Abstract
Aims:
By virtue of being a specialized field by itself, the science of clinical trials (CTs) may not be well understood by doctors who are not specifically trained in it. A lack of knowledge may translate to a negative perception toward CT. With the idea of getting a situational snapshot, we estimated the knowledge and perception of CTs among doctors from government medical colleges of West Bengal who are not trained on CT in their postgraduate curriculum. Several determinants of knowledge and perception regarding CT were also evaluated.
Methods:
We have quantified the knowledge and perception of CTs by a structured validated questionnaire. Development and validation of the questionnaire was performed prior to the study.
Results:
Among 133 participants, 7.5% received focused training on CT and 16.5% participated in CTs as investigators. Majority of the doctors were unfamiliar with the basic terminologies such as, “adverse event” and “good clinical practice.” Encouragingly, 93.3% doctors advised that a detailed discussion of CT methodology should be incorporated in the under graduate medical science curriculum. They had an overall positive attitude toward CTs conducted in India, with a mean score that is 72.6% of the maximum positive score. However, a large number of the doctors were skeptical about the primary motivation and operations of pharmaceutical industry sponsored CTs, with 45% of them believing that patients are exploited in these sponsored CTs.
Conclusion:
Participant doctors had a basic knowledge of CT methodology. The study has revealed specific areas of deficient knowledge, which might be emphasized while designing focused training on CT methodology.
Keywords: Clinical research, clinical trial, ethics, knowledge, perception
INTRODUCTION
The science of clinical development of a chemical entity into a drug is of indispensible importance for progression of healthcare. Regulatory approval for marketing of new molecules is typically subject to production of high quality data from clinical trials (CTs) comparing the novel molecule with standard comparators.[1] Eventual aim of this exercise is the percolation of the benefits of the intervention to patients and the society at large. Thus, the benevolent scientific motive behind CTs and the positive effects produced by the same in human society is indisputable. However, in essence, trials are a form of scientific exploration, thus not being free of risks inherent to any experimentation, including ineffectiveness or harm. While the conduct of a CT is permitted only when there is enough evidence to suggest that the anticipated benefits of the intervention will supersede the associated risks, this does not always happen in reality. Thus, interpretation of the results of a CT, especially those with “disappointing” outcomes, is a matter of utmost scientific sensitivity, with ample scope of being misconstrued by the lay media and public.[2,3,4] The situation has been complicated by the recent incidences of conduct of several studies with questionable integrity and ethics, thus casting a shadow of doubt on the scientific community.[5,6] That the CT subjects are not “guinea pigs”, and that participation of pharmaceutical industry in the clinical development of a molecule is generally of crucial importance,[7] are facts often beyond the scope of casual understanding of the public. Closer home, the perception of CTs inside the medical community also has not remained untarnished. This is important in the Indian context, as doubt around the integrity and usefulness of trials amongst members of the medical community (one of the highest stakeholders in the process), can only be of detrimental significance in the already languished indigenous scientific scenario of the country.
Data regarding the knowledge and perception of CTs among Indian doctors is scanty. Much of the negative perception within the medical community might stem from a lack of clear knowledge regarding the scientific, logistic and procedural intricacies of CTs. Accordingly we have conducted a questionnaire-based study to assess the level of knowledge and perception of CTs among doctors employed in three government teaching hospitals in Kolkata, India. We have also attempted to identify the important factors determining the knowledge and perception of CTs.
METHODS
This anonymous questionnaire based cross sectional study conducted in three Government Teaching Hospitals in West Bengal, India, during the period of June 2014–August 2014. Ethics Committee approvals were obtained prior to the initiation of the study. The sample size was chosen as a convenient figure of 150 doctors. We included all consenting doctors who are employees of these hospitals except Pharmacology residents and faculty who are trained in CT methodology in their Postgraduate curriculum. The survey data was collected by the researchers including one undergraduate medical student. Data was transcribed to a MS Excel (Microsoft Excel, Redmond, Washington, 2010) database and analyzed using SPSS version 20 (IBM SPSS Statistics 20.0 software (IBM Corporation software group, Armonk, New York, USA). The items of the questionnaire were selected from previous literature on similar survey and standard CT guidelines. The construct validity was done by formal opinion on each item by six subject experts on the appropriateness of the items and wording adequacy. Accordingly, addition, deletion or alteration of the individual items was performed. The questionnaire was piloted among 20 doctors from same setting (one of the teaching hospitals). The data generated from pilot study was excluded from the analysis. The questionnaire thus prepared had 21 items on knowledge and 30 items on the perception of CTs.
Knowledge was quantified with a cumulative score with a scoring scheme of +1 for a correct response, −1 for an incorrect response and 0 for no response. The level of perception of individual respondents was assessed using the cumulative score of a five point agreement scale, scoring one to five on each item, with an increased order of agreement. The scores of negatively worded questions were reversed while calculating the cumulative score. The sample knowledge and perception was assessed using the mean score and its proportion with maximum possible score. The response distributions of the items were presented with frequency. The possible factors influencing the level of knowledge and perception was assessed by comparing groups, using a statistical test of significance (unpaired t-test, one-way ANOVA). Multivariate analysis was performed with multiple linear regression analysis, considering the total knowledge and perception score as dependent variables and selected personal information of the respondents as independent variables. The internal consistency of the two segments of the questionnaire was calculated for the data collected from the colleges separately by cronbach's alpha method for the perception questionnaire (Likert scales) and Kuder–Richardson (KR20) coefficient for knowledge questionnaire (binary response).[8]
RESULTS
In our study, we have individually approached 150 doctor employees from three Government Medical Colleges in West Bengal. In the three Medical Colleges, 86, 31 and 16 doctors consented to participate and returned the questionnaire, making a total of 133 (88.67%) participants. The mean age was 33.41 years (standard deviation [SD] = 8.60). The proportion of male respondent was 83 (62.4%). The doctors working from various departments were approached by the survey coordinator. Of the doctors, 52.6% were from medicine or allied disciplines. 21.8% from surgical fields and the rest 24.8% from pre- and paraclinical fields. The respondents were classified according to their designations as senior level faculties (9.8%), mid-level faculties (30.8%) and residents (58.6%). Among the respondents, 59.4% doctors were postgraduates while the rest were pursuing their post-graduate degrees. Formal training related to CTs had been received by 7.5% of the participants. Of all the doctors, 2.3% and 15.8% were currently involved in industry sponsored and investigator initiated CTs respectively. Overall 16.5% doctors were involved in CTs of either type.
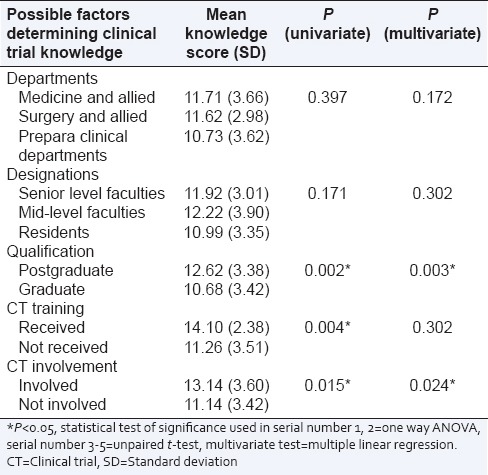
The KR20 coefficient (measure of internal consistency) for knowledge questionnaire were 0.469, 0.682, 0.732 respectively from the data obtained from three medical colleges. The frequencies of correct responders to individual items testing the knowledge related to CT are presented in Table 1. The mean score of the knowledge questionnaire was 11.47 (SD = 3.51), which is 54.62% of the maximum possible score. The possible factors determining the knowledge of CTs were assessed by comparing mean obtained scores among determining groups [Table 2].
Table 1.
Frequencies of correct responders to individual items testing the knowledge related to clinical trial
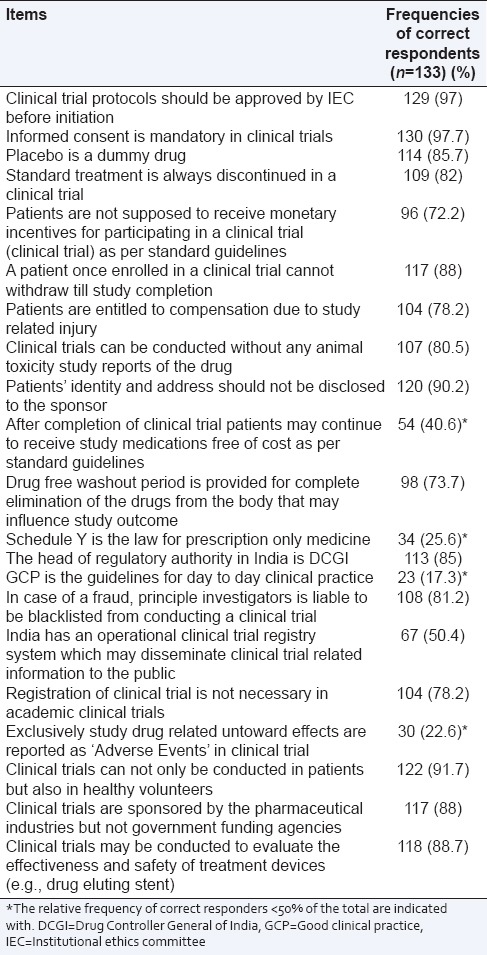
Table 2.
The cumulative score of questionnaire assessing the knowledge regarding clinical trial in various determinant groups in univariate and multivariate analysis
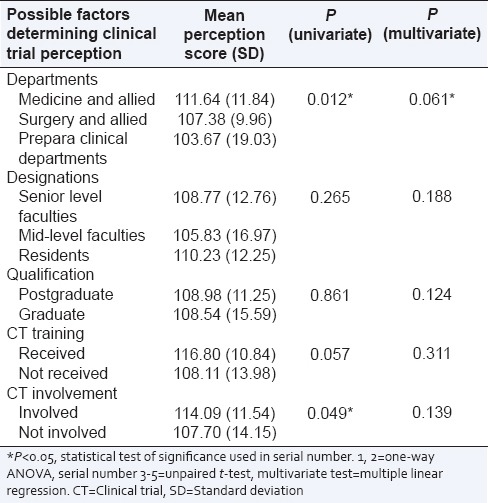
The cronbach's alpha values (measure of internal consistency) of the perception scale were 0.73, 0.59 and 0.64 respectively from the data collected from three medical colleges. A negative perception towards CT was defined as a score of 1–2 of five point Likert scale. The perceptions regarding CTs among the doctors are mixed. Among the respondents 93.3% doctors think that a detailed discussion of CTs should be incorporated in the UG medical science curriculum. Ten items were identified with highest negative attitude based on the frequency of doctors perceive them to be unscientific or unethically conducted. This result is presented in Table 3. The mean CT perception score was 108.76 (SD =13.92), which is 72.5% of the maximum possible score. The possible factors determining the perception of CT are presented in Table 4. The group of doctors (n = 48) who scored <50% marks in the knowledge test had a significantly (P value 0.005) lower perception score (mean sore 104.27 vs., 111.29) compared to those who scored over 50% (n = 85), although a clear association between knowledge and perception was not reflected from the analysis.
Table 3.
Top ten selected items based on perception assessment questionnaire regarding clinical trial with most negative perception in descending order

Table 4.
The cumulative score of questionnaire assessing perception regarding clinical trial in various determinant groups and its level of significance by univariate and multivariate analysis
DISCUSSION
In the present study, we objectively evaluated the knowledge of CT and assessed the perception of the physicians toward the same with the help of a validated questionnaire. We identified specific areas of deficient knowledge including pharmacovigilance and technical aspects where training can potentially improve knowledge. Moreover, we have observed a negative perception among doctors toward CTs sponsored by the pharmaceutical industry when compared with investigator initiated trials in academic settings.
Previous studies determining awareness of doctors or public on CTs were either qualitative or used nonvalidated questionnaires.[9,10,11,12,13] Furthermore, most studies incompletely covered the major domains of scientific and ethical aspects of CTs. A high response rate and an acceptable cronbach's alpha (≥0.6) and KR20 coefficient (≥0.5) of the perception and knowledge questionnaires respectively, together suggest the questionnaire developed for the present study is reliable and the items are internally consistent.
We observed that only 7.5% of the doctors had received focused training on CT science and operations. This is in sharp contrast to the 36.3% doctors receiving such training as reported by Dhodi et al. which was conducted in Mumbai.[9] In a teaching hospital in Koyto, Japan, the figure was <20% in 2009.[11] An expert committee report on the approval of new drugs by Prof. Ranjit Roychoudhury recommends that government hospitals should be included as CT sites for regulatory trials.[14] However, implementation of this clause will require a greater degree of structured training amongst doctors employed in government settings, at a level uniform throughout the country. The pharmaceutical and clinical research industries should also proactively provide logistic and technical support to train doctors in academic settings in CT science. Ignorance of the relevant science could also be a possible explanation of the low rate of CTs being conducted by doctors in government medical colleges as reported in the present study, in spite of the potentially high patient turnover in such settings.
The mean knowledge score of the study participants was 54.62% of the maximum possible. This reflects that the study participants possess a basic level of knowledge regarding CTs with a scope for further improvement. Knowledge regarding technical aspects of CTs was inadequate among the doctors in our sample as suggested by low correct response rates in items pertaining to good clinical practice (GCP) and schedule Y. This concurs with findings of Yanagawa et al. among Japanese nurses, wherein <50% of nurses had knowledge of GCP.[15]
In our study, only 22.3% doctors were familiar with the term “adverse event”, which is a basic pharmacovigilance terminology, discussed in undergraduate Pharmacology and in training for “adverse drug reaction reporting” thereafter. This highlights the importance a relatively detailed discussion of pharmacovigilance in the undergraduate curriculum and reinforcement of the same thereafter. In contrast, in the study by Bhowmick et al. where 68.75% of medical members of Ethics Committees were aware of pharmacovigilance terminology and activities.[16] In all likelihood, the higher awareness among the medical members of Ethics Committee has developed due to training and regular exposure. Yet, it is desirable that all medical personnel should know the basic terminologies of CT methodology.
Expectedly, it was observed that doctors holding faculty positions were significantly more knowledgeable than the postgraduate trainees regarding CTs. Thus, training on CT science might be emphasized and incorporated in postgraduate curricula irrespective of disciplines. In our study, univariate analysis showed doctors having CT training and involvement in trials fared better in the area of knowledge. However, this advantage did not sustain in multivariate analysis. This observation indicates that repeated training, as opposed to standalone courses, might be necessary for the generation of sustained knowledge regarding CTs. Currently, most doctors receive training on CT methodology with an average duration of 2 days, which seems inadequate for long-term knowledge gain.
About 38% of the doctors believed that the informed consent procedure followed in our country is inadequate and one fourth of them thought that industry sponsored trials are conducted because of commercial gains rather than scientific interests. Nearly 40% respondents thought patients are at a disadvantage by participating in CTs. Similar to our observations, a study by Jadhav and Bhatt reported that over half of the CT professionals from India believe that the risk of participation is inadequately explained to the trial subjects during the consent process.[17] On the other hand, in the study by Bindra and Kochhar 2010, almost a third of the doctors thought that some form of conflict of interest existed in being a CT investigator.[10] However, in spite of negative attitude regarding CTs in some specific items the overall mean perception score was found to be satisfactory (72.5% of maximum possible perception score). This result is encouraging as in spite of deficient knowledge, the overall attitude and perception of doctors towards CTs have not been affected.
On a subgroup analysis, the pre/paraclinical departments were found to having a negative attitude towards CTs in comparison to clinical departments. Dhodi et al. observed that, doctors from prepara clinical departments were more knowledgeable regarding clinical research, although they did not compare their perceptions regarding the same.[9] In the present study, a comparable level of knowledge was found between pre/paraclinical departments and clinical ones. One of the reasons of this relatively negative perception could be a lack of involvement in CTs, resulting in ignorance. This indicates that sensitization specifically to these group of doctors may change their perception.
We observed that over 90% of doctors believed that CT training may be incorporated in the undergraduate curriculum. This might be regarded as an indication of a felt need for the requirement of training, which they have not experienced during their undergraduate course. Similar to our findings, in a study conducted in Mumbai over 70% doctors and medical students were in favor of including clinical research training in the undergraduate curriculum. In Koyto all doctors believed that clinical research is necessary for physicians.[11]
Some limitations of the study merit discussion. First, a statistically derived sample size and sampling strategy could conclude the results with more confidence. Furthermore, the selection of the participating medical colleges was purposive.
CONCLUSION
Our report is the first data in the poorly researched area of the knowledge and perception of doctors regarding CTs in India using a validated questionnaire with high internal consistency. While a basic understanding regarding CTs exists among the participants, knowledge in the technical, logistic and quality control aspects is deficient. A greater degree of involvement along with structured courses can be specific interventions used to target these areas. Although the overall perception of CT is positive, many retain a negative outlook toward the pharmaceutical industry sponsored trials. Given the crucial role of the industry in the conduct of CTs, misconceptions regarding its role cannot bring positive outcomes in the scientific growth of a nation and needs to be cleared. Taken together, our data uncovers important deficits in the knowledge and perception regarding CTs among doctors in India, and identifies specific areas with a need for targeted interventions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank all the doctors who participated in the study. The authors would also like to thank Ms. Rituparna De (MBBS student) and Md. Rashid Alam (MBBS student) for their contribution in data collection and informed consent for non-English speakers translation respectively. We place on record, our sincere thanks to Prof. Dr. R K Roy, HOD (Pharmacology), College of Medicine and Sagore Dutta Hospital (CMSDH), Prof. Dr. Avijit Hazra, Department of Pharmacology, IPGME&R and Dr. Gairik Sengupta, Department of Pharmacology, CMSDH for their contribution in construct validation of the questionnaire used in this study.
REFERENCES
- 1.Imran M, Najmi AK, Rashid MF, Tabrez S, Shah MA. Clinical research regulation in India-history, development, initiatives, challenges and controversies: Still long way to go. J Pharm Bioallied Sci. 2013;5:2–9. doi: 10.4103/0975-7406.106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nundy S, Gulhati CM. A new colonialism? – Conducting clinical trials in India. N Engl J Med. 2005;352:1633–6. doi: 10.1056/NEJMp048361. [DOI] [PubMed] [Google Scholar]
- 3.Shah S. The Body Hunters: Testing New Drugs on the World’s Poorest Patients. New York: New Press; 2006. [Google Scholar]
- 4.Kille LW. Misrepresentation of Randomized Controlled Trials in Press Releases and News. Journalist’s Resource. 2012. [Last accessed on 2015 Feb 10]. Available from: http://www.journalistsresource.org/studies/society/publichealth/misrepresentation-of-randomized-controlled-trials-in-pressreleases-and-news-coverage# .
- 5.Sarojini N, Deepa V. Trials and tribulations: An expose of the HPV vaccine trials by the 72nd Parliamentary Standing Committee Report. Indian J Med Ethics. 2013;10:220–2. doi: 10.20529/IJME.2013.069. [DOI] [PubMed] [Google Scholar]
- 6.Sree SP. How ethical are clinical trials in India? [Last accessed on 2015 Feb 02];Indian J Law. 2005 2:3. Available from: http://www.indialawjournal.com/volume2/issue_3/article_by_sreesudha.html . [Google Scholar]
- 7.Leisinger KM. Meeting the global health challenge: The role of the pharmaceutical industry. Making it Magazine. 2012. [Last accessed on 2015 Feb 10]. Available from: http://www.makingitmagazine.net/?p=6046 .
- 8.Streiner DL. Starting at the beginning: An introduction to coefficient alpha and internal consistency. J Pers Assess. 2003;80:99–103. doi: 10.1207/S15327752JPA8001_18. [DOI] [PubMed] [Google Scholar]
- 9.Dhodi DK, Thakkar KB, Billa G, Khobragade AA, Sinha SR, Patel SB. Knowledge, attitude and practices of medical students and teachers towards clinical research in a tertiary care hospital in Mumbai – Cross sectional survey. J Contemp Med Educ. 2013;1:238–44. [Google Scholar]
- 10.Bindra S, Kochhar P. Survey on perceptions of Indian investigators on research ethics. Perspect Clin Res. 2010;1:94–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Sumi E, Murayama T, Yokode M. A survey of attitudes toward clinical research among physicians at Kyoto University Hospital. BMC Med Educ. 2009;9:75. doi: 10.1186/1472-6920-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi V, Kulkarni AA. Public awareness of clinical trials: A qualitative pilot study in Pune. Perspect Clin Res. 2012;3:125–32. doi: 10.4103/2229-3485.103593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi VD, Oka GA, Kulkarni AA, Bivalkar VV. Public awareness and perception of clinical trials: Quantitative study in Pune. Perspect Clin Res. 2013;4:169–74. doi: 10.4103/2229-3485.115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Report of the Prof Ranjit Roy Chaudhury Expert Committee to Formulate Policy and Guidelines for Approval of New Drugs, Clinical Trials and Banning of Drugs. [Last accessed on 2015 Feb 17]. Available from: http://www.indiaenvironmentportal.org.in/files/file/clinical%20trials1.pdf .
- 15.Yanagawa H, Takai S, Yoshimaru M, Miyamoto T, Katashima R, Kida K. Nurse awareness of clinical research: A survey in a Japanese University Hospital. BMC Med Res Methodol. 2014;14:85. doi: 10.1186/1471-2288-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhowmick S, Banerjee K, Sikdar S, Chatterjee TK. An evaluation of knowledge, attitude, and practice of institutional ethics committee members from eastern India regarding ethics committee functioning and pharmacovigilance activities conducted during clinical trials: A pilot study. Perspect Clin Res. 2014;5:115–20. doi: 10.4103/2229-3485.134310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadhav M, Bhatt A. Ethics in clinical research in India: A survey of clinical research professionals' perceptions. Perspect Clin Res. 2013;4:4–8. doi: 10.4103/2229-3485.106368. [DOI] [PMC free article] [PubMed] [Google Scholar]


