Abstract
Objective:
To compare the effects of 3% hypertonic saline (HS) and 0.9% normal saline with nebulized 0.9% normal saline with salbutamol in patients of acute viral bronchiolitis.
Materials and Methods:
Participants were divided into three groups, that is, 3% HS group, 0.9% normal saline group and 0.9% saline with salbutamol group. Four doses at interval of 6 h were given daily until discharge. Average CS score and length of hospital stay were compared. One-way analysis of variance paired t-test and Chi-square test were utilized for statistical analysis.
Results:
The mean ages of the patients in three groups were 6.03 ± 3.71, 5.69 ± 3.34 and 5.48 ± 3.35 respectively. The 3rd day CS scores for all the groups were 1.0 ± 1.1, 1.9 ± 1.1 and 3.3 ± 0.5 respectively (P = 0.000). The average length of hospital stay was 3.4 ± 1.7, 3.7 ± 1.9 and 4.9 ± 1.4 days respectively (P = 0.001).
Conclusion:
The present study concludes that 3% HS nebulization (without additional bronchodilators) is an effective and safe treatment for nonasthmatic, moderately ill patients of acute bronchiolitis. The economic benefit of this comparably priced modality of treatment can be enormous in terms of hospital costs with parents returning to work sooner.
Keywords: 0.9% normal saline, 0.9% saline with salbutamol, 3% hypertonic saline, acute bronchiolitis
INTRODUCTION
Bronchiolitis is regarded as the most common lower respiratory tract infection among infants in both developed and developing countries.[1] The reported attack rates in western literature are as high as 11.6 per 100 children in the 1st year and 6 per 100 children in the 2nd year of life.[2] The mortality rate is as high as 0.5–1.5% in hospitalized patients, increasing to 3–4% for patients with underlying cardiac or pulmonary disease.[3] In our country too, it is a significant problem judging by the frequency of wheezing episodes among young infants, though it is difficult to routinely identify the causative viruses.[4]
Acute bronchiolitis is defined as the first episode of acute wheezing in children <2 years of age, starting as a viral upper respiratory infection (coryza, cough or fever).[5] Most of the cases occur in the 1–6 months age group. It usually occurs in winter and early spring season.[6] It is more common in males as compared to females and males are 1.25 times more likely to be admitted than females.[7] Respiratory syncytial virus (RSV) infection causes bronchiolitis in 50–90% of cases.[8] Other less common pathogens include para-influenza virus, adenovirus, influenza A and B, rhinovirus, human metapneumovirus and Mycoplasma pneumoniae.[6]
Acute bronchiolitis usually occurs following exposure to a patient with minor respiratory symptoms within the previous week. Infant first develops a mild upper respiratory tract infection with sneezing and rhinorrhea. This is followed by decreased appetite and moderate grade fever. After a few days respiratory distress ensues. The infant is often tachypneic, which may interfere with feeding. The physical examination is characterized most prominently by wheezing, prolonged expiration, fine rales and ronchi. The work of breathing increases characterized by nasal flaring, intercostal and subcostal retractions, hyperexpansion of chest, restlessness and peripheral cyanosis.[7] Most of the infants show improvement within 3–4 days after the onset of the disease.[3]
The diagnosis is usually a clinical one and investigations are not generally needed to confirm it. However, confirmation of RSV infection can be made by enzyme-linked immunofluorescence assay and fluorescent antibody techniques for detection of the viral antigen, amplification of the virus using the shell vial method and amplification of viral genome by polymerase chain reaction or viral culture.[9] A rapid test using monoclonal antibodies against RSV on nasopharyngeal aspirates can identify RSV by the bedside.[3]
Management of bronchiolitis is often frustrating for physicians and care-givers because “nothing seems to work” in most cases.[4] There is lack of robust evidence for almost all the interventions that are usually tried including inhaled epinephrine, bronchodilators, steroids, anticholinergics, antibiotics, surfactant and chest physiotherapy. Some experts have questioned whether bronchiolitis can be treated at all and current research data is far from adequate to draw definite conclusions. It has been suggested that hypertonic saline (HS) nebulization may be useful in making secretions less viscous and promoting their excretion, thereby resulting in clinical improvement. Despite the lack of sufficient data, many physicians use this, though sometimes more for psychological than clinical benefit. Against such a background, it is relevant to ask the clinical question, “in infants with bronchiolitis (population), does HS nebulization (intervention) result in better clinical response (outcome) compared to no intervention or nebulization with normal saline (comparison).”[4] A few studies have been done to see the effectiveness of 3% HS nebulization in acute bronchiolitis. A recent Cochrane review suggested that HS nebulization resulted in shorter duration of hospitalization among admitted infants and better clinical severity score among nonadmitted infants, although it failed to reduce the rate of hospitalization among them. No adverse effects were reported.[10]
Most of the data on this topic originated from Israel, Canada, and UAE. Hence, we carried out this study with the objective to find out the effectiveness of 3% HS nebulization in acute bronchiolitis among Indian children. We hypothesized that 3% HS nebulization would lead to early recovery of admitted infants.
MATERIALS AND METHODS
This prospective study was conducted on one hundred children admitted in the Pediatrics Department of GGS Medical College and Hospital, Faridkot with the diagnosis of acute bronchiolitis between the months of October to February for 2 consecutive years, the typical bronchiolitis season in this part of the country. A written and informed consent was taken from the parents on a prescribed format. The study was approved by the ethical committee of GGS Medical College and Hospital, Faridkot.
Selection of cases
Inclusion criteria
All children with acute bronchiolitis having the following features were included in the study:
Age 2 months to 24 months
History of preceding viral upper respiratory infection that is, fever >38°C or coryza
First episode of respiratory distress associated with wheezing
Clinical severity score >3
No evidence of bacterial infection.
Exclusion criteria
Age <1 month or >24 months
History of two or more episodes of respiratory distress in the past
Chronic cardiopulmonary disease viz. congenital heart disease, cystic lung disease, etc
Preterm birth or history of mechanical ventilation in the neonatal period
Family history of asthma
Critical illness at presentation suggesting incipient respiratory failure.
The data of all the cases were recorded on a predetermined performa. The diagnosis of acute bronchiolitis was clinical, based on the history and physical examination. The following investigations were performed on all patients:
Hb, total leucocyte count, differential leukocyte count
X-ray chest PA and lateral views at the time of admission and discharge.
The severity of the illness was assessed by using CS score described by Wang et al.[11]
The children were randomly assigned to three groups designated as “A,” “B” and “C.”
Children in Group A were given nebulized 3% HS (4 ml) with an oxygen flow rate of 8 L/min
Children in Group B were given nebulized 0.9% normal saline (4 ml) with an oxygen flow rate of 8 L/min
Children in Group C were given nebulized salbutamol in a dose of 0.15 mg/kg body weight (minimum dose 1 mg) in normal saline to make a total volume of 4 ml using an oxygen flow rate of 8 L/min.
Four doses at interval of 6 h were given daily until discharge. CS scores were recorded before and 30 min after first nebulization at the time of admission and then every morning before and 30 min after first nebulization till the patient was discharged. Any add-on therapy given by the consulting physician was recorded.
Criteria for discharge
CS score of <3
Child feeding well.
Statistical methods
Spreadsheets were created for data entry. The data was analyzed using SPSS 15 (SPSS Inc., Chicago, IL, USA) Windows software program.
Two major outcomes of interest were considered that is, change in the CS score after 3% HS, 0.9% saline, and salbutamol (with 0.9% saline solution) inhalation each day, and length of hospital stay. One-way analysis of variance paired t-test and Chi-square test were utilized for statistical analysis.
RESULTS
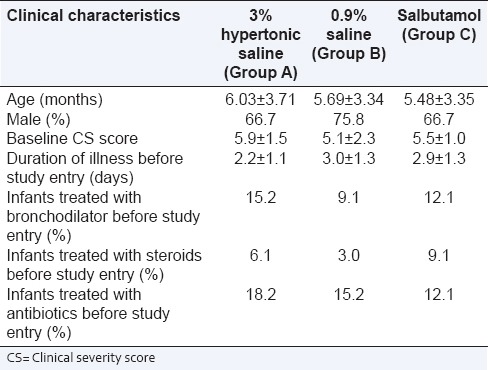
The mean age of the patients was 6.03, 5.69 and 5.48 respectively. Thus, all three groups were alike. The percentage of males was 66.7%, 75.8% and 66.7 respectively. The mean baseline CS scores and duration of illness were (5.9, 5.1, 5.5) and (2.2, 3, 2.9) respectively. Infants treated with bronchodilator therapy, oral steroids and antibiotics before admission was (15.2%, 9.1% and 12.1%), (6.1%, 3.9% and 9.1%) and (18.2%, 15.2% and 12.1%) respectively [Table 1].
Table 1.
Demographic details and illness status at the baseline
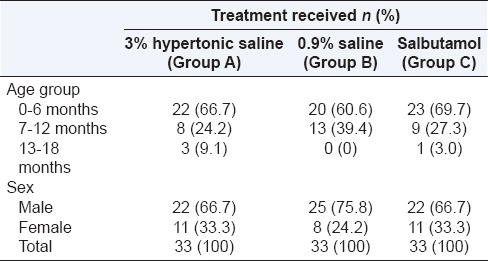
The maximum numbers of cases in each treatment group were reported in the age group 0–6 months and male preponderance in all the treatment groups was observed [Table 2].
Table 2.
Table 2: Distribution of cases according to age, sex and type of treatment given
The CS scores before giving the specified therapy in the study population were 5.9 ± 1.5, 5.1 ± 2.3 and 5.5 ± 1.0 respectively (P = 0.146) no significant association was observed. The 3rd day CS scores for all the groups were 1.0 ± 1.1, 1.9 ± 1.1 and 3.3 ± 0.5 respectively. (P = 0.000) post-hoc analysis revealed that there was a significant reduction in the CS scores in 3% HS group as compared with 0.9% saline and salbutamol groups after 3 days of treatment [Table 3].
Table 3.
Comparison of CS scores before and 3 days after treatment

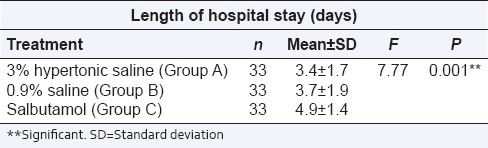
The average length of hospital stay was 3.4 ± 1.7, 3.7 ± 1.9 and 4.9 ± 1.4 days in 3% HS, 0.9% saline and salbutamol groups respectively (P = 0.001) which was significant. Post-hoc analysis depicted significant reduction in the length of hospital stay in 3% HS and 0.9% saline groups as compared to salbutamol group [Table 4].
Table 4.
Length of hospital stay according to type of treatment in each group
DISCUSSION
This prospective study was conducted on one hundred children to assess the effectiveness of nebulized 3% HS in acute bronchiolitis by measuring the clinical severity score before and after treatment and at the time of discharge and length of hospital stay. The results were compared with 0.9% saline and salbutamol nebulizations.
The mean age of the patients in our study population was 5.7 ± 3.4 months, the youngest being 2 months and the eldest being 14 months. In our study, most of the children (65.7%) presented in the first 6 months of age. This is in accordance with the peak age of presentation of acute bronchiolitis.[6] The mean age observed by Sarrell et al. is 12.5 ± 6 months (range, 3–24 months),[12] Mandelberg et al. is 2.9 ± 2.1 months (range, 0.5–12 months),[13] Tal et al. is 2.6 ± 1 months (range, 1–5 months)[14] and Kuzik et al. is 4.7 ± 4.2 months (range, 10 days to 18 months).[15]
In our study, 69.7% of the patients were males and 30.3% were females. In our study, males were more affected than females from acute bronchiolitis. This might be attributed to presence of two X chromosomes which produces greater genetic diversity to the female immunologic defense.
In our study, the mean duration of illness before study entry in 3% HS, 0.9% saline and salbutamol group was 2.2 ± 1.1, 3.0 ± 1.3 and 2.9 ± 1.3 days respectively. The mean duration of illness before study entry observed by Mandelberg et al. is 3.0 ± 1.6 days in 0.9% saline group and 3.9 ± 2.9 days in 3% HS group;[13] by Tal et al. is 4.5 ± 2.2 days in 0.9% saline group and 4.0 ± 2.2 days in 3% HS group[14] and by Kuzik et al. is 4.0 ± 2.4 days in 0.9% saline group and 4.5 ± 2.3 days in 3% HS group.[15]
In the present study, the percentage of infants treated with bronchodilators before study entry in 3% HS, 0.9% saline and salbutamol groups were 15.2%, 9.1% and 12.1% respectively. This was much less as compared to Kuzik et al. who reported use of bronchodilators before study entry in 86% in 3% HS group and 91% in 0.9% saline group.[15]
In our study, the percentage of infants treated with steroids before study entry in 3% HS, 0.9% saline and salbutamol groups were 6.1%, 3.0% and 9.1% respectively. Kuzik et al. reported use of steroids before study entry in 2.5% in 3% HS group and 2.4% in 0.9% saline group.[15]
In our study, the percentage of infants treated with antibiotics before study entry in 3% HS, 0.9% saline and salbutamol groups were 18.2%, 15.2% and 12.1% respectively [Table 1]. Kuzik et al. observed this in 15.0% in 3% HS group and 9.8% in 0.9% saline group.[15]
The baseline CS scores at the time of enrollment in the study in 3% HS, 0.9% saline and salbutamol groups were 5.9 ± 1.5, 5.1 ± 2.3 and 5.5 ± 1.0 respectively. After treatment the 3rd day CS scores dropped to 1.0 ± 1.1, 1.9 ± 1.1 and 3.3 ± 0.5. Hence, there was 82.57%, 61.89% and 40% reduction in the CS scores in 3% HS, 0.9% saline and salbutamol groups respectively. Thus, the effect of treatment was evident as a significant fall in CS scores in all the three groups. However, the comparison in the CS scores by post-hoc analysis showed that the difference was much greater in 3% HS group as compared to 0.9% saline and salbutamol groups.
Sarrell et al. in a study of seventy patients with mild to moderate bronchiolitis on an outdoor basis observed a baseline CS score of 6.6 ± 1.5 in 3% HS (with terbutaline) group and 6.4 ± 1.8 in 0.9% saline (with terbutaline) group.[12] After 3 days of treatment, the CS score dropped to 2.1 ± 2.2 and 4.8 ± 2.3 in 3% HS (with terbutaline) and 0.9% saline (with terbutaline) groups respectively. Hence, they concluded that in nonasthmatic, nonseverely ill ambulatory children with bronchiolitis, nebulized 3% HS plus terbutaline is more effective in decreasing symptoms as compared to 0.9% normal saline plus terbutaline.[12]
In a study conducted by Mandelberg et al. the percentage improvement in the CS scores after inhalation therapy was not significant in 0.9% saline (with epinephrine) group on the first, second and 3rd days after hospital admission (3.5%, 2%, and 4%, respectively). In the 3% HS (with epinephrine) group, significant improvement was observed on these days (7.3%, 8.9% and 10%, respectively; P < 0.001). Also, the improvement in CS scores differed significantly on each of these days between the two groups. Hence, they concluded that in nonasthmatic nonseverely ill patients with acute bronchiolitis nebulized 3% HS (with epinephrine) decreases the symptoms significantly.[13]
Another study done by Kuzik et al. used 3% HS nebulization alone and compared it with 0.9% saline nebulization, but they did not report its effect on the clinical severity score. They only measured the length of hospital stay in response to therapy.[15] In our study, we have compared the effect of 3% HS nebulization with 0.9% saline and salbutamol without any addition of bronchodilators to 3% HS and 0.9% saline groups and compared it with bronchodilator (i.e. salbutamol) in 0.9% saline.
It is possible that, in our study, an improvement in mucociliary transport and a better elimination of intracellular debris may have reduced viral load and decreased ongoing inflammation within the airways. This might also have reduced an opportunity for secondary bacterial overgrowth and hereby may contribute to the favorable effect of decreasing post inhalation therapy clinical severity scores.
In our study, the mean duration of hospital stay was 3.4 ± 1.7, 3.7 ± 1.9 and 4.9 ± 1.4 days in 3% HS, 0.9% saline and salbutamol groups respectively. Length of hospital stay was found to be statistically significant (P = 0.001). Post-hoc analysis showed that length of stay was significantly reduced in 3% HS and 0.9% saline groups. In fact, the length of stay was reduced by as much as 30.6% in 3% HS Group and 24.9% in 0.9% saline group as compared to salbutamol group.
A similar reduction in the length of hospital stay has been observed by various other authors.
Mandelberg et al. observed a mean duration of hospital stay of 3 ± 1.2 and 4 ± 1.9 days in 3% HS (with epinephrine) and 0.9% saline (with epinephrine) groups respectively.[13] Tal et al. observed a mean duration of hospital stay of 2.6 ± 1.4 and 3.5 ± 1.7 days in 3% HS (with epinephrine) and 0.9% saline (with epinephrine) groups respectively.[14] Luo et al. observed a mean duration of hospital stay of 6 ± 1.2 and 7.4 ± 1.5 days in 3% HS (with salbutamol) and 0.9% saline (with salbutamol) groups respectively.[16]
In our study protocol, we did not encourage the use of bronchodilators along with the study solution. However, the attending physician felt the need to introduce them in only 4 patients in the 3% HS group and 6 patients in the 0.9% saline group. No side effects viz. tremors, severe bronchospasm, tachycardia were noted in the study in all the three groups.
CONCLUSION
The present study concludes that 3% HS nebulization (without additional bronchodilators) is an effective and safe treatment for nonasthmatic, moderately ill patients of acute bronchiolitis. It significantly reduces the CS scores and length of hospital stay as compared to 0.9% saline and salbutamol in 0.9% saline nebulization. The economic benefit of this comparably priced modality of treatment can be enormous in terms of hospital costs with parents returning to work sooner.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Klassen TP. Recent advances in the treatment of bronchiolitis and laryngitis. Pediatr Clin North Am. 1997;44:249–61. doi: 10.1016/s0031-3955(05)70472-7. [DOI] [PubMed] [Google Scholar]
- 2.Ray MS, Singh V. Comparison of nebulized adrenaline versus salbutamol in wheeze associated respiratory tract infection in infants. Indian Pediatr. 2002;39:12–22. [PubMed] [Google Scholar]
- 3.Dennis MM. Bonchiolitis. Arch Dis Child Educ Pract. 2005;90:81–6. [Google Scholar]
- 4.Mathew JL. Hypertonic saline nebulization for bronchiolitis. Indian Pediatr. 2008;45:987–9. [PubMed] [Google Scholar]
- 5.Panitch HB, Callahan CW, Jr, Schidlow DV. Bronchiolitis in children. Clin Chest Med. 1993;14:715–31. [PubMed] [Google Scholar]
- 6.Kabra SK, Ghai OP. Respiratory disorders. In: Ghai OP, Gupta P, Paul VK, editors. Ghai Essentials Pediatrics. 6th ed. New Delhi: CBS Publishers and Distributors; 2004. pp. 352–4. [Google Scholar]
- 7.Kimberly DW, Goodman DM. Wheezing, bronchiolitis and bronchitis. In: Kliegman RM, Behram RE, Jenson HB, Stanton BF, editors. Nelson Text Book of Pediatrics. 18th ed. New Delhi: Elsevier; 2007. p. 1774. [Google Scholar]
- 8.Handforth J, Friedland JS, Sharland M. Basic epidemiology and immunopathology of RSV in children. Paediatr Respir Rev. 2000;1:210–4. doi: 10.1053/prrv.2000.0050. [DOI] [PubMed] [Google Scholar]
- 9.Leung AK, Kellner JD, Davies HD. Respiratory syncytial virus bronchiolitis. J Natl Med Assoc. 2005;97:1708–13. [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulized hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. 2013;31(7):CD006458. doi: 10.1002/14651858.CD006458.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Wang EE, Milner RA, Navas L, Maj H. Observer agreement for respiratory signs and oximetry in infants hospitalized with lower respiratory infections. Am Rev Respir Dis. 1992;145:106–9. doi: 10.1164/ajrccm/145.1.106. [DOI] [PubMed] [Google Scholar]
- 12.Sarrell EM, Tal G, Witzling M, Someck E, Houri S, Cohen HA, et al. Nebulized 3% hypertonic saline solution treatment in ambulatory children with viral bronchiolitis decreases symptoms. Chest. 2002;122:2015–20. doi: 10.1378/chest.122.6.2015. [DOI] [PubMed] [Google Scholar]
- 13.Mandelberg A, Tal G, Witzling M, Someck E, Houri S, Balin A, et al. Nebulized 3% hypertonic saline solution treatment in hospitalized infants with viral bronchiolitis. Chest. 2003;123:481–7. doi: 10.1378/chest.123.2.481. [DOI] [PubMed] [Google Scholar]
- 14.Tal G, Cesar K, Oron A, Houri S, Ballin A, Mandelberg A. Hypertonic saline/epinephrine treatment in hospitalized infants with viral bronchiolitis reduces hospitalization stay: 2 years experience. Isr Med Assoc J. 2006;8:169–73. [PubMed] [Google Scholar]
- 15.Kuzik BA, Al-Qadhi SA, Kent S, Flavin MP, Hopman W, Hotte S, et al. Nebulized hypertonic saline in the treatment of viral bronchiolitis in infants. J Pediatr. 2007;151:266–70. doi: 10.1016/j.jpeds.2007.04.010. 270.e1. [DOI] [PubMed] [Google Scholar]
- 16.Luo Z, Liu E, Luo J, Li S, Zeng F, Yang X, et al. Nebulized hypertonic saline/salbutamol solution treatment in hospitalized children with mild to moderate bronchiolitis. Pediatr Int. 2010;52:199–202. doi: 10.1111/j.1442-200X.2009.02941.x. [DOI] [PubMed] [Google Scholar]


