Medicines Agency. EMA/INS/GCP/154352/2010. Clinical Trials Submitted in Marketing Authorization Applications to the EMA. 2010. Nov 5, [Last accessed on 2015 Aug 17]. Available from: http://www.ema.europa. eu/docs/en_GB/document_library/Other/2009/12/WC500016819.pdf .
- 13.International Conference on Harmonization, Good Clinical Practice, ICH GCP E6. [Last accessed on 2015 Aug 15]. Available from: http://www.ichgcp.net .
- 14.World Health Organization. Guidelines for Good Clinical Practice (GCP) for Trials on Pharmaceutical Products. Technical Report Series, No. 850, Annex3. 1995. [Last accessed on 2015 Aug 17]. Available from: http://www.apps.who.int/medicinedocs/pdf/whozip13e/whozip13e.pdf .


