Abstract
Non-obstetric surgery during pregnancy posts additional concerns to anaesthesiologists. The chief goals are to preserve maternal safety, maintain the pregnant state and achieve the best possible foetal outcome. The choice of anaesthetic technique and the selection of appropriate anaesthetic drugs should be guided by indication for surgery, nature, and site of the surgical procedure. Anaesthesiologist must consider the effects of the disease process itself and inhibit uterine contractions and avoid preterm labour and delivery. Foetal safety requires avoidance of potentially dangerous drugs and assurance of continuation of adequate uteroplacental perfusion. Until date, no anaesthetic drug has been shown to be clearly dangerous to the human foetus. The decision on proceeding with surgery should be made by multidisciplinary team involving anaesthesiologists, obstetricians, surgeons and perinatologists. This review describes the general anaesthetic principles, concerns regarding anaesthetic drugs and outlines some specific conditions of non-obstetric surgeries.
Keywords: Anaesthesia, foetal development, non-obstetric surgery, pregnancy, teratogenicity
INTRODUCTION
Anaesthesia for non-obstetric procedures is frequently needed for maternal procedures like cervical encirclage, as well as foetal procedures like ex utero intrapartum treatment, at varying times during pregnancy and even for in vitro fertilisation procedures or assisted reproductive techniques. When pregnant patients require surgery the ‘two-in-one package’, comprising of the patient herself and the unborn foetus in utero, presents substantial anaesthetic challenges. Currently, surgical mortality is not significantly greater in women who are pregnant compared to those that are not.[1] In addition to preserving maternal safety, our goals extend to maintaining the pregnant state and achieving the best possible foetal outcome.
The estimated incidence of pregnant women requiring non-obstetric surgery is around 1–2%.[2] Appendicitis, ovarian disorders (torsion or neoplasm) and trauma constitute the most common non-obstetric conditions requiring surgery during pregnancy. Obstetric patients presenting for neurosurgery[3] or admitted in intensive care unit[4] for various indications require appropriate changes in the management plans by the anaesthesiologist or intensivist. In the largest single series exploring incidental surgeries during pregnancy, trimester-wise breakup is reported to be 42%, 35% and 23% during the first, second and third, respectively.[5]
PRINCIPLES OF ANAESTHETIC MANAGEMENT
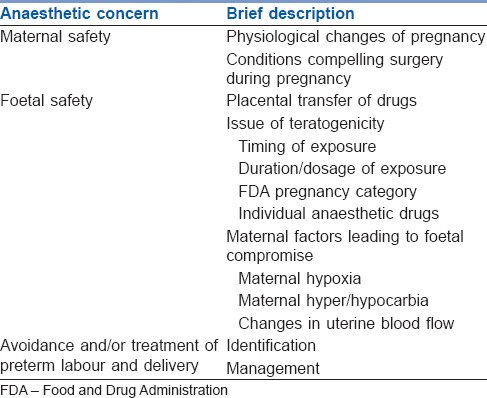
To ensure maternal safety and to maintain the pregnant state, in-depth understanding of the physiological changes and pharmacological adaptations to pregnancy is required. Avoidance of potentially dangerous drugs at critical times during foetal development and maintenance of adequate uteroplacental perfusion are imperative for foetal safety. More importantly, the anaesthesiologist must consider the effects of the disease process itself, inhibit uterine contractions and avoid preterm labour and delivery. The key anaesthetic concerns may be summarised as given in Table 1.
Table 1.
The key anaesthetic concerns for non-obstetric surgery during pregnancy
Maternal safety
According to American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, regardless of trimester, pregnant woman should not be denied indicated surgery. The choice of anaesthetic technique(s), and the selection of appropriate drugs of anaesthesia should be guided by maternal indications for surgery and the location of the surgical procedure. Resuscitation, if required, should be vigorously performed following the standard advanced life support or advanced trauma life support protocols, with the addition of left lateral tilt to avoid supine hypotension.
Rapid-sequence intravenous induction and intubation, with effective cricoid pressure, should be preceded by meticulous pre-oxygenation with 100% oxygen for 5 min. However, in cases of failed intubation, laryngeal mask airway has been used to ventilate successfully and safely in the reverse Trendelenburg's position for brief periods. As changes in maternal position can have profound haemodynamic effects, positioning during anaesthesia should be carried out slowly.
The effects of light general anaesthesia and its associated catecholamine surge with resulting impaired uteroplacental perfusion are considerably more dangerous to foetus. Positive pressure ventilation should be used with care and end-tidal carbon dioxide levels should be maintained within the limits. Since there is a good correlation between end-tidal CO2 (ETCO2) and PaCO2 in pregnancy, ETCO2 can be used to guide ventilation in pregnant patients.[6] Patients should be extubated fully awake as the risk of aspiration persists until protective airway reflexes have returned.
Foetal safety
Depending on the dose administered, the timing of exposure with respect to development, and the route of administration of any drug given during pregnancy can potentially jeopardise the development of the foetus. Until date, no anaesthetic drug has been proven to be clearly hazardous to the human foetus. It may be noted that no animal model perfectly simulates human gestation and a randomised trial on pregnant patients in this regard would be definitely unethical. Hence, definitive evidence seems elusive.
Placental transfer of drugs
The placental drug transfer depends on various factors. High lipid solubility allows the rapid transfer, but may result in trapping of the drug in the placenta. Protein binding has a variable effect depending on the particular drug and protein interaction.
The drugs crossing placenta may be categorised into three types. In type 1 (e.g., thiopental), complete transfer occurs with equilibrating concentrations in maternal and foetal blood. In type 2 (e.g., ketamine), the drug reaches a higher concentration in foetal blood compared to maternal blood. In type 3 (e.g., succinylcholine), only minimal amount reaches the foetal blood.
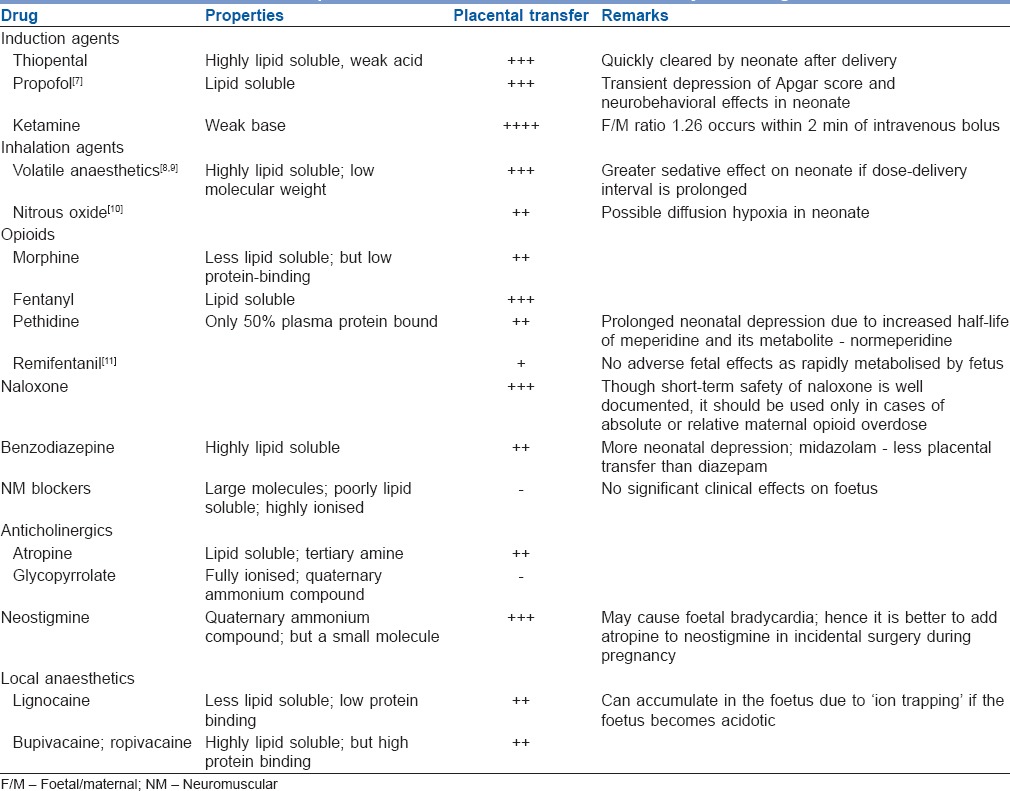
The mechanism of placental drug transfer may be (i) simple diffusion e.g., paracetamol, midazolam; (ii) facilitated diffusion e.g., glucocorticoids, cephalosporins; (iii) active transport e.g., dopamine, norepinephrine; or (iv) pinocytosis. Table 2 depicts the commonly used drugs and their placental transfer characteristics.
Table 2.
The placental transfer characteristics of commonly used drugs
Issue of teratogenicity
A teratogen is defined as a substance that causes an increase in the incidence of a particular defect in a foetus that cannot be attributed to chance. The teratogen must be given in a sufficient dose for a substantial period of time at a critical developmental point to produce the defect.
When considering the possible teratogenicity of various anaesthetic agents, several important points must be kept in mind. First, the background incidence of congenital anomalies in humans is approximately 3%. Second, physiologic derangements such as hypoxaemia, hypercarbia, stress and hypotension may be teratogenic themselves. These problems can occur during anaesthesia and surgery and sometimes exist pre-operatively.
As early as 1960s, Tuchmann-Duplessis[12] observed that major congenital malformations were most likely to occur from exposures between 2 weeks and 2 months of gestation. During the first 15 days of human gestation, the embryo is typically aborted or preserved fully intact (an all-or-nothing phenomenon). Among the multitude of case–control studies, rather than excess birth defects, most have shown a small increase in the risk of miscarriage or preterm delivery.[1,5,13]
Food and drug administration pregnancy risk categories
The Food and Drug Administration (FDA) final rule requires the removal of the pregnancy categories A, B, C, D and X from all human prescription drug and biological product labelling.[14] The new regulation requires that the labeling includes a summary of the risks of using a drug during pregnancy and lactation, a discussion of the data supporting that summary, and relevant information to help health care providers make prescribing decisions and counsel women about the use of drugs during pregnancy and lactation.
FDA appeals healthcare professionals to continue to follow the existing recommendations in current drug labels regarding the use of analgesics during pregnancy. Current drug labels state that pregnant women should not use non-steroidal anti-inflammatory drugs (NSAIDs) in their third trimester of pregnancy because of the risk of premature closure of the ductus arteriosus in the foetus. FDA evaluated research studies published in the medical literature and determined they are too limited to make any recommendations at this time.
Teratogenicity of common anaesthetic drugs
N2O inhibits methionine synthetase, an enzyme necessary for DNA synthesis. Teratogenic effects are shown in animals after administering high concentrations for prolonged periods.[15] However, such high required doses are not encountered in clinical practice. However, some recommend avoiding nitrous oxide in pregnant women.[16,17] In modern day practice, it is rarely necessary to use nitrous oxide in a pregnant patient, and we have so many alternatives for general anaesthesia.
General anaesthetic drugs inhibit synaptic transmission and may lead to abnormal synaptic connections and inappropriate apoptosis.[18] Many anaesthetic agents have effects on neuronal receptors which are necessary for neuronal differentiation, synaptogenesis, and survival during development. In humans, the phase of rapid synaptogenesis extends from mid-gestation to several years after birth, and most of the perinatal anaesthetic exposure will be only for a brief fraction of the susceptible phase. There is no definite evidence to show the teratogenicity of any volatile anaesthetic. However, it is prudent to use the lowest effective concentrations for the shortest possible time, especially because many of these drugs do cause significant maternal hypotension.
Benzodiazepine use in pregnancy has been associated with cleft palate and cardiac anomalies. However, many recent controlled studies have countered this association.[19,20] It is usually recommended to avoid benzodiazepine use throughout gestation and most especially during the first trimester. However, it may be appropriate to provide judicious pre-operative anxiolysis so as to avoid increases in circulating catecholamine levels, which impair uteroplacental perfusion.
Most other anaesthetic medications, including barbiturates, propofol, opioids, muscle relaxants, and local anaesthetics have been widely used during pregnancy with a good safety record. Nonetheless, delicate associations cannot be ruled out.
Maternal factors linked to foetal compromise
Since autoregulation is absent for the uteroplacental circulation, any reduction in maternal arterial pressure can compromise uteroplacental blood flow leading to foetal ischaemia. Therefore, except under unusual circumstances such as severe maternal cardiac or renal disease, the intravenous fluid administration can be liberal. Both ephedrine and phenylephrine are currently considered safe and effective vasopressors during pregnancy for control of maternal hypotension.[21]
Because foetal haemoglobin has a high affinity for oxygen, transient decreases in maternal oxygenation are well tolerated by the foetus.[22] However, prolonged or significant maternal hypoxaemia leads to uteroplacental vasoconstriction, reduced perfusion and subsequent in foetal hypoxaemia, acidosis, and death.[23] Maternal hypercarbia also can produce uterine artery vasoconstriction compromising uterine blood flow.[24] It can also directly lead to foetal respiratory acidosis. Similarly, hypocapnoea causes reduced uterine blood flow and can eventually trigger foetal acidosis. Uterine hypertension, as occurs with increased uterine irritability, can also lead to a reduction in the uteroplacental blood flow.
Risk of preterm labour
Many studies have reported an increased incidence of spontaneous abortion, premature labour, and preterm delivery after non-obstetric surgery during pregnancy. This may be attributed to the surgery itself, manipulation of the uterus, or the underlying condition of the patient, mainly sepsis. As early as 1977, one study showed that foetal mortality was 8.7% when appendicitis occurred without perforation, but was 35.7% when peritonitis was present.[25] The lowest risk for preterm labour is reported during the second trimester and for surgeries that do not manipulate the uterus.[26]
When premature labour occurs, tocolytics are indicated to preserve the pregnancy. Although its efficacy during non-obstetric surgery is debatable, prophylactic tocolytics may be considered in the third trimester for lower abdominal or pelvic surgery for inflammatory conditions. Volatile anaesthetic agents may help relaxing the uterus, although high concentrations can cause undesirable hypotension. Other alternatives include, but not limited to, β-mimetics (e.g., terbutaline), magnesium sulphate, vasodilators (e.g., GTN) and calcium-channel blockers (e.g., nifedipine).
DECISION-MAKING ALGORITHM FOR NON-OBSTETRIC SURGERY DURING PREGNANCY
Elective surgery should be delayed until as late as 6 weeks postpartum. This will allow resolution of physiological changes of pregnancy. An overall miscarriage rate following surgery is reported as 5.8%, increasing to 10.5% during the first trimester.[27] A multidisciplinary team-involving surgeons, anaesthesiologists, obstetricians and perinatologists should be involved in the decision on proceeding with surgery. The second trimester is chosen for semi-elective surgery, which cannot be postponed. Urgent surgery should not be delayed because secondary complications may increase the risk to the mother and/or foetus. Greater risk of uterine irritability and preterm labour are encountered in the advanced stages of pregnancy. This is believed to result from the direct manipulation of the uterus during surgery or the disease process itself, as there is no evidence to suggest that any anaesthetic technique, agent or dose influences this risk. Conditions associated with a particularly high risk include lower abdominal and pelvic inflammatory conditions, such as acute appendicitis with peritonitis. Figure 1 summarises the decision-making algorithm in this regard.
Figure 1.
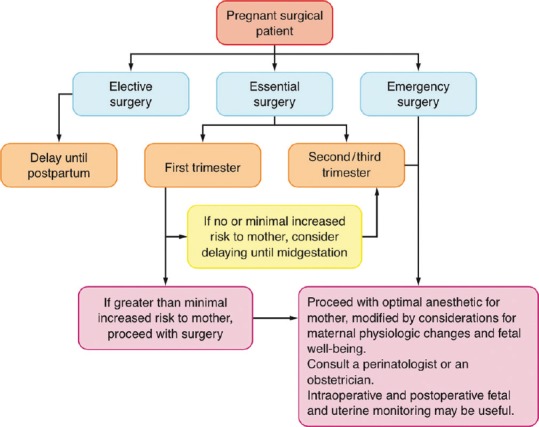
Decision-making algorithm for non-obstetric surgery during pregnancy
GENERAL PRINCIPLES OF ANAESTHESIA MANAGEMENT
Pre-operative preparation
Pregnant patients who require surgery should be evaluated pre-operatively in the same manner as non-pregnant patients. Laboratory and other testing should be performed as indicated by the patient's comorbidities and the proposed surgery. In addition to standard pre-operative procedures, preparation of pregnant women takes into account risks of aspiration, difficult intubation, thromboembolism, and the well-being of the foetus. Standard adult fasting guidelines, i.e., 6-8 h for solid food, depending on the type of food ingested (e.g., fat content) are applicable to these patients.
Aspiration prophylaxis: The gastric emptying has recently been shown to be normal during pregnancy until the onset of labour. However, the risk of aspiration is still higher due to reduced gastric barrier pressure and lower oesophageal sphincter tone (a progesterone effect).[28] The presence of additional risk for regurgitation and aspiration, including active reflux or obesity should be surveyed. Prophylaxis against aspiration pneumonitis should be administered from 16 weeks gestation with H2-receptor antagonists and non-particulate antacids.
Antibiotic prophylaxis: The need for antibiotic prophylaxis depends on the specific procedure. However, attention should be paid in selecting antibiotics with good safety profile in pregnancy.
Prophylactic glucocorticoids: Administration of a course of antenatal glucocorticoids 24–48 h before surgery between 24 and 34 weeks of gestation can reduce perinatal morbidity/mortality if preterm birth occurs. Despite the potential benefits to the foetus, however, antenatal glucocorticoids are best avoided in the setting of systemic infection (such as sepsis or a ruptured appendix), because they may impair the ability of the maternal immune system to contain the infection.
Thromboprophylaxis: Pregnancy is a hypercoagulable state. The 2012 American College of Chest Physicians clinical practice guideline on prevention and treatment of thrombosis recommends mechanical or pharmacologic thromboprophylaxis for all pregnant patients undergoing surgery.[29]
Prophylactic tocolytics: There is no proven benefit to routine administration of prophylactic perioperative tocolytic therapy. Minimising uterine manipulation may reduce the risk of development of uterine contractions and preterm labour. Tocolytics are indicated for the treatment of preterm labour until resolution of the underlying, self-limited condition that may have caused the contractions.
Conduct of anaesthesia
Pregnant patients beyond 18–20 weeks of gestation should be positioned with a 15° left lateral tilt, to reduce aortocaval compression and supine hypotension syndrome. Alternatively, a wedge may be placed under the right hip. Regional anaesthesia, which includes peripheral nerve blocks as well as neuraxial anaesthesia, is an option for some surgical procedures, particularly those involving the extremities. Regional anaesthesia does reduce the exposure of foetuses to potential teratogens, avoids the potential risk of failed intubation and aspiration, and provides excellent post-operative analgesia. Management of neuraxial anaesthesia for non-obstetric surgery in the pregnant patient is no different than its management for caesarean delivery. The major concern with neuraxial anaesthesia is maternal hypotension, which may reduce placental perfusion. However, there is no conclusive evidence demonstrating superior safety for regional anaesthesia, and general anaesthesia is frequently required. Thiopentone in late pregnancy had no significant effect on intra-uterine pressure. Ketamine was found to cause uterine contraction (equal to ergometrine) in early pregnancy. However, ketamine exerts no effect in late pregnancy.[30] Therefore, ketamine's excellent analgesic property may be utilised in late pregnancy. Volatile anaesthetics such as halothane, sevoflurane, desflurane and isoflurane are shown to inhibit the uterine contractility, which may prove beneficial in preventing preterm contractions.[31] During anaesthesia and surgery, foetal well-being is best ensured by careful maintenance of stable maternal haemodynamic parameters and oxygenation. Close monitoring of foetal responses for signs of distress is strongly advocated.
Foetal monitoring
Foetal heart monitoring should be interpreted by an experienced operator with an understanding of the changes encountered during surgery and anaesthesia. Foetal heart rate (FHR) monitoring is practical from 18 to 22 weeks, and from 25 weeks, heart rate variability (HRV) can be readily observed. Anaesthetic agents reduce both baseline FHR and HRV, so readings must be interpreted in the context of administered drugs. The value of intraoperative FHR monitoring is that it detects an early compromise, allowing optimization of maternal haemodynamics and oxygenation with appropriate fluid therapy, vasopressors, blood product administration, hyperventilation or position adjustment.
Monitoring for uterine contractions
When external tocodynamometer can be placed outside of the surgical field, uterine contractions may be monitored intraoperatively. If uterine contractions are detected, maternal haemodynamics should be improved by giving more intravenous fluids and also consider tocolytic treatment in consultation with the perinatologist/obstetrician. Tocometry during post-operative period is useful as post-operative analgesia may mask awareness of mild early contractions and delay tocolysis.
Recovery from anaesthesia
Recovery from anaesthesia requires close monitoring, particularly of the airway and respiratory system, because most severe anaesthetic complications due to hypoventilation or airway obstruction occur during emergence, extubation, or recovery.
Post-operative analgesia
Provision of adequate analgesia is important in the post-operative period as well, since the pain has been shown to increase the risk of premature labour. Regional nerve or plexus blockade or epidural analgesia can provide excellent post-operative analgesia and reduce the risk of opioid-induced hypoventilation when compared with intravenous opioids. Opioids can be used, as needed, to control post-operative pain. Paracetamol is the analgesic of choice for the treatment of mild to moderate pain during any stage of pregnancy. NSAIDs should be avoided, especially after 32 weeks of gestation, because they may cause premature closure of the foetal ductus arteriosus (if given for more than 48 h). They are also associated with oligohydramnios with reduced foetal renal function. NSAIDs can also inhibit uterine contraction.
CLINICAL PEARLS REGARDING NON-OBSTETRIC SURGERY DURING PREGNANCY
Based on current evidence, pregnancy testing is a cost-effective method and should be offered to all verbally consenting females of childbearing potential. This does not substitute for an appropriate pregnancy history.
Regarding fluid resuscitation during pregnancy, rather than the type of fluid, maintenance of hemodynamic goals take higher priority. Positioning concerns to avoid aortocaval compression by the gravid uterus is an important consideration while managing persistent hypotension.
While contemplating on blood transfusion during pregnancy, additional concerns arise due to maternal physiological changes and the risks to foetus due to alloimmunisation and infective complications. Expected physiological changes in vital signs during pregnancy and some pregnancy-related comorbids such as pre-eclampsia, thrombocytopaenia, coagulation changes, etc., make the blood loss estimations a thorny problem.[32]
Pregnancy is no longer considered a contraindication to laparoscopic surgery. Laparoscopic techniques may be preferred when abdominal surgery is undertaken of reduced morbidity for the mother, and potentially a decreased incidence of preterm labour as a result of reduced manipulation of the uterus. The advantages include smaller incisions, decreased pain, less need for analgesics, and more rapid recovery and mobilisation.
Trauma in itself complicates 6–7% of pregnancies. The emphasis is on maternal resuscitation and in life-threatening multi-trauma, caesarean section may be performed to improve maternal haemodynamics.
Neuro-anaesthesia may be indicated during pregnancy for procedures such as intracranial surgery, spinal surgery and diagnostic and therapeutic interventions. Some tumours such as meningiomas may express oestrogen or progesterone receptors and as such rapidly increase in size in the pregnant state. Spinal surgery represents a particular challenge regarding patient positioning. While the prone position provides good uteroplacental perfusion, the mechanics are challenging in the pregnant population.
Cardiac surgery with cardiopulmonary bypass involves certain pathophysiologic effects, such as hypothermia, haemodilution, inhibition of coagulation, haemolysis, complement activation, and non-pulsatile flow, as well as acid-base changes that affect the uteroplacental circulation and the foetus. The choice of inotropic or vasoactive drug should be based predominantly on the underlying pathology and haemodynamic goals.
The ex-utero intrapartum procedure is used to secure a foetal airway while gas exchange continues via the placenta. Historically, Ex utero intrapartum therapy (EXIT) was described using general anaesthesia with high end-tidal concentrations of volatile anaesthetic agents, while resulting hypotension and insufficient uteroplacental blood flow are managed with vasopressor. Alternatively, EXIT procedures can be performed using neuraxial anaesthesia with intravenous nitroglycerine to achieve uterine relaxation.[33,34]
SUMMARY
While dealing with non-obstetric surgery or some maternal procedures during pregnancy, anaesthesiologist is dealing with “two clients in one” scenario, where the safety of both is very important and challenging. Anaesthesia management, including post-operative analgesia, should be planned well to preserve the pregnancy and to ensure the safety of the mother as well as the foetus. A multidisciplinary team approach is highly recommended to ensure an adequate standard of care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Coleman MT, Trianfo VA, Rund DA. Nonobstetric emergencies in pregnancy: Trauma and surgical conditions. Am J Obstet Gynecol. 1997;177:497–502. doi: 10.1016/s0002-9378(97)70135-2. [DOI] [PubMed] [Google Scholar]
- 2.Crowhurst JA. Anaesthesia for non-obstetric surgery during pregnancy. Acta Anaesthesiol Belg. 2002;53:295–7. [PubMed] [Google Scholar]
- 3.Marulasiddappa V, Raghavendra B, Nethra H. Anaesthetic management of a pregnant patient with intracranial space occupying lesion for craniotomy. Indian J Anaesth. 2014;58:739–41. doi: 10.4103/0019-5049.147170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trikha A, Singh P. The critically ill obstetric patient - Recent concepts. Indian J Anaesth. 2010;54:421–7. doi: 10.4103/0019-5049.71041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazze RI, Källén B. Reproductive outcome after anesthesia and operation during pregnancy: A registry study of 5405 cases. Am J Obstet Gynecol. 1989;161:1178–85. doi: 10.1016/0002-9378(89)90659-5. [DOI] [PubMed] [Google Scholar]
- 6.Bhavani-Shankar K, Steinbrook RA, Brooks DC, Datta S. Arterial to end-tidal carbon dioxide pressure difference during laparoscopic surgery in pregnancy. Anesthesiology. 2000;93:370–3. doi: 10.1097/00000542-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Zakowski MI, Herman NL. The placenta: Anatomy, physiology, and transfer of drugs. In: Chestnut DH, editor. Obstetric Anesthesia: Principles and Practice. 3rd ed. Philadelphia: Elsevier-Mosby; 2004. pp. 49–65. [Google Scholar]
- 8.Kangas L, Erkkola R, Kanto J, Mansikka M. Halothane anaesthesia in caesarean section. Acta Anaesthesiol Scand. 1976;20:189–94. doi: 10.1111/j.1399-6576.1976.tb05027.x. [DOI] [PubMed] [Google Scholar]
- 9.Dwyer R, Fee JP, Moore J. Uptake of halothane and isoflurane by mother and baby during caesarean section. Br J Anaesth. 1995;74:379–83. doi: 10.1093/bja/74.4.379. [DOI] [PubMed] [Google Scholar]
- 10.Polvi HJ, Pirhonen JP, Erkkola RU. Nitrous oxide inhalation: Effects on maternal and fetal circulations at term. Obstet Gynecol. 1996;87:1045–8. doi: 10.1016/0029-7844(96)00060-9. [DOI] [PubMed] [Google Scholar]
- 11.Kan RE, Hughes SC, Rosen MA, Kessin C, Preston PG, Lobo EP. Intravenous remifentanil: Placental transfer, maternal and neonatal effects. Anesthesiology. 1998;88:1467–74. doi: 10.1097/00000542-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Tuchmann-Duplessis H. The teratogenic risk. Am J Ind Med. 1983;4:245–58. [PubMed] [Google Scholar]
- 13.Shnider SM, Webster GM. Maternal and fetal hazards of surgery during pregnancy. Am J Obstet Gynecol. 1965;92:891–900. doi: 10.1016/0002-9378(65)90722-2. [DOI] [PubMed] [Google Scholar]
- 14. [Last accessed on 2015 Nov 14]. Available from: https://www.federalregister.gov/articles/2014/12/04/2014-28241/content-and-format-oflabeling-for-human-prescription-drug-and-biologicalproducts-requirements-for?utm_campaign=email+a+friend and utm_medium=email and utm_source=federalregister.gov .
- 15.Fujinaga M, Baden JM. Methionine prevents nitrous oxide-induced teratogenicity in rat embryos grown in culture. Anesthesiology. 1994;81:184–9. doi: 10.1097/00000542-199407000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Aldridge LM, Tunstall ME. Nitrous oxide and the fetus. A review and the results of a retrospective study of 175 cases of anaesthesia for insertion of Shirodkar suture. Br J Anaesth. 1986;58:1348–56. doi: 10.1093/bja/58.12.1348. [DOI] [PubMed] [Google Scholar]
- 17.Crawford JS, Lewis M. Nitrous oxide in early human pregnancy. Anaesthesia. 1986;41:900–5. doi: 10.1111/j.1365-2044.1986.tb12912.x. [DOI] [PubMed] [Google Scholar]
- 18.Sonner JM, Antognini JF, Dutton RC, Flood P, Gray AT, Harris RA, et al. Inhaled anaesthetics and immobility: Mechanisms, mysteries, and minimum alveolar anesthetic concentration. Anesth Analg. 2003;97:718–40. doi: 10.1213/01.ANE.0000081063.76651.33. [DOI] [PubMed] [Google Scholar]
- 19.Safra MJ, Oakley GP., Jr Association between cleft lip with or without cleft palate and prenatal exposure to diazepam. Lancet. 1975;2:478–80. doi: 10.1016/s0140-6736(75)90548-6. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg L, Mitchell AA, Parsells JL, Pashayan H, Louik C, Shapiro S. Lack of relation of oral clefts to diazepam use during pregnancy. N Engl J Med. 1983;309:1282–5. doi: 10.1056/NEJM198311243092103. [DOI] [PubMed] [Google Scholar]
- 21.Ngan Kee WD, Khaw KS. Vasopressors in obstetrics: What should we be using? Curr Opin Anesthesiol. 2006;19:238–43. doi: 10.1097/01.aco.0000192816.22989.ba. [DOI] [PubMed] [Google Scholar]
- 22.Itskovitz J, LaGamma EF, Rudolph AM. The effect of reducing umbilical blood flow on fetal oxygenation. Am J Obstet Gynecol. 1983;145:813–8. doi: 10.1016/0002-9378(83)90684-1. [DOI] [PubMed] [Google Scholar]
- 23.Dilts PV, Jr, Brinkman CR, 3rd, Kirschbaum TH, Assali NS. Uterine and systemic hemodynamic interrelationships and their response to hypoxia. Am J Obstet Gynecol. 1969;103:138–57. doi: 10.1016/s0002-9378(16)34357-5. [DOI] [PubMed] [Google Scholar]
- 24.Walker AM, Oakes GK, Ehrenkranz R, McLaughlin M, Chez RA. Effects of hypercapnia on uterine and umbilical circulations in conscious pregnant sheep. J Appl Physiol. 1976;41(5 Pt 1):727–33. doi: 10.1152/jappl.1976.41.5.727. [DOI] [PubMed] [Google Scholar]
- 25.Babaknia A, Parsa H, Woodruff JD. Appendicitis during pregnancy. Obstet Gynecol. 1977;50:40–4. [PubMed] [Google Scholar]
- 26.Saunders P, Milton PJ. Laparotomy during pregnancy: An assessment of diagnostic accuracy and fetal wastage. Br Med J. 1973;3:165–7. doi: 10.1136/bmj.3.5872.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen-Kerem R, Railton C, Oren D, Lishner M, Koren G. Pregnancy outcome following non-obstetric surgical intervention. Am J Surg. 2005;190:467–73. doi: 10.1016/j.amjsurg.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 28.Wong CA, McCarthy RJ, Fitzgerald PC, Raikoff K, Avram MJ. Gastric emptying of water in obese pregnant women at term. Anesth Analg. 2007;105:751–5. doi: 10.1213/01.ane.0000278136.98611.d6. [DOI] [PubMed] [Google Scholar]
- 29.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ. American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9 th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S–47S. doi: 10.1378/chest.1412S3. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oats JN, Vasey DP, Waldron BA. Effects of ketamine on the pregnant uterus. Br J Anaesth. 1979;51:1163–6. doi: 10.1093/bja/51.12.1163. [DOI] [PubMed] [Google Scholar]
- 31.Yoo KY, Lee JC, Yoon MH, Shin MH, Kim SJ, Kim YH, et al. The effects of volatile anaesthetics on spontaneous contractility of isolated human pregnant uterine muscle: A comparison among sevoflurane, desflurane, isoflurane, and halothane. Anesth Analg. 2006;103:443–7. doi: 10.1213/01.ane.0000236785.17606.58. [DOI] [PubMed] [Google Scholar]
- 32.Jadon A, Bagai R. Blood transfusion practices in obstetric anaesthesia. Indian J Anaesth. 2014;58:629–36. doi: 10.4103/0019-5049.144674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia PJ, Olutoye OO, Ivey RT, Olutoye OA. Case scenario: Anesthesia for maternal-fetal surgery: The ex utero intrapartum therapy (EXIT) procedure. Anesthesiology. 2011;114:1446–52. doi: 10.1097/ALN.0b013e31821b173e. [DOI] [PubMed] [Google Scholar]
- 34.George RB, Melnick AH, Rose EC, Habib AS. Case series: Combined spinal epidural anesthesia for Cesarean delivery and ex utero intrapartum treatment procedure. Can J Anaesth. 2007;54:218–22. doi: 10.1007/BF03022643. [DOI] [PubMed] [Google Scholar]


