Abstract
Explosive devices have been the most frequent cause of traumatic brain injury (TBI) among deployed contemporary U.S. service members. The purpose of this study was to examine the influence of previous cumulative blast exposures (that did or did not result in TBI) on later post-concussion and post-traumatic symptom reporting after sustaining a mild TBI (MTBI). Participants were 573 service members who sustained MTBI divided into four groups by number of blast exposures (1, 2, 3, and 4–10) and a nonblast control group. Post-concussion symptoms were measured using the Neurobehavioral Symptom Inventory (NSI) and post-traumatic stress disorder (PTSD) symptoms using the Post-traumatic Checklist-Civilian version (PCL-C). Results show groups significantly differed on total NSI scores (p<0.001), where symptom endorsement increased as number of reported blast exposures increased. Total NSI scores were significantly higher for the 3– and 4–10 blast groups compared with the 1- and 2-blast groups with effect sizes ranging from small to moderate (d=0.31 to 0.63). After controlling for PTSD symptoms using the PCL-C total score, NSI total score differences remained between the 4–10-blast group and the 1- and 2-blast groups, but were less pronounced (d=0.35 and d=0.24, respectively). Analyses of NSI subscale scores using PCL-C scores as a covariate revealed significant between-blast group differences on cognitive, sensory, and somatic, but not affective symptoms. Regression analyses revealed that cumulative blast exposures accounted for a small but significant amount of the variance in total NSI scores (4.8%; p=0.009) and total PCL-C scores (2.3%; p<0.001). Among service members exposed to blast, post-concussion symptom reporting increased as a function of cumulative blast exposures. Future research will need to determine the relationship between cumulative blast exposures, symptom reporting, and neuropathological changes.
Key words: : blast, mild traumatic brain injury, neurobehavioral symptom inventory, post-concussion, post-traumatic stress
Introduction
More than 290,000 U.S. military service members sustained a traumatic brain injury (TBI) between 2000–2013.1 Mild TBI (MTBI), or concussion, predominates in the U.S. military, with 82.5% of all reported TBI cases between 2000–2013 being classified as MTBI.1 Investigations using diagnostic codes from selected samples of military medical records estimate that between 10% and 20% of all returned veterans from Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) sustained at least one MTBI.2–5 Although noncombat accidents, primarily motor vehicle accidents, are the most frequent causes of moderate and severe TBI,6 blast-related TBI represents a significant proportion of the MTBIs sustained by deployed U.S. military personnel, with nearly 80% of all deployment-related MTBI in U.S. military personnel serving in Iraq and Afghanistan being blast related.7
A study of MTBI in a non-combat injured military population found increased symptom reporting among soldiers with two or more MTBIs compared with soldiers who had experienced a single MTBI, but only within the first 3 months post-injury.8 A study of deployed U.S. service members found that 87% of second concussions occurred within 30 days of the initial injury, and that severity of the second injury (but not the first) was associated with higher use of mental health and neurological services during the 2 years after injury.9 This is of concern because early evidence suggests that close temporal proximity of multiple head injuries is associated with poorer recovery. Another study found that Florida National Guard personnel were more likely to receive a diagnosis of probable major depression, anxiety, post-traumatic stress disorder (PTSD), and post-concussion syndrome if they reported sustaining multiple MTBIs, as opposed to a single injury.10
Many soldiers have experienced multiple concussive and subconcussive blast exposures.11 While data suggest that multiple concussions may be problematic, little is known about the effect of blast exposure. That is, does simple exposure to blast, if it does not reach the threshold of an MTBI, have an effect on symptom reporting? A recent study in a small sample of veterans suggests that exposure to primary blast rather than diagnosis of MTBI after exposure to primary blast confers white matter abnormality with associated decrements in set shifting and spatial working memory.12 Still, much is unknown about the impact and consequences of multiple blast injuries in military personnel. Given the current literature, it is reasonable to hypothesize that multiple blast exposures might increase post-concussion symptom reporting, possibly demonstrating a cumulative effect regarding number of blast exposures and severity of symptom burden. Thus, the purpose of this investigation was to characterize post-concussion symptomatology after MTBI with varying numbers of previous blast exposures in a multicenter cohort of previously deployed military service members. PTSD symptomatology was also examined to determine what the additive burden of psychological comorbidity might contribute to overall symptom reporting.
Methods
Participants
Participants were derived from a larger sample of 3205 service members evaluated at one of six Military Medical Centers: San Antonio Military Medical Center, San Antonio, TX (SAMMC; n=1053); Walter Reed National Military Medical Center, Bethesda, MD (WRNMMC; n=861); Naval Medical Center San Diego, San Diego, CA (NMCSD; n=437); Marine Corps Base Camp Pendleton, Camp Pendleton, CA (MCBCP; n=476); Marine Corps Base Twenty Nine Palms, Twenty Nine Palms, CA (MCB29P; n=108); and Wilford Hall Air Force Medical Center, San Antonio, TX (WHAFMC; n=222), and 48 cases were missing a location variable.
Inclusion criteria for selecting the current sample consisted of closed head injury only (excluded n=71), valid and complete Post-traumatic Checklist-Civilian version (PCL-C) and Neurobehavioral Symptom Inventory (NSI) inventories (excluded n=350), injury sustained in OEF/OIF (excluded n=493), time tested post-injury between 1 and 24 months (excluded n=984), sustained mild TBI only (excluded n=596), and were male (excluded n=28). Females were excluded only because of the relatively small number who met inclusion criteria. There were 104 persons who were excluded because of missing data regarding number of previous blast exposures. Finally, six additional service members were excluded because they reported exposure to more than 10 blasts. The Wilford Hall Air Force Medical Center Internal Review Board approved this study.
Final sample selection included 573 male active duty military service members (mean age=26.8 years, standard deviation=7.0, range=18–55), who sustained combat related MTBI, grouped into five categories: 0 blast exposures (n=68), 1 blast exposure (n=123), 2 blast exposures (n=178), 3 blast exposures (n=106), and 4–10 blast exposures (n=98). The number of blasts that characterize each group reflects the number of previous blast exposures subjectively reported by the service member plus the current mechanism of injury. Whereas diagnosis and verification of MTBI was required for inclusion in the study, it is unknown whether previous blast exposures resulted in undocumented MTBI. Previous blast exposure was simply operationalized as any blast that knocked the service member off his feet or resulted in injury. Number of previous blast exposures therefore does not necessarily reflect the number of previous concussions or MTBIs in this study.
The mechanism of injury/cause of current MTBI in all groups occurred in the context of a blast event except the 0 blast group. The primary blast pressure wave, however, may not have explicitly caused the MTBI in this sample. Primary, secondary, or tertiary blast injuries may have been the “blast-related” cause of MTBI in members of any of our groups (excluding the 0-blast group).
The distribution of participants by military service branch was as follows; Army (n=3 59, 62.7%), Marines (n=170, 29.6%), Navy (n=43, 7.5%) and Air Force (n=1, 0.2%).
MTBI was classified according to Department of Defense (DoD) criteria with the exception of “complicated” mild cases. DoD guidelines suggest that any TBI resulting in abnormal brain imaging be classified as at least moderate in severity. For purposes of this research, however, we chose to include cases in which loss of consciousness (LOC) and post-traumatic amnesia (PTA) fell in the mild range (<30 min and <24 h, respectively), including those few with identified neuroimaging abnormalities (n=15).
For the 0 blast group (i.e., those with nonblast-related MTBI and no exposure to blast) the primary mechanism of injury was distributed as follows (more than one mechanism could be endorsed by the service members): fall (n=28, 41.2%), motor vehicle accident (n=23, 33.8%), bullet (n=2, 2.9%), and “other” (n=18, 26%). For the remaining groups, the source of blast-related MTBI was (again, more than one mechanism could be endorsed by the service members): improvised explosive device (n=412, 81.6%), land mine (n=7, 1.4%), rocket-propelled grenade (n=43, 8.5%), grenade (n=6, 1.2%), bomb (n=10, 2.0%), mortar (n=14, 2.8%), “other” (n=29, 5.7%).
Measures
Details of the service members' TBI and demographic data were obtained via the Clinical Tracking Form (CTF) and medical chart review. Post-concussion symptoms were measured with the NSI and PTSD symptoms with the PCL-C.
CTF
The CTF is a structured questionnaire detailing the service member's TBI and was administered via a semi-structured clinical interview at one of the aforementioned Military Medical Centers by a physician assistant, nurse, neuropsychologist, or social worker trained in the assessment of TBI. The CTF queries demographic information such as the service member's age, sex, rank, and branch of military service. It also covers injury-related information, such as date, time, location, and mechanism of injury, directionality of impacts (if any), specific sensory deficits, presence and duration of LOC, and amnesia.
Post-concussive symptoms
The NSI is a 22-item self-report inventory of common post-concussion sequelae.13 Subjects are instructed to rate the presence/severity of each symptom within the past 2 weeks on a five-item Likert scale ranging from 0 (none) to 4 (very severe). Analyses in this study were conducted on both NSI total scores as well as the four subscale scores identified in the original Cicerone factor analysis (cognitive, affective, sensory, and somatic) to facilitate comparison of present results with existing literature.
Post-traumatic stress symptoms
The PCL-C is a self-rated interval-level rating scale used to screen for PTSD.14 The PCL-C consists of 17 items, each designed to capture one of three distinct clusters of symptoms representing PTSD diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). These three clusters are labeled reexperiencing (items 1–5), avoidance or numbing (items 6–12) and hyperarousal (items 13–17). This self-reported measure requires the subject to rate how much he/she has been bothered by that symptom in the past month, using a 1 (not at all) to 5 (extremely) Likert-scale value. Scores are derived by summing the weighted frequencies for all items and can range from 17 to 85. For the current study, PCL-C total scores were used to capture the degree of PTSD symptom severity.
Statistical analyses
Differences in the demographic makeup of the five blast-exposure groups were explored using analysis of variance (ANOVA) for continuous variables and chi-square or Fisher exact (for TBI severity and LOC duration comparisons only) tests for categorical variables. Between-group differences on demographic and injury-related variables were tested with univariate ANOVAs and effect sizes calculated for significant differences. PTSD and post-concussive symptom endorsement were compared between groups using analysis of covariance (ANCOVA) with the demographic variables found to discriminate between groups included as covariates. An additional ANCOVA was conducted on post-concussion symptom endorsement, controlling for both demographic variables and PTSD symptom report. Finally, hierarchical multiple linear regression analyses were conducted to ascertain the amount of variance in symptom reporting accounted for by number of blast exposures. For the purposes of symptom analyses in this investigation, “demographic” is defined to include age, number of deployments, time since injury (TSI), and duration of LOC. All analyses were conducted using SPSS version 19.
Results
Demographics
Significant differences between blast groups (Table 1) were present in measures of TSI (F=2.452, df(4568), p=0.049) and number of deployments (F=2.414, df(4540), p=0.048), while the proportion of LOC was significantly different (χ2=11.659, df(4), p=0.02). A trend toward significance was seen for age (F=2.115, df(4568), p=0.078). There were no differences seen across blast groups for duration of PTA, TBI severity, or rank (all p>0.05).
Table 1.
Demographic and Injury Related Variables across Blast Exposure Groups
| 0 blasts(n=68) | 1 blast(n=123) | 2 blasts(n=178) | 3 blasts(n=106) | 4–10 blasts(n=98) | |||
|---|---|---|---|---|---|---|---|
| M(SD) | M(SD) | M(SD) | M(SD) | M(SD) | F (df) | p | |
| Age | 28.1 (9.1) | 25.5 (5.8) | 27.4 (7.4) | 26.9 (7.1) | 26.5 (5.7) | 2.11 (4,568) | 0.078 |
| Time since injury (days) | 218 (183) | 255 (206) | 220 (193) | 204 (165) | 181 (156) | 2.40 (4,565) | 0.049 |
| Deployment number | 1.5 (0.8) | 1.7 (1.2) | 1.5 (0.7) | 1.7 (0.9) | 1.8 (0.9) | 2.41 (4,540) | 0.048 |
| N (%) | N (%) | N (%) | N (%) | N (%) | p | |
|---|---|---|---|---|---|---|
| LOC | ||||||
| Yes | 60 (88.2) | 86 (69.9) | 148 (83.1) | 84 (79.2) | 76 (77.6) | 0.020 |
| No | 8 (11.8) | 37 (30.1) | 30 (16.9) | 22 (20.8) | 22 (22.4) | |
| LOC (Duration) | ||||||
| None | 8 (11.8) | 37 (30.1) | 30 (16.9) | 22 (20.8) | 22 (22.4) | 0.035 |
| <1 min | 37 (54.2) | 46 (37.4) | 107 (60.1) | 51 (48.1) | 49 (50.0) | |
| 1–15 min | 22 (32.6) | 38 (30.9) | 39 (21.9) | 30 (28.3) | 24 (24.5) | |
| 16–30 min | 1 (1.4) | 2 (1.6) | 2 (1.1) | 3 (2.8) | 3 (3.1) | |
| PTA (duration) | ||||||
| <1 min | 27 (39.6) | 37 (30.1) | 82 (46.0) | 40 (37.6) | 35 (35.7) | 0.237 |
| 1–15 min | 22 (32.4) | 54 (43.9) | 56 (31.5) | 36 (34.0) | 29 (29.6) | |
| 16–59 min | 8 (11.8) | 14 (11.4) | 22 (12.4) | 15 (14.2) | 19 (19.4) | |
| 1–24 h | 11 (16.2) | 18 (14.6) | 18 (10.1) | 15 (14.2) | 15 (15.3) | |
| TBI severity | ||||||
| Complicated | 4 (5.9) | 2 (1.6) | 7 (3.9) | 1 (.9) | 1 (1.0) | 0.182 |
| Uncomplicated | 64 (94.1) | 121 (98.4) | 171 (96.1) | 105 (99.1) | 97 (99.0) | |
| Rank | ||||||
| E1–4 | 42 (61.8) | 80 (65.0) | 107 (60.1) | 59 (55.7) | 49 (50.0) | 0.208 |
| E5+ | 26 (38.2) | 43 (35.0) | 71 (39.9) | 47 (44.3) | 49 (50.0) | |
SD, standard deviation; LOC, loss of consciousness; PTA, post-traumatic amnesia; TBI, traumatic brain injury.
NSI and PCL-C
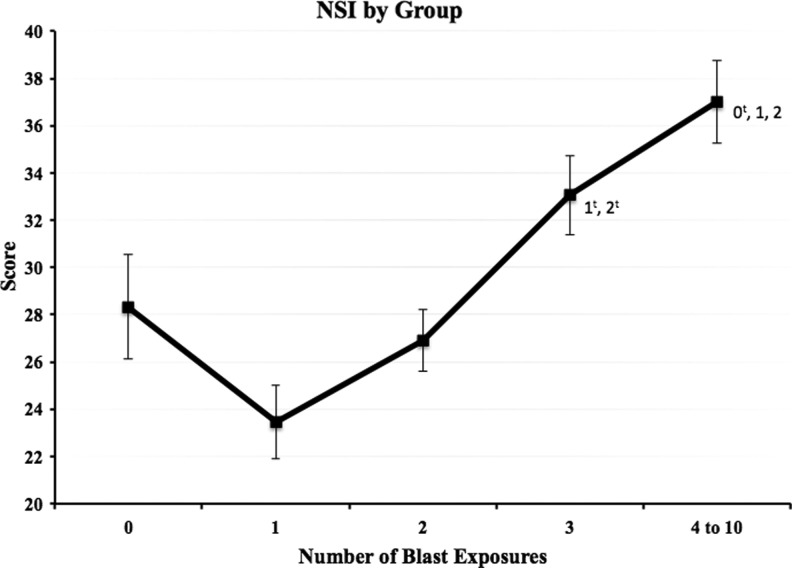
Examination of mean total NSI scores by blast group revealed a linear increase in scores from the 1 blast to the 4–10 blast group (Fig. 1). Time since injury, number of deployments, LOC, and age were included as covariates in between-group comparisons of the NSI and its subscales (Table 2). These analyses revealed a main effect of blast group (F=10.5, p<0.001, η2=0.072) on symptom reporting (total NSI score). Scores among the four groups with blast-related MTBI increased as a function of blast exposure. Bonferroni-corrected post hoc analysis (Fig. 1) revealed significantly higher scores in the 4–10 blast group (M=36.0) than the 1 blast (M=23.3) and 2 blast (M=27.4) groups (p<0.001, d=0.76, and p<0.001, d=0.50, respectively) and significantly higher scores in the 3 blast group (M=32.8) than the 1 blast and 2 Blast groups (p<0.001, d=0.56 and p=0.038, d=0.31, respectively). Scores from the 0 blast group (M=29.4) were significantly lower than the 4–10 blast group (p=0.023, d=0.37). The 0 blast group (MTBI from a no-blast mechanism with no reported previous blast exposures) had a mean score that fell between the 2 blast and 3 blast groups.
FIG. 1.
Raw Neurobehavioral Symptom Inventory (NSI) total mean score for each blast exposure group. Error bars reflect standard error, and values adjacent to group mean markers represent blast exposure groups for which significant differences were present. tsignifies group mean differences that were no longer statistically significant after the addition of demographic variables and Post-traumatic Checklist-Civilian version scores as covariates.
Table 2.
Analysis of Variance and Analysis of Covariance Comparisons of Neurobehavioral Symptom Inventory and Post-Traumatic Checklist-Civilian version Symptom Endorsement Scores across Blast Exposure Groups
| Group | 0 blasts(n=68) | 1 blast(n=123) | 2 blasts(n=178) | 3 blasts(n=106) | 4–10 blasts(n=98) | ||
|---|---|---|---|---|---|---|---|
| M(SD) | M(SD) | M(SD) | M(SD) | M(SD) | F (df) | p | |
| ANOVA | |||||||
| NSI total | 29.3 (17.9) | 24.1 (16.6) | 27.6 (17.2) | 32.9 (17.7) | 35.0 (17.8) | 7.02 (4,568) | <0.001 |
| Somatic | 5.5 (4.8) | 4.2 (4.2) | 5.4 (4.4) | 6.6 (4.8) | 7.4 (4.9) | 7.81 (4,568) | <0.001 |
| Cognitive | 8.3 (5.0) | 6.5 (5.0) | 7.3 (5.2) | 9.0 (5.3) | 9.6 (5.2) | 6.58 (4,568) | <0.001 |
| Affective | 6.1 (4.7) | 5.1 (4.4) | 5.6 (4.4) | 6. 8 (4.6) | 6.5 (4.5) | 2.59 (4,568) | 0.036 |
| Sensory | 9.5 (5.5) | 8.2 (5.0) | 9.2 (5.5) | 10.5 (5.5) | 11.5 (5.4) | 6.24 (4,568) | <0.001 |
| PCL-C total | 42.9 (18.2) | 39.9 (17.1) | 41.5 (16.5) | 45.8 (17.5) | 46.5 (17.5) | 3.08 (4,568) | 0.016 |
| Re-experience | 11.7 (5.9) | 11.3 (5.3) | 12.4 (5.6) | 13.5 (5.6) | 13.6 (6.1) | 3.38 (4,568) | 0.009 |
| Avoidance | 16.6 (7.9) | 14.9 (7.3) | 14.8 (6.8) | 16.4 (7.6) | 16.8 (7.6) | 2.14 (4,568) | 0.074 |
| Hyperarousal | 14.6 (5.7) | 13.7 (5.6) | 14.3 (5.6) | 15.9 (5.4) | 16.1 (5.5) | 3.97 (4,568) | 0.003 |
| ANCOVA (demographics) | Marginal Mean (SD) | Marginal Mean (SD) | Marginal Mean (SD) | Marginal Mean (SD) | Marginal Mean (SD) | ||
| NSI total | 28.3 (18.7) | 23.5 (16.0) | 26.9 (17.0) | 33.1 (17.9) | 37.0 (17.5) | 10.45 (4,537) | <0.001 |
| Somatic | 5.3 (4.9) | 4.1 (4.0) | 5.3 (4.3) | 6.7 (4.9) | 7.8 (4.9) | 10.54 (4,537) | <0.001 |
| Cognitive | 7.9 (5.2) | 6.3 (4.9) | 7.2 (5.2) | 9.1 (5.3) | 10.1 (5.1) | 9.72 (4,537) | <0.001 |
| Affective | 5.7 (4.8) | 4.9 (4.2) | 5.4 (4.3) | 6.9 (4.6) | 7.0 (4.5) | 4.73 (4,537) | 0.001 |
| Sensory | 9.4 (5.5) | 8.1 (4.9) | 9.0 (5.5) | 10.4 (5.5) | 12.1 (5.4) | 8.52 (4,537) | <0.001 |
| ANCOVA (demographics and PCL-C) | Marginal Mean (SD) | Marginal Mean (SD) | Marginal Mean (SD) | Marginal Mean (SD) | Marginal Mean (SD) | ||
| NSI total | 29.2 (18.7) | 26.7 (16.0) | 28.4 (17.0) | 30.4 (17.9) | 32.5 (17.5) | 4.71 (4,536) | 0.001 |
| Somatic | 5.5 (4.9) | 4.7 (4.0) | 5.6 (4.3) | 6.1 (4.9) | 6.9 (4.9) | 4.99 (4,536) | 0.001 |
| Cognitive | 8.1 (5.2) | 7.2 (4.9) | 7.6 (5.2) | 8.4 (5.3) | 8.9 (5.1) | 3.98 (4,536) | 0.003 |
| Affective | 5.9 (4.9) | 5.8 (4.2) | 5.8 (4.3) | 6.2 (4.6) | 5.8 (4.5) | 0.52 (4,536) | 0.724 |
| Sensory | 9.6 (5.5) | 9.0 (4.9) | 9.4 (5.5) | 9.7 (5.5) | 10.9 (5.4) | 3.22 (4,536) | 0.013 |
ANOVA, analysis of variance; NSI, Neurobehavioral Symptom Inventory; PCL-C, Post-traumatic Stress Checklist, Civilian version; ANCOVA, analysis of covariance. Items in parentheses were included as covariates in the analyses.
Demographic variables used as covariates were as follows: time since injury, number of deployments, duration of loss of consciousness, and age.
Not surprisingly, an analysis of the individual NSI items across blast exposure groups (controlled for age, TSI, LOC, and number of deployments) also revealed a graded pattern of symptom reporting for a majority of the 22 items (Table 3) with main effects of blast group present for all but three items (i.e., numbness or tingling in parts of my body; Change in taste and/or smell; Feeling depressed or sad).
Table 3.
Individual Item Analysis of the Neurobehavioral Symptom Inventory across Blast Exposure Groups*
| Blast Group | 0 | 1 | 2 | 3 | 4 to 10 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NSI Item | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | η2 |
| 1. Feeling dizzy | 1.03 | 1.07 | 0.72 | 0.86 | 0.87 | 0.95 | 1.18 | 1.08 | 1.28 | 0.91 | <0.001 | 0.044 |
| 2. Loss of balance | 0.98 | 1.07 | 0.66 | 0.90 | 0.96 | 0.96 | 1.07 | 1.06 | 1.41 | 1.02 | <0.001 | 0.058 |
| 3. Poor coordination, clumsy | 0.92 | 0.95 | 0.75 | 0.91 | 0.92 | 0.92 | 1.21 | 1.07 | 1.41 | 1.07 | <0.001 | 0.064 |
| 4. Headaches | 2.22 | 1.23 | 1.69 | 1.20 | 1.71 | 1.28 | 2.02 | 1.23 | 2.21 | 1.26 | 0.001 | 0.035 |
| 5. Nausea | 0.73 | 0.85 | 0.49 | 0.82 | 0.66 | 0.94 | 0.84 | 1.13 | 1.10 | 1.02 | <0.001 | 0.043 |
| 6. Vision problems, blurring, trouble seeing | 0.83 | 1.02 | 0.58 | 0.84 | 0.97 | 1.12 | 1.14 | 1.17 | 1.20 | 1.21 | <0.001 | 0.042 |
| 7. Sensitivity to light | 1.25 | 1.20 | 0.85 | 1.08 | 1.02 | 1.12 | 1.36 | 1.31 | 1.57 | 1.20 | <0.001 | 0.049 |
| 8. Hearing difficulty | 1.10 | 0.99 | 1.48 | 1.16 | 1.34 | 1.19 | 1.53 | 1.17 | 1.84 | 1.08 | <0.001 | 0.038 |
| 9. Sensitivity to noise | 1.22 | 1.20 | 1.05 | 1.05 | 1.20 | 1.20 | 1.65 | 1.31 | 1.84 | 1.20 | <0.001 | 0.066 |
| 10. Numbness or tingling in parts of my body | 1.12 | 1.04 | 0.88 | 1.01 | 1.12 | 1.20 | 1.05 | 1.13 | 1.23 | 1.20 | 0.309 | 0.009 |
| 11. Change in taste and/or smell | 0.46 | 0.77 | 0.24 | 0.65 | 0.51 | 0.88 | 0.42 | 0.81 | 0.52 | 0.94 | 0.099 | 0.014 |
| 12. Increased or decreased appetite | 1.12 | 1.10 | 0.80 | 1.00 | 1.04 | 1.07 | 1.18 | 1.08 | 1.24 | 1.22 | 0.016 | 0.022 |
| 13. Poor concentration, cant pay attention | 1.75 | 1.21 | 1.48 | 1.18 | 1.55 | 1.31 | 1.98 | 1.18 | 2.15 | 1.15 | <0.001 | 0.054 |
| 14. Forgetfulness, cant remember things | 1.86 | 1.20 | 1.66 | 1.18 | 1.95 | 1.26 | 2.20 | 1.24 | 2.46 | 1.13 | <0.001 | 0.057 |
| 15. Difficulty making decisions | 1.19 | 1.12 | 0.92 | 1.12 | 1.05 | 1.19 | 1.36 | 1.23 | 1.57 | 1.24 | <0.001 | 0.044 |
| 16. Slowed thinking | 1.46 | 1.22 | 1.06 | 1.16 | 1.29 | 1.21 | 1.67 | 1.31 | 1.19 | 1.25 | <0.001 | 0.053 |
| 17. Fatigue, loss of energy | 1.46 | 1.19 | 1.06 | 1.13 | 1.29 | 1.20 | 1.67 | 1.27 | 1.82 | 1.22 | <0.001 | 0.048 |
| 18. Difficulty falling or staying asleep | 2.37 | 1.19 | 1.82 | 1.30 | 2.21 | 1.32 | 2.34 | 1.28 | 2.55 | 1.28 | <0.001 | 0.038 |
| 19. Feeling anxious or tense | 1.41 | 1.35 | 1.22 | 1.23 | 1.44 | 1.28 | 1.82 | 1.32 | 1.76 | 1.34 | <0.001 | 0.037 |
| 20. Feeling depressed or sad | 1.14 | 1.33 | 0.88 | 1.11 | 1.01 | 1.11 | 1.21 | 1.24 | 1.17 | 1.22 | 0.087 | 0.015 |
| 21. Easily annoyed/irritability | 1.81 | 1.35 | 1.68 | 1.19 | 1.72 | 1.29 | 2.09 | 1.34 | 2.05 | 1.26 | 0.005 | 0.027 |
| 22. Poor frustration tolerance | 1.53 | 1.37 | 1.23 | 1.24 | 1.36 | 1.31 | 1.69 | 1.34 | 1.70 | 1.37 | 0.005 | 0.027 |
Comparisons were controlled for age, time since injury, deployment number, and loss of consciousness.
NSI, Neurobehavioral Symptom Inventory; SD, standard deviation.
Because TSI was significantly different between blast groups and TSI relates to symptom endorsement,5,15,16 we dichotomized TSI using a median (TSI=155 days) split and tested whether there was an interaction between blast exposure group and TSI (TSI <155 days vs. TSI >155 days) group. While a main effect of both TSI group and blast group on NSI score was present, there was no interaction between the two groups. In addition, we compared the proportion of those in each blast group that were members of these dichotomized groups (TSI <155 days vs. TSI >155 days) using a chi-square analysis that yielded no differences.
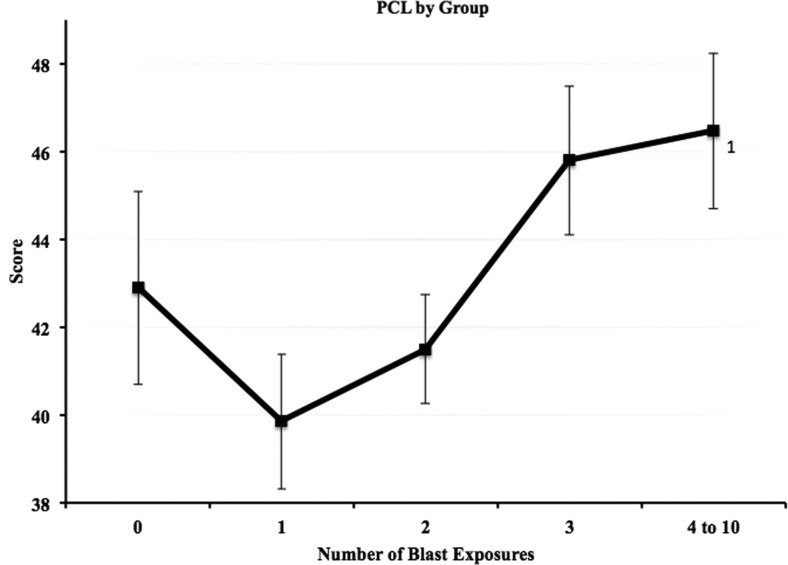
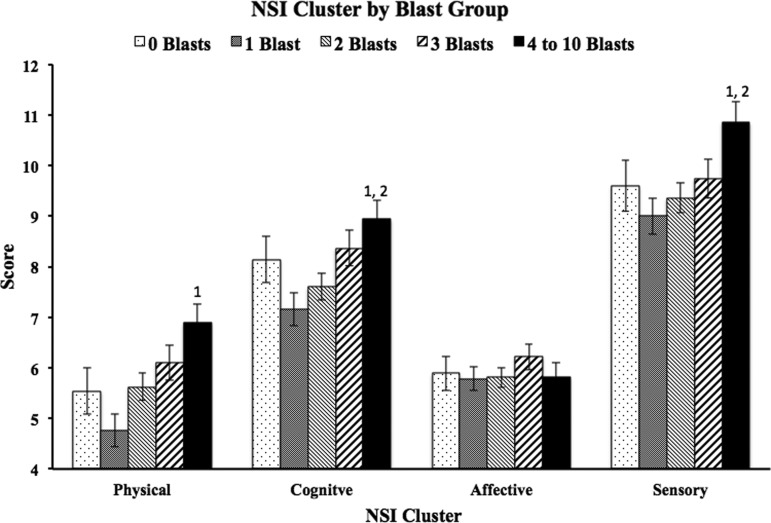
Analysis of PCL-C scores also revealed a main effect of blast group (F=3.1, p=0.016, Fig. 2). Therefore, the NSI ANCOVAs were run again adding total PCL-C score as an additional covariate. Significant differences remained on total NSI scores between the 4–10 blast group and the 1 blast and 2 blast groups (p=0.001, d=0.35 and p=0.020, d=0.24, respectively). Additional analyses of NSI subscales while controlling for PCL-C scores as well as the other covariates revealed a main effect of blast group in three of the four clusters (cognitive, F=3.98, p=0.003; sensory, F=3.22, p=0.013; somatic, F=4.99, p=0.001; affective, F=0.516, p=0.724). Bonferroni-corrected post hoc analyses of the subscales (Fig. 3) revealed significant differences in the cognitive cluster between the 4–10 blast group (M=8.94) and the 1 blast (M=7.16) and 2 blast (M=7.61) groups (p=0.004, d=0.36 and p=0.039, d=0.26, respectively); in the sensory cluster between the 4–10 blast group (M=10.85) and the 1 blast (M=9.0) and 2 blast (M=9.36) groups (p=0.007, d=0.36 and p=0.032, d=0.27, respectively); in the somatic cluster between the 4–10 blast group (M=6.89) and the 1 blast group (M=4.76, p<0.001, d=0.48). Affective symptoms did not differ as a function of blast exposure.
FIG. 2.
Raw Post-traumatic Checklist-Civilian version (PCL-C) total mean score for each blast exposure group. Error bars reflect standard error and values adjacent to group mean markers represent blast exposure groups for which significant differences were present (analysis of variance).
FIG. 3.
Raw Neurobehavioral Symptom Inventory (NSI) subscale mean score for each blast exposure group. Error bars reflect standard error and values above bars represent blast exposure groups for which significant differences were present when using demographic variables and Post-traumatic Checklist-Civilian version scores as covariates.
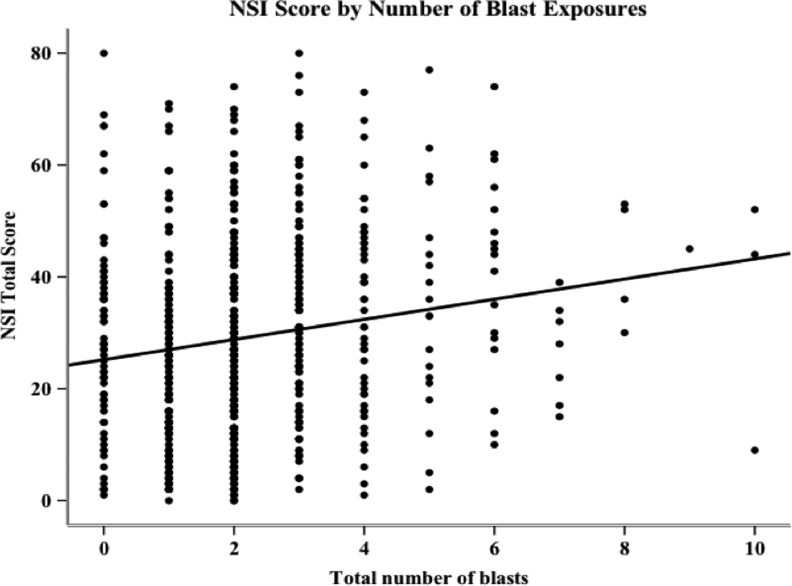
A series of regression analyses were performed on NSI and PCL-C total scores to determine the amount of variance in symptom reporting accounted for by number of blast exposures (Tables 4 and 5). A simple regression revealed that number of blast exposures accounted for 3.0% (p<0.001) of the variance in NSI scores (Fig. 4). After controlling for demographic variables by entering them first in a hierarchical regression analysis, number of blast exposures accounted for 4.8% (p=0.009) of the variance in NSI scores. An increase of one blast exposure resulted in an average total NSI score increase of 2.3 points. When both PCL-C score and demographics were entered before blast exposure in the regression, number of blasts accounted for only 0.09% (p<0.001) of the variance in NSI symptom endorsement.
Table 4.
Summary of Hierarchical Regression Analyses: Percent of Post-Traumatic Stress Checklist, Civilian Version Variance Accounted for by Number of Blasts before and after Accounting for Demographics and Neurobehavioral Symptom Inventory Score
| Regression 1 Step 1: Blast | Regression 2 Step 1: Demographics Step 2: Blasts | Regression 3 Step 1: NSI Step 2: Demographics Step 3: Blasts | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | R2 | p | β | R2 (or Δ) | p (or Δ) | β | R2 (or Δ) | p (or Δ) | β |
| NSI | 0.666 | <0.001 | 0.797 | ||||||
| Demographics | 0.043 | 0.001 | 0.010 | 0.014 | |||||
| Blasts | 0.010 | 0.017 | 1.012 | 0.023 | <0.001 | 1.58 | 0.001 | 0.277 | 0.283 |
Demographics consisted of age, time since injury, duration of loss of consciousness and number of deployments. Demographic predictors were entered simultaneously in regressions two and three.
NSI, Neurobehavioral Symptom Inventory.
Table 5.
Summary of Hierarchical Regression Analyses: Percent of Neurobehavioral Symptom Inventory Variance Accounted for by Number of Blasts before and after Accounting for Demographics and Post-Traumatic Stress Checklist, Civilian Version Score
| Regression 1 Step 1: Blast | Regression 2 Step 1: Demographics Step 2: Blasts | Regression 3 Step 1: PCL-C Step 2: Demographics Step 3: Blasts | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | R2 | p | β | R2 (or Δ) | p (or Δ) | β | R2 (or Δ) | p (or Δ) | β |
| PCL-C | 0.666 | <0.001 | 0.836 | ||||||
| Demographics | 0.046 | <0.001 | 0.011 | 0.006 | |||||
| Blasts | 0.030 | <0.001 | 1.802 | 0.048 | <0.001 | 2.323 | 0.009 | <0.001 | 1.035 |
deployments. Demographic predictors were entered simultaneously in regressions two and three.
PCL-C, Post-traumatic Stress Checklist, Civilian version.
FIG. 4.
Scatterplot of Neurobehavioral Symptom Inventory (NSI) total scores and number of blast exposures, including index event, with regression line (number of blast exposures is the sole predictor; R2=0.03, β=1.8, see Table 3).
The same series of analyses on PCL-C scores revealed a similar pattern of results. Number of blast exposures alone accounted for 1.0% (p=0.017) of the variance in PCL-C scores. When demographic variables were entered first, number of blasts accounted for 2.3% (p<0.001) of the variance in PCL-C scores. An increase of one blast exposure resulted in an average total PCL-C score increase of 1.6 points. When both NSI score and demographics were entered before blast exposure, number of blasts accounted for only 0.01% (p=0.277) of the variance in PCL-C symptom endorsement.
Discussion
This study explored the association between cumulative blast exposures and neurobehavioral symptom reporting in the context of comorbid PTSD symptomatology. Without accounting for PTSD symptom reporting, a graded increase in NSI reporting was found as number of blast exposures increased. Service members that reported being exposed to more blasts endorsed a significantly greater number of symptoms across the NSI and its four subscales. A similarly graded pattern of PTSD symptom endorsement was also present. When controlling for PTSD symptom reporting, these significant findings remained present with the exception of the affective subscale. These results indicate that a dose effect of blast exposures may exist in terms of neurobehavioral symptom reporting. A recent study by Kontos and associates17 found a similarly graded pattern of post-concussion symptom endorsement after cumulative blast-related MTBIs with those experiencing more MTBIs endorsing more post-concussion symptoms. While they report on multiple blast-related MTBIs and the present study explored single episodes of MTBI with or without accompanying previous blast exposures, a similar pattern of increased symptom reporting is evident across our two studies. These similar findings support the notion that repeated blast exposure increases symptom endorsement in a graded fashion.
No interaction of NSI endorsement was detected between TSI and blast exposure. In addition, the proportion of persons belonging to a high or low TSI group within each blast group was not significantly different statistically; therefore, we propose that blast exposure is positively associated with symptom endorsement independent of TSI. It would be interesting, however, to explore how each individual item comprising the NSI varies as a function of TSI. Such analyses may better inform programmatic rehabilitative planning, and although further individual item by TSI analysis is an important and interesting topic, it is beyond the scope of this article and left for future research.
The issue of comorbid PTSD and MTBI is not easily addressed using the current study methods. Nonetheless, we attempted to demonstrate the range of variance in post-concussion and post-traumatic symptom reporting that is attributable to blast exposure before and after taking PTSD or post-concussion symptomatology and demographic variables into account (Tables 4 and 5). These analyses show blast exposure accounts for 4.8% of the variance in post-concussion symptom reporting after accounting for demographic variables only and 0.09% of the variance in post-concussion symptom reporting after accounting for both PCL-C score and demographic variables.
Blast exposure likewise accounts for 2.3% (p<0.001) of the variance in PCL-C score after accounting for demographics and 0.01% (p=0.277) of the variance after accounting for NSI score as well. Because the correlation between NSI and PCL-C scores in our sample is 0.81 (Pearson R), it is not surprising that accounting for PCL-C or NSI (depending on the dependent variable in the regression) reduces the variance explained by blast exposure considerably. These analyses reflect the significant degree to which multiple blast exposures contribute to both PTSD and post-concussion symptom endorsement. These analyses also reflect the high degree of PCL-C and NSI symptom endorsement overlap that exists in this population and underscores the difficulty of trying to isolate one from the other.
Much research supports the notion that post-concussion and stress-related symptom endorsement overlap in the context of MTBI. It has been reported that up to 43.9% of service members who present with either MTBI or PTSD3,18 endorse both combat stress-related and post-concussion symptoms, making it difficult to assign causality to either—i.e., post-concussion or combat-stress related. PTSD symptom reporting increases in service members sustaining an MTBI,3,19 and post-concussion symptom endorsement increases in service members with ongoing PTSD.2,20,21 There is a growing clinical movement to address the commonalities between these maladies22–24 rather than trying to separate the two phenomena into their component parts.
As such, the “burden of adversity” hypothesis,22 based on cumulative disadvantage,25 is well suited to account for the increase in symptom reporting across both phenomena. This hypothesis proposes that treatment of short-term or acute symptoms be performed independent of primary diagnosis in an effort to reduce the physical/psychological/emotional burden of the patient. In addition, this theory takes into account the events leading up to a diagnosis. In the context of blast-related MTBI, service members experience highly intense environments in which the possibility of injury and/or death of one's self and/or one's colleagues is often present before exposure to a blast event occurs. These environments lend themselves to potentially traumatic experiences even if physical injury is not sustained. As such, on injury, these service members become the bearers of “global” distress, not simply psychological, emotional, physical, etc., but a conglomeration of symptoms. In the present context, this may drive the similarity in symptom reporting across these two measures (NSI and PCL-C). While MTBI and PTSD are distinct entities their etiologies are not mutually exclusive.
The current study is one of the largest to date to explore the association between multiple blast exposures and post-concussion symptom reporting in service members with MTBI. In addition, the large sample of recently deployed service members from six regionally distinct national military medical centers makes this study sample extremely representative of its intended population. Despite these strengths, we acknowledge the current study has several limitations that warrant comment. First, and most importantly, the dependent and independent variables in this study are self-reported, which may affect the validity of our findings. Because the NSI and PCL-C have no formal embedded validity scale (although embedded validity has been explored in the NSI),26 it is possible that a significant portion of our data reflects over- or underreporting. In addition, while the number of blast exposures a service member reported to have experienced was gathered through semi-structured clinical interview, the possibility of inaccurate recollection on the part of the service member is still a possibility. Second, the structured interview through which information about blast exposure and MTBI was acquired did not probe for accounts of MTBI (i.e., concussion) before enlistment in the military. Therefore, it is possible some of these service members had sustained other remote MTBI at the time they volunteered to participate in this study.
Third is the retrospective nature of the data collection. Because of this methodology, it is impossible to explore factors that may have contributed to the findings, such as compensation seeking status, or motivation to return to duty, either of which can potentially mediate symptom reporting. Finally, we propose a hypothesis based on “global” distress comprised of multiple etiologies, yet have no measure of psychological or physical distress or combat exposure intensity in our sample aside from the dependent variables. Future prospective studies should include independent measures of global distress and combat exposure intensity to further examine this hypothesis.
While there is much room for improvement with regard to similar studies in the future, the present study demonstrates a probable dose dependent increase in MTBI and PTSD symptom endorsement because of retrospectively recalled blast exposure that may be clinically useful for inferring symptom severity and required level of care for service members who have been exposed to varying numbers of explosive blasts before sustaining MTBI. Even in the absence of objective confirmation of their report, those service members who report more exposure before the index injury may be at risk for increased symptom burden.
Acknowledgments
Portions of these data were presented at the International Brain Injury Association annual conference, March 2014, San Francisco, CA.
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Defense, the Department of Veterans' Affairs, or the U.S. Government.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.DVBIC (2014). DoD worldwide numbers for TBI. Defense Medical Surveillance System. Available at: http://dvbic.dcoe.mil/dod-worldwide-numbers-tbi Accessed October6, 2014
- 2.Schneiderman A.I., Braver E.R., and Kang H.K. (2008). Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am. J. Epidemiol. 167, 1446–1452 [DOI] [PubMed] [Google Scholar]
- 3.Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 [DOI] [PubMed] [Google Scholar]
- 4.Pietrzak R.H., Johnson D.C., Goldstein M.B., Malley J.C., and Southwick S.M. (2009). Posttraumatic stress disorder mediates the relationship between mild traumatic brain injury and health and psychosocial functioning in veterans of Operations Enduring Freedom and Iraqi Freedom. J. Nerv. Ment. Dis. 197, 748–753 [DOI] [PubMed] [Google Scholar]
- 5.Terrio H., Brenner L.A., Ivins B.J., Cho J.M., Helmick K., Schwab K., Scally K., Bretthauer R., and Warden D. (2009). Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J. Head Trauma Rehabil. 24, 14–23 [DOI] [PubMed] [Google Scholar]
- 6.Anonymous (2013). External causes of traumatic brain injury, 2000–2011. MSMR 20, 9–14 [PubMed] [Google Scholar]
- 7.Rigg J.L., and Mooney S.R. (2011). Concussions and the military: issues specific to service members. PM R 3, Suppl 2, S380–S386 [DOI] [PubMed] [Google Scholar]
- 8.Miller K.J., Ivins B.J., and Schwab K.A. (2013). Self-reported mild TBI and postconcussive symptoms in a peacetime active duty military population: effect of multiple TBI history versus single mild TBI. J Head Trauma Rehabil 28, 31–38 [DOI] [PubMed] [Google Scholar]
- 9.MacGregor A.J., Dougherty A.L., Morrison R.H., Quinn K.H., and Galarneau M.R. (2011). Repeated concussion among U.S. military personnel during Operation Iraqi Freedom. J. Rehabil. Res. Dev. 48, 1269–1278 [DOI] [PubMed] [Google Scholar]
- 10.Vanderploeg R.D., Belanger H.G., Horner R.D., Spehar A.M., Powell-Cope G., Luther S.L., and Scott S.G. (2012). Health outcomes associated with military deployment: mild traumatic brain injury, blast, trauma, and combat associations in the Florida National Guard. Arch. Phys.Med. Rehabil. 93, 1887–1895 [DOI] [PubMed] [Google Scholar]
- 11.Elder G.A., Mitsis E.M., Ahlers S.T., and Cristian A. (2010). Blast-induced mild traumatic brain injury. Psychiatr Clin. North Am. 33, 757–781 [DOI] [PubMed] [Google Scholar]
- 12.Taber K.H., Hurley R.A., Haswell C.C., Rowland J.A., Hurt S.D., Lamar C.D., and Morey R.A. (2014). White matter compromise in veterans exposed to primary blast forces. J. Head Trauma Rehabil. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicerone K.D., and Kalmar K. (1995). Persistent postconcussion syndrome: The structure of subjective complaints after mild traumatic brain injury. J. Head Trauma Rehabil. 10, 1–17 [Google Scholar]
- 14.Blanchard E.B., Jones-Alexander J., Buckley T.C., and Forneris C.A. (1996). Psychometric properties of the PTSD Checklist (PCL). Behav. Res. Ther. 34, 669–673 [DOI] [PubMed] [Google Scholar]
- 15.Drag L.L., Spencer R.J., Walker S.J., Pangilinan P.H., and Bieliauskas L.A. (2012). The contributions of self-reported injury characteristics and psychiatric symptoms to cognitive functioning in OEF/OIF Veterans with mild traumatic brain injury. J. Int. Neuropsychol. Soc. 18, 576–584 [DOI] [PubMed] [Google Scholar]
- 16.Dolan S., Martindale S., Robinson J., Kimbrel N., Meyer E., Kruse M., Morissette S., Young K., and Gulliver S. (2012). Neuropsychological sequelae of PTSD and TBI following war deployment among OEF/OIF veterans. Neuropsychol. Rev. 22, 21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kontos A.P., Kotwal R.S., Elbin R.J., Lutz R.H., Forsten R.D., Benson P.J., and Guskiewicz K.M. (2013). Residual effects of combat-related mild traumatic brain injury. J. Neurotrauma 30, 680–686 [DOI] [PubMed] [Google Scholar]
- 18.Lew H.L., Vanderploeg R.D., Moore D.F., Schwab K., Friedman L., Yesavage J., Keane T.M., Warden D.L., and Sigford B.J. (2008). Overlap of mild TBI and mental health conditions in returning OIF/OEF service members and veterans. J. Rehabil. Res. Dev. 45, xi–xvi [PubMed] [Google Scholar]
- 19.Kennedy J.E., Leal F.O., Lewis J.D., Cullen M.A., and Amador R.R. (2010). Posttraumatic stress symptoms in OIF/OEF service members with blast-related and non-blast-related mild TBI. NeuroRehabilitation 26, 223–231 [DOI] [PubMed] [Google Scholar]
- 20.Hoge C.W., Terhakopian A., Castro C.A., Messer S.C., and Engel C.C. (2007). Association of posttraumatic stress disorder with somatic symptoms, health care visits, and absenteeism among Iraq war veterans. Am. J. Psychiatry 164, 150–153 [DOI] [PubMed] [Google Scholar]
- 21.Vanderploeg R.D., Belanger H.G., and Curtiss G. (2009). Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Arch.Phys. Med. Rehabil. 90, 1084–1093 [DOI] [PubMed] [Google Scholar]
- 22.Brenner L.A., Vanderploeg R.D., and Terrio H. (2009). Assessment and diagnosis of mild traumatic brain injury, posttraumatic stress disorder, and other polytrauma conditions: burden of adversity hypothesis. Rehabil Psychol 54, 239–246 [DOI] [PubMed] [Google Scholar]
- 23.King N.S. (2008). PTSD and traumatic brain injury: folklore and fact? Brain Inj. 22, 1–5 [DOI] [PubMed] [Google Scholar]
- 24.Brenner L.A., Ivins B.J., Schwab K., Warden D., Nelson L.A., Jaffee M., and Terrio H. (2010). Traumatic brain injury, posttraumatic stress disorder, and postconcussive symptom reporting among troops returning from Iraq. J. Head Trauma Rehabil. 25, 307–312 [DOI] [PubMed] [Google Scholar]
- 25.Merton R.K. (1968). The Matthew effect in science. The reward and communication systems of science are considered. Science 159, 56–63 [PubMed] [Google Scholar]
- 26.Vanderploeg R.D., Cooper D.B., Belanger H.G., Donnell A.J., Kennedy J.E., Hopewell C.A., and Scott S.G. (2014). Screening for postdeployment conditions: development and cross-validation of an embedded validity scale in the neurobehavioral symptom inventory. J. Head Trauma Rehabil. 29, 1–10 [DOI] [PubMed] [Google Scholar]