Abstract
The pain-signaling molecules, nitric oxide synthase (NOS) and calcitonin gene-related peptide (CGRP), are implicated in the pathophysiology of post-traumatic headache (PTH) as they are for migraine. This study assessed the changes of inducible NOS (iNOS) and its cellular source in the trigeminal pain circuit, as well as the relationship between iNOS and CGRP after controlled cortical impact (CCI) injury in mice. The effects of a CGRP antagonist (MK8825) and sumatriptan on iNOS messenger RNA (mRNA) and protein were compared to vehicle at 2 weeks postinjury. Changes in CGRP levels in the trigeminal nucleus caudalis (TNC) in iNOS knockouts with CCI were compared to wild-type (WT) mice at 3 days and 2 weeks post injury. Trigeminal allodynia and photosensitivity were measured. MK8825 and sumatriptan increased allodynic thresholds in CCI groups compared to vehicle (p < 0.01), whereas iNOS knockouts were not different from WT. Photosensitivity was attenuated in MK8825 mice and iNOS knockouts compared to WT (p < 0.05). MK8825 and sumatriptan reduced levels of iNOS mRNA and iNOS immunoreactivity in the TNC and ganglia (p < 0.01). Differences in iNOS cellular localization were found between the trigeminal ganglia and TNC. Although the knockout of iNOS attenuated CGRP at 3 days (p < 0.05), it did not reduce CGRP at 2 weeks. CGRP immunoreactivity was found in the meningeal layers post-CCI, while negligible in controls. Findings support the importance of interactions between CGRP and iNOS in mediating allodynia, as well as the individual roles in photosensitivity. Mitigating prolonged increases in CGRP may be a promising intervention for treating acute PTH.
Key words: : pain, post-traumatic headache, post-traumatic migraine, traumatic brain injury, trigeminovascular system
Introduction
Headache after traumatic brain injury (TBI) is highly prevalent and the most common and persistent symptom of postconcussion syndrome.1 The majority of post-traumatic headache (PTH) will resolve within a couple of weeks postinjury; however, in a subset of patients, headache becomes a chronic disorder contributing to a prolonged recovery from injury, poor quality of life, and disability. PTH commonly shares features of either migraine- or tension-type headache.2 Migraine is the predominant headache phenotype in patients experiencing frequent headaches after mild traumatic brain injury (TBI), as well as among military service members with concussion at prevalence rates ranging from 49% to 89%.1,3,4 Diagnostic criteria for migraine includes sensitivity to light and sound (photo- and phonophobia), nausea/vomiting, a unilateral headache, pulsate quality, moderate-to-severe intensity, and headache aggravated by routine physical activity.2 Mechanical allodynia (cutaneous hypersensitivity to a mechanical stimulus that is otherwise innocuous under normal conditions) is a pain response common in patients with migraine and has also been reported in patients with PTH.5–7 Trigeminal allodynia and photophobia have been studied in animal models of TBI and migraine.7–10
Many of the postulated biological mechanisms of PTH may be drawn from research on primary headache disorders, such as migraine. Migraine is generally regarded as a neurovascular disorder, derived, in part, from the former vascular hypothesis.11–13 Controversy surrounding the vascular theory of migraine continues because of imaging studies showing conflicting results for the meningeal vasculature during migraine attacks.13 Activation of the trigeminovascular system during a migraine attack involves a phenomenon known as sensitization (peripheral and central).14 In PTH, it is hypothesized that the persistent inflammation post-TBI sensitizes trigeminal pain neurons.8,9,15,16 The precise mechanisms of inflammatory-induced sensitization of the trigeminal system post-TBI remains poorly understood.
Key pain-signaling molecules, calcitonin gene-related peptide (CGRP) and nitric oxide synthase (NOS), play a role in the development of PTH pathophysiology as they do for migraine.8,17–19 CGRP and nitric oxide (NO)/NOS are proposed to have reciprocal feedback mechanisms in the trigeminovascular system.20–23 NO and CGRP activate the trigeminovascular system and trigger headache in humans and animal models of migraine.24–28 NO is converted from l-arginine by different isoforms of the NOS enzymes; the two constitutive NOS isoforms include endothelial (eNOS) and neuronal (nNOS), whereas there is one inducible NOS isoform, iNOS. Of note, a role of iNOS is well demonstrated in inflammatory pain pathogenesis, whereas it is not supported in migraine23,29,30; hence, of the NOS isoforms responsible for NO production, the inducible isoform has been investigated initially for PTH because inflammation is a predominant feature of TBI. The aims of this research were to determine whether iNOS gene and protein expression were altered in the trigeminal pathway in a murine model of controlled cortical injury (CCI) along with the cellular source and to examine the relationship between CGRP and iNOS. Two agents known to block the actions of CGRP (sumatriptan and a CGRP antagonist, MK8825) and genetic knockout of iNOS were used to investigate pain neurochemicals in the trigeminal pathway and behaviors indicative of headache (trigeminal allodynia and photosensitivity) in mice with cortical injury.
Methods
Experimental design
All research involving use of male C57BL/6 mice (Charles River Laboratories Inc., Wilmington, MA) and C57BL/6Ai-[KO] Inos N12 (iNOS KO) mice (Taconic, Germantown, NY)31 at approximately 8 weeks of age was approved and monitored by the Thomas Jefferson University Institutional Animal Care and Use Committee to assure compliance with the provisions of federal regulations and the National Institute of Health's ”Guide for the Care and Use of Laboratory Animals.” All animals were housed under a 12-hour light/dark cycle in the Thomas Jefferson University (Philadelphia, PA), Laboratory Animal Services Facility (Association for Assessment and Accreditation of Laboratory Animal Care accredited). All animals underwent a 1-week period of acclimation after arriving at Thomas Jefferson University animal facility before handling.
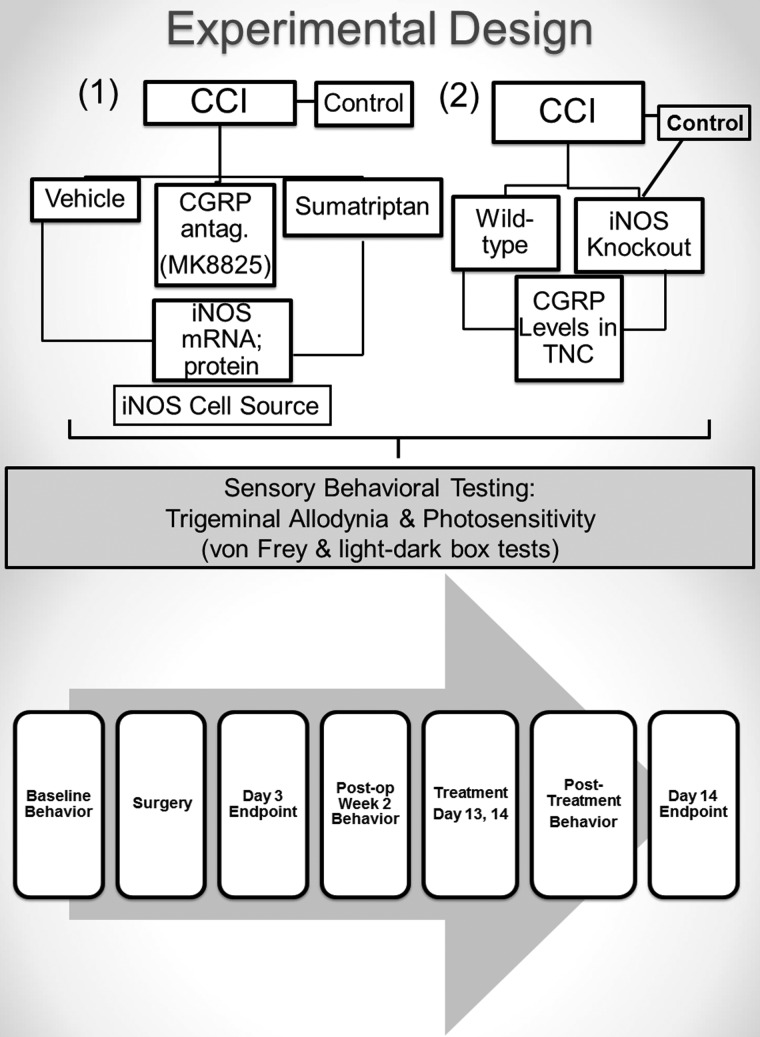
Sixty wild-type (WT) male mice were randomly assigned to CCI injury (n = 44) or incision-only controls (n = 16) groups for a 3-day or 2-week study endpoint (Fig. 1). There were two experimental arms for the CCI groups: 1) pharmacological modulation and 2) genetic modulation. In the pharmacological modulation study arm, CCI groups were randomly assigned to receive vehicle (n = 20), sumatriptan (n = 12), or MK8825 (n = 12). In the genetic modulation study arm, CCI or incision control procedures were performed in iNOS KO mice (n = 11) and compared to WT CCI mice (n = 14) at either a 3- or 14-day endpoint. Naïve WT and iNOS KO were also included as controls (n = 4). The incision control group received a skin incision without craniotomy for CCI because craniotomy results in transient cutaneous and dural hypersensitivity, neuroinflammation, and increases in CGRP, indicating a mild tissue injury.8,32,33 In order to determine whether a treatment returns an outcome measure to “normal levels,” an incision control group was necessary for this study. All groups underwent behavioral testing and tissue assessments for respective outcomes, as described below. To test the effects of CGRP on iNOS, iNOS messenger RNA (mRNA) and protein synthesis were assessed for the pharmacologic arm because MK8825 and sumatriptan inhibit the actions of CGRP. To test the effects of iNOS on CGRP, CGRP levels were assessed in iNOS knockouts and WT with CCI.
FIG. 1.
Experimental design diagram shows group assignment, treatment, and outcomes along with the experimental paradigm, including endpoints. There were two experimental arms for the CCI groups: 1) pharmacological modulation and 2) genetic modulation. In the pharmacological modulation study arm, CCI groups were randomly assigned to receive vehicle, sumatriptan, or MK8825. In the genetic modulation study arm, CCI or incision control procedures were performed in iNOS KO mice and compared to wild-type CCI mice with either a 3- or 14-day endpoint. Naïve wild-type and iNOS KO were also included as controls. All groups underwent behavioral testing and tissue assessments for respective outcomes. To test the effects of CGRP on iNOS, iNOS mRNA and protein synthesis were assessed for the pharmacologic arm because MK8825 and sumatriptan inhibit the actions of CGRP. To test the effects of iNOS on CGRP, CGRP levels were assessed in iNOS knockouts and wild-type with CCI. CCI, controlled cortical injury; CGRP, calcitonin gene-related peptide; iNOS, inducible nitric oxide synthase; KO, knockout; mRNA, messenger RNA; TNC, trigeminal nucleus caudalis.
All behavioral tests and euthanasia occurred between the hours of 9 am and 12 pm. Animals were allowed to recover from anesthesia and kept in separate cages postoperatively with unrestricted food and water. Animals were euthanized with isoflurane overdose for postmortem analysis and cervical dislocation
Surgical procedure
Traumatic brain injury (TBI) was induced in mice using a mild CCI injury model as described previously by our laboratory.8,9,34–36 Animals were anesthetized with isoflurane (3% induction; 2–2.5% maintenance), and body temperature was maintained at 37+ 0.5°C. A right-sided, 4-mm craniotomy at 1.0 mm posterior to bregma exposing the right somatosensory cortex was performed. CCI was induced using an electromagnetic stereotaxic impactor (Leica Biosystems Richmond, formerly MyNeuroLab, Richmond, IL) at 1.0-mm depth and 3.0-m/sec velocity (100-ms contact time) at an impact angle perpendicular to the cortical surface. Duration of surgery was approximately 20 ± 5 min plus 5 min for induction. To control for the effects of anesthesia, incision controls were kept under anesthesia for approximately the same duration (20 min) as experimental animals. Postinjury, the bone flap was replaced, sealed with permanent cyanoacrylate-based fast-acting adhesive, and the skin sutured. A single dose of short-acting buprenorphine (0.05 mg/kg; subcutaneous injection) was administered before removal from isoflurane to facilitate postoperative recovery.
Treatments
WT mice were treated with a 100-μL intraperioteneal injection of either sumatriptan (1 mg/kg; Sigma-Aldrich, St. Louis, MO), a CGRP antagonist (MK8825; 100 mg/kg in 0.9% NaCl; Merck and Co. Inc., Summit, NJ), or vehicle (0.9% NaCl; Ricca Chemical Company, Arlington, TX). MK8825 is a rat-specific CGRP receptor antagonist with suitable potency, selectivity, and pharmacokinetics to clarify the in vivo effect of the CGRP receptor.37 Sumatriptan is a migraine therapeutic, a serotonin (5-HT1) agonist, and reduces the release of CGRP from trigeminal ganglia neurons.38,39 Doses for MK882537 and sumatriptan28,40 were selected based on the literature and pilot studies in our laboratory. Treatments were administered on 2 consecutive days in postoperative week 2, on days 13 and 14 before euthanasia. The 2-week delayed time point for treatment administrations was selected to allow local tissue ischemia to resolve considering the potential for cerebral vasoconstriction elicited by sumatriptan and a CGRP antagonist. Treatment administration at week 2 post-injury was also selected for clinically relevance given time to see the headache symptomology persist beyond the first week of injury, and in consideration of the fact that there may be a narrow therapeutic window to prevent the chronification of PTH. On the day of euthanasia, a final treatment dose of either vehicle, or the experimental drug, was given at 1 h before behavior testing and approximately 2 h before euthanasia procedures for either enzyme-linked immunosorbent assay/quantitative reverse-transcriptase polymerase chain reaction (ELISA/qRT-PCR) or immunohistochemistry (IHC).
von Frey mechanical allodynia testing
Cutaneous sensory testing with von Frey monofilaments were used to measure allodynia—a painful response characterized by cutaneous hypersensitivity to a mechanical stimulus that is otherwise innocuous under normal conditions. von Frey sensory testing is a validated method to test for allodynia in humans and rodents to evaluate sensory changes after central nervous system injury.41 The periorbital region was tested in this study because this region is innervated by the ophthalmic (V1) division of the trigeminal nerve and trigeminal allodynia is implicated in headache disorders. Periorbital allodynia was correlated with persistent increases in CGRP post-CCI in mice8,9 and has been a useful outcome reported for patients with PTH and migraine.5–7
A trained technician performed von Frey (North Coast Medical Inc., Gilroy, CA) testing blinded to experimental conditions. Testing was performed during the morning daylight portion of their circadian cycle (9 am to 12 pm), as described previously.8,9 A universal plastic tube rodent restraint was used for periorbital testing in which mice entered in a gentle guided, unforced manner; there was a 5-min acclimation period to the test chamber. Animals that show signs of excessive stress, such as prolonged (>5-min acclimation period) biting or clawing at restraint, vocalization, or baseline allodynia were to be omitted from periorbital testing. Note to readers: When using restraint to test mice, handling before testing is of utmost importance to prevent meeting the exclusion criteria. Periorbital thresholds (grams) were determined by applying the von Frey monofilaments over the rostral portion of the eyes distal from the suture site for five stimulations bilaterally. The smallest force that elicited a limb withdrawal was considered the threshold force. The filament must make firm perpendicular contact with the skin causing the filament to bend, thereby producing a precise bending force as calibrated by the manufacturer. A positive response for testing was characterized by the following: vigorous forepaw strokes of face; head withdrawal from the stimulus; or head shaking. Force thresholds were defined as greater than a 60% (3 of 5) response frequency for a von Frey stimulus.
The normal baseline thresholds for mice determined from our previous studies include 0.07 and 0.16.9 von Frey forces that induce a positive response in the ophthalmic trigeminal zone in naïve mice are typically lower (<1.0 g), compared to that of rats (<10 g).8,9 Allodynia was defined as a reduction in threshold below baseline ≤0.04 g because mice normally do not respond at this stimulation force. The goal of treatments in this study was to alleviate allodynia as shown by an increase in threshold. In general, for a change in threshold to be considered significant, there should be a change in threshold of at least two steps in filament grade; for example, moving from 0.02 to 0.04 g (one filament) would not qualify as a significant change, whereas moving from 0.02 to 0.07 g would be considered significant.9
Photosensitivity testing
Testing for photosensitivity was adapted from methods by Russo and colleagues in which aversion to bright light has been shown in mice overexpressing CGRP receptors.10,42 Mice were acclimated for at least 1 h in their cages with the lights on (∼400 lux inside the cage). The light-dark test apparatus consists of an open-topped rectangular plexiglass box divided into a small (27 × 28 × 19 cm) and a large (45 × 29 × 30 cm) compartment with an entryway/opening (8 × 8 cm) located in between the two compartments. The small compartment is black, whereas the large compartment is white plexiglass illuminated with either ambient ceiling lighting at approximately 400 lux, or with an additional bright light of approximately 4000 lux measured using a Digital Light Level Meter LX1010B Illuminance Meter (SF Cable, Inc., Hayward, CA). Average light intensity for normal office spaces is estimated to be 500 lux, whereas full daylight to sunlight conditions can range from 1000 to 10,000 lux or greater.
Each test session begins with the mice placed in the center of the light compartment facing away from the opening and allowed to explore for 5 min. Mice are tracked and behaviors, including time spent in the light compartment, the number of transitions between the light and dark compartment, and the number of rears, were recorded and analyzed using the ANY-maze™ software (Stoelting Co., Wood Dale, IL). In separate experiments, an ambient light trial was included to distinguish between photosensitivity and anxiety behaviors post-TBI. Two testing sessions of an ambient light and a subsequent bright light session were separated by a 15- to 20-min break in between sessions. Mice have stereotyped behavior of spending more time in the dark compared to light, which becomes more pronounced when anxious based on the concept that rodents find novel environments and large, open spaces stressful or anxiety provoking. The ambient light test was included as the first session of the test to determine the presence of anxiety. The bright light test was conducted second, once mice had acclimated under ambient conditions, reducing novelty and making the chamber less stressful.
Enzyme-linked immunoassay for calcitonin gene-related peptide
After mice were euthanized with overdose of isoflurane and cervical dislocation, fresh frozen specimens of brainstem tissues were rapidly created in liquid nitrogen and stored at −80°C. Brainstems were sectioned according to the mouse brain atlas by Frankin and Paxinos (−5.8 to −8.2 mm bregma) to include the spinal trigeminal nucleus (i.e., trigeminal nucleus caudalis; TNC). All brainstem samples were homogenized and unpooled with ethylenediaminetetraacetic acid–free complete protease inhibitor cocktail tablets using 50 μL/10 mg of tissue (Roche Diagnostics GmbH, Mannheim, Germany). Homogenates were centrifuged at 14,000 rpm for 15 min at 4°C. Total protein content for each sample was determined using BCA-200 protein assays (bicinchoninic acid [BCA]; Pierce, Rockford, IL). Tissue lysates were analyzed for CGRP according to the manufacturer's protocol (CGRP Elisa Kit, catalog no.: 589001; Cayman Chemicals, Ann Arbor, MI). Each sample was run in duplicate and data (pg CGRP protein) was normalized to milligrams total protein, then reported as fold change above control levels. MK8825 was not included in the CGRP ELISA as treatment with MK8825 because it was not expected to reduce the CGRP levels in the TNC; the CGRP antagonist mechanism of action works by blocking the CGRP receptors and this was confirmed by our lab with pilot data.
Quantitative reverse-transcriptase polymerase chain reaction
The pellets from ELISA samples were used for RNA isolation. Total RNA was extracted with the RNeasy Miniprep Kit (Qiagen, Valencia, CA), reverse transcribed into complementary DNA (cDNA) with iScript reverse transcriptase (Bio-Rad, Hercules, CA), and then measured by qRT-PCR with gene-specific primers and probes.88 The sequences of the primers were: L13 forward, 5′-AGATTGGCCGGACTCCCTAC-3′; L13 reverse, 5′-AGTATCATGCCATTCCGGCT-3′; iNOS forward, 5′-AACCCAAGGTCTACGTTCAGG-3′; iNOS reverse, 5′-TGGCCACCAGCTTCTTCAAT-3′. All primers were run using an annealing temperature of 59°C. SsoAdvanced Universal SYBR Green Supermix and the C1000 Thermal Cycler real-time detection system were also used for quantification (Bio-Rad). Thermal cycle conditions were: 3 min at 95°C; 40 cycles (15 sec at 95°C, 30 sec at 59°C, and 30 sec at 72°C); and a melt curve from 55 to 95°C at 0.5°C increments. All samples were run in triplicate and compared to cDNA gene standards to determine copy numbers, which were normalized to the copy number of each sample's housekeeping gene (L13).
Immunohistochemical analysis and cell counting
Isoflurane euthanized mice were treated with heparinized saline followed by 4% paraformaldehyde (PFA) treatment. Brains and trigeminal ganglia were postfixed in 4% PFA for 24 h then transferred to 30% sucrose for storage. Frozen sections were cut coronally with a cryostat at −24°C (20-μm thickness) and air dried overnight. Sections −2.0 to −2.5 mm to bregma were used to assess the meningeal changes, whereas sections −5.8 to −8.4 mm to bregma were used for the TNC. Tissues were incubated in 10% normal goat serum in 0.3% Triton-100 for 30 min. Alternate sections were labeled using the following primary antibodies overnight at room temperature: rabbit anti-CGRP (1:200; Sigma-Aldrich); rabbit anti-iNOS (1:100; Enzo Life Sciences, Farmingdale, NY); rabbit IBA-1 (1:250; Wako, Richmond, VA); rabbit glial fibrillary acidic protein (GFAP; 1:200; Millipore, Billerica, MA); and rabbit NeuN (1:1000; Millipore). Slides were incubated in fluorescent secondary antibodies DyLight 488– or 549–conjugated AffiniPure Goat anti-rabbit immunoglobulin G (Jackson ImmunoResearch, West Grove, PA) (diluted 1:100 in 1× phosphate-buffered saline) for 2 hours at room temperature. 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen Life Science, Carlsbad, CA) mounting media was applied to visualize the nucleus for cell counting. Negative control staining was performed by omitting the primary antibodies. Each IHC experiment includes both controls and experimental groups processed at the same time to control for variability with processing.
Stereological methods were used to count cells positive for iNOS or CGRP in trigeminal ganglia cells (right trigeminal ganglia ipsilateral to injury only) by an observer blinded to experimental conditions. Cell counts were measured using a Nikon NIU microscope (Nikon, Tokyo, Japan) at a 20× objective for three sampling frames (100 μm2 each frame) from two serial sections for each animal (six total counting frames).43 A grid overlay was used to count cells, and only cells with a DAPI-labeled nucleus were counted per frame. When capturing images, exposure times were held constant between specimens and cells with fluorescent staining selected using the thresholding tool from Nis-Elements software (Nikon). All six frames per section were averaged and are reported as the mean ± standard deviation (SD) number of iNOS-positive ganglia cells. For CGRP cell counts, mean percentage of CGRP-positive cells ± SD out of all cells in the ganglia were reported. Although bilateral changes were observed in the trigeminal ganglia and TNC, only the right side (ipsilateral to the injury) was included in the analysis with the exception of the side comparisons for CGRP
Quantification of CGRP percent immunoreactivity was performed, as described previously, by our laboratory.8,44 Briefly, the ophthalmic (V1) region of the TNC was imaged using a Nikon NIU microscope and a 20× objective in which three frames from three sections were captured for two sections per animal. When capturing images, exposure times were held constant as was done for cell counting. Threshold parameters were set to select the immunofluorescent CGRP product while omitting the background using the Nis-Elements software (Nikon) slider tools. The pre-set threshold levels were held constant for measuring pixels counts for both control and experimental groups to avoid experimenter bias. The total number of pixels was then measured by selecting all the pixels in the region of interest to ensure consistent sampling between specimens. Percent immunoreactivity was calculated as the measured threshold CGRP immunofluorescent product in pixels divided by the total pixels in the V1 region of interest. Percent immunoreactivity for the six sampling frames (two sections × three frames) were averaged and are reported as mean ± SD.
Statistical analysis
To determine the group effects on mechanical von Frey thresholds and histological outcomes, one-way analyses of variance (ANOVAs) were used at matched time points. ANOVAs were followed by Bonferroni's post-hoc tests for multiple comparisons with adjusted p values. A Student's t-test was used to determine side differences for von Frey thresholds. All data were analyzed using the GraphPad Prism 5 statistical program (GraphPad Software Inc., La Jolla, CA). Significance levels were set at p < 0.05 for all statistical analyses, adjusted p values are reported, and data are reported as the mean and standard error of the mean.
Results
Trigeminal allodynia and photosensitivity
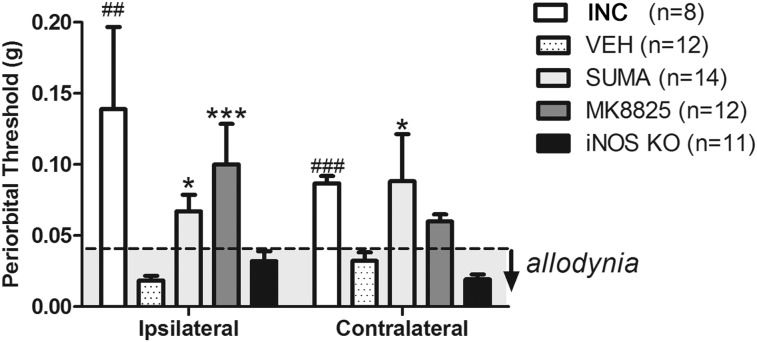
Periorbital von Frey sensory testing was used to assess changes in trigeminal allodynia at 2 weeks post-CCI injury for groups treated with sumatriptan, MK8825 compared to vehicle, and incision controls, as well as in iNOS knockout mice (Fig. 2). Allodynic thresholds were present in 100% of mice with CCI, with bilateral allodynia occurring in 83% of mice. CCI injury resulted in a significant reduction in periorbital thresholds ipsi- and contralateral to injury compared to baseline ANOVA (p < 0.0001; F = 6.2 and 10.34, respectively). Sumatriptan and MK8825 significantly increased thresholds (reduced allodynia) on the ipsilateral side compared to vehicle-treated controls (p < 0.01 and p < 0.05, respectively). Sumatriptan also increased thresholds contralaterally (p < 0.05), but MK8825 did not. Treatment with sumatriptan and MK8825 abolished allodynia in 57% and 75% of mice with CCI, respectively. In contrast, iNOS knockouts with CCI did not show a difference in von Frey thresholds from WT CCI mice. Ipsilateral thresholds for CCI mice treated with vehicle (0.02 ± 0.01 g) and iNOS KO (0.02 ± 0.01) were significantly reduced compared to baseline thresholds (0.2 ± 0.2 g; ANOVA, p < 0.0001). In mice showing a treatment effect, ipsilateral thresholds were returned to baseline thresholds in sumatriptan- (0.07 ± 0.04 g) and MK8825-treated groups (0.1 ± 0.02 g). One mouse in the incision group was excluded from testing during baseline measures. Animals administered the CGRP antagonist, MK8825, and sumatriptan showed significant improvements in trigeminal sensitivity (reduced sensitivity/increased thresholds), in which there may be an enhanced treatment efficacy for MK8825 compared to sumatriptan.
FIG. 2.
Wild-type CCI mice treated with sumatriptan (SUMA) and MK8825 compared to vehicle (VEH; *p < 0.05; ***p < 0.001) and incision control (INC) compared to VEH (##p < 0.01 and ###p < 0.001) compared to VEH. iNOS knockout (KO) compared to CCI wild-type mice treated with vehicle were not statistically significant. Gray shaded area below dotted line indicates allodynia (≤0.04 g). iNOS, inducible nitric oxide synthase.
Testing for photosensitivity was measured as light aversion at two different light intensities, ambient (∼400 lux) and bright light (∼4000 lux) in CCI and naïve groups (Fig. 3A). Avoidant behavior to bright light conditions was determined in mice treated with vehicle, sumatriptan, MK8825, and iNOS knockouts (Fig. 3B–D). The ambient light test was included as the first session of the test to determine the presence of anxiety in the novel, open chamber. Subsequently, the bright light test was conducted once mice had acclimated to ambient conditions, reducing novelty and making the chamber less stressful. CCI injury resulted in a significant reduction in the percentage of time spent in the bright light compared to naïve values, whereas there was no significant difference between groups under the ambient light conditions (Fig. 3A; p < 0.001; F = 8.1). Treatment with MK8825 and iNOS KO significantly increased the CCI-induced reduction in the time spent in the bright light (p = 0.02), whereas sumatriptan had no effect on time spent in the bright light compartment. Analysis of the additional exploratory behaviors during the bright light showed reduced rearing, but not transitions in CCI groups treated with vehicle (p < 0.001; F = 6.58). Sumatriptan significantly reduced the transitions and rears compared to vehicle treated mice in bright light conditions (p < 0.001; Fig. 3C,D), whereas MK8825 and iNOS showed an increase in rearing activity (p < 0.01 and p < 0.05) in the bright light, compared to vehicle, that was similar to control levels (3D). In summary, injury induced an avoidance of bright light that was not present under ambient light conditions; light avoidance behavior indicative of photosensitivity and rearing activity were improved by MK8825 and genetic knockout of iNOS, but not by sumatriptan. Rearing in the ambient light conditions was significantly reduced for CCI (8.25 ± 5.5 rears/session) compared to baseline means (17.23 ± 2.7 rears/session; p < 0.001), indicating that there may be spontaneous pain behavior after injury.
FIG. 3.
Photophobia assessed as a change in the percentage of time or an aversion to bright light (∼4000 lux) compared to a less intense, ambient light (∼400 lux) condition. (A) Percentage of time spent in the bright light compartment during ambient and bright light exposure for wild-type CCI mice treated with vehicle (VEH) compared to naïve mice (**p < 0.01). (B) Percentage of time spent in the bright light compartment for CCI groups treated with sumatriptan (SUMA), MK8825, and iNOS KO compared to VEH controls (*p < 0.05 and **p < 0.01) and compared to naïve (###p < 0.001). (C) Transitions between the bright light and dark compartment for all groups compared to VEH (**p < 0.01). (D) Number of rears in the light compartment for all groups compared to VEH (*p < 0.05; **p < 0.01; **p < 0.001) and compared to naïve (*p < 0.05). CCI, controlled cortical injury; iNOS, inducible nitric oxide synthase; KO, knockout.
Inducible nitric oxide synthase messenger RNA and immunohistochemistry
iNOS immunoreactivity appeared as granulated or homogenous cytoplasmic staining in the trigeminal ganglia and TNC post-CCI injury, whereas there was negligible expression in the control tissue (Figs. 4–6). iNOS has been described as cytosolic small vesicles or granules, cytoskeletal attachments, mitochondrial, nuclear, and perinuclear aggresomes, although the full characterization of these distinct molecular entities and their significance remain elusive.45 CCI injury increased the number of cells positive for iNOS immunoreactive protein expression in the trigeminal ganglia (ANOVA, p < 0.0001; F = 32.32; Fig. 4). iNOS was found in both neuronal and non-neuronal glial cells in the ganglia of CCI injured mice (Figs. 4, 5, and 6). Ionized calcium-binding adapter molecule 1 (IBA-1) microglia and GFAP-labeled satellite cells have a distinct cellular morphology and sit juxtaposed between neurons in the trigeminal ganglia (Fig. 5). There was a close colocalization between iNOS and CGRP (Fig. 6); in the ganglia, some cells colabeled for both CGRP and iNOS, whereas some labeled for iNOS and CGRP independently (Fig. 6A–C). In the TNC, the distribution of CGRP and iNOS showed a close overlap in the superficial lamina (Fig. 6D–F). CCI injury resulted in significant increases in iNOS mRNA compared to controls (p < 0.01; F = 5.98) and increased iNOS immunoreactivity iNOS in the TNC (Fig. 7). Treatment with sumatriptan and MK8825 significantly reduced iNOS mRNA in the TNC (p < 0.01; Fig. 7B–D) and similarly reduced the number of iNOS-positive ganglia cells (p < 0.0001; Fig. 4D–F). Data show that injury to the sensory cortex induces increases in the inducible isoform of NOS gene and protein levels in the trigeminal ganglia and caudal brainstem nuclei, changes that are attenuated with two drugs, sumatriptan and MK8825, that also inhibit the actions of CGRP though blocking its release or receptor binding, respectively.
FIG. 4.
Immunofluorescent iNOS-labeled trigeminal ganglia cells. (A) The number of iNOS-positive cells at 2 weeks postoperatively in incision control, CCI treated with vehicle (VEH), sumatriptan (SUMA), and MK8825 (####p < 0.0001) compared to control and (****p < 0.0001) compared to vehicle. (B–F) Images showing iNOS immunofluorescently labeled ganglia cells. (B) Low power image showing a trigeminal ganglia section with the opthalamic V1 region of interest (box) indicated; scale bar = 200 μm. High-power images of the ganglia from (C) control and CCI groups treated with (D) VEH, (E) SUMA, and (F) MK8825; scale bar = 10 μm. CCI, controlled cortical injury; iNOS, inducible nitric oxide synthase. Color image is available online at www.liebertpub.com/neu
FIG. 5.
Trigeminal ganglia cells labeled for ionized calcium-binding adapter molecule 1 (IBA-1)-positive microglia, glial fibrillary acidic protein (GFAP)-positive satellite cells, and inducible nitric oxide synthase (iNOS) markers. (A) GFAP satellite cells and (B) IBA-1 microglia cells show distinct cellular morphologies and sit juxtaposed between neurons in the trigeminal ganglia. Postinjury, iNOS is expressed by cells with a neuronal morphology indicated by arrows and by non-neuronal cells indicated by arrow heads (C and D); 4′,6-diamidino-2-phenylindole is labeled blue. Scale bars = 10 μm. Color image is available online at www.liebertpub.com/neu
FIG. 6.
Immunofluorescent images showing inducible nitric oxide synthase (iNOS; red), calcitonin gene-related peptide (CGRP; green), and colocalization (yellow) in the trigeminal nucleus caudalis (A–C) and trigeminal ganglia (D–F). iNOS and CGRP (yellow) are tightly colocalized in the trigeminal nucleus caudalis (F). In trigeminal ganglia cells, some CGRP-positive cells do not colocalize with iNOS. Bar = 100 μm. Color image is available online at www.liebertpub.com/neu
FIG. 7.
Inducible nitric oxide synthase (iNOS) messenger RNA (mRNA; left) and iNOS immunoreactive protein expression of trigeminal nucleus caudalis (TNC). Quantitative reverse-transcriptase polymerase chain reaction showed iNOS mRNA levels at 2 weeks after incision control (CONT), controlled cortical impact (CCI) injury treated with vehicle (VEH), MK8825, and sumatriptan (SUMA). iNOS mRNA levels post-CCI compared to control: ##p < 0.01. iNOS mRNA post-CCI treated with MK8825 and SUMA compared to vehicle: **p < 0.01 and ***p < 0.01, respectively. Lower left image shows the topographical representation of the ophthalamic VI division of the trigeminal nerve where central afferents of the ganglia enter the TNC (Source: website: ©2014 Allen Institute for Brain Science, http://atlas.brain-map.org/atlas?atlas=1). Immunofluorescent images of the TNC show iNOS immunoreactivity for (A) incision control mice, (B) CCI mice treated with VEH, (C) CCI treated with SUMA, and (D) CCI treated with MK8825; scale bar = 100 μm. Color image is available online at www.liebertpub.com/neu
IHC examination of trigeminal ganglia and TNC tissues showed differences in the cellular localization of iNOS. Whereas in the ganglia iNOS had a clear cellular localization, iNOS expression in the TNC was not specifically localized with any cell type, except for a few microglia, but was distributed between cells in granular appearance with occasionally overlapping cellular processes (Fig. 8). In the superficial lamina, iNOS appears strikingly similar to CGRP, as a highly granulated or vesicular product without distinct cellular localization, and is presumed to be housed in pre-synaptic afferent terminals. CGRP and iNOS are tightly colocalized in the trigeminal ganglia, and the distribution in the TNC suggests coexpression in the afferent terminal. The CGRP receptors, calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein 1 (RAMP1), localize to the central axons of ganglion neurons, but not the peripheral axons.46 In the trigeminal ganglia soma and central afferent terminals, CGRP and its receptors are colocalized, making a subset of cells “autoreceptors.”47 The finding of CGRP-iNOS colocalization, combined with the potential of the ganglia cells working as autoreceptors supports the proposed positive feed-forward CGRP-iNOS/NO circuit within the ganglia. CGRP receptors localize to form “glomerular structures” in the TNC and has been previously proposed to be part of the neuronal afferent terminals.47 A limitation of this study is that the precise glial cell source in the ganglia as being either microglia or glial satellite cells could not be determined owing to species interactions among certain commercially available antibodies for mouse in the ganglia. Expression of iNOS between the two regions in the trigeminal pain system appears to be from different cellular sources (neurons and glia in the ganglia compared to the central afferent terminals in the TNC) and is notably similar to the pattern and localization for CGRP.
FIG. 8.
Immunofluorescent Images show inducible nitric oxide synthase (iNOS) labeling surrounded by microglia and astrocytes. Immunofluorescent antibodies against (A) iNOS in the trigeminal nucleus caudalis (TNC) double labeled with (B) microglial-specific antibodies against the ionized calcium-binding adaptor molecule (IBA-1) and (C) astrocyte-specific antibodies against the glial fibrillary acidic protein (GFAP). (A) iNOS appears as highly granulated or vesicular product without distinct cellular localization in the superficial lamina of the TNC, presumed to be housed in pre-synaptic afferent terminals. (B) iNOS was absent from most cell bodies except for a few IBA-1-labeled microglia (arrow heads), which appeared in an activated phenotype, as did the (C) GFAP-positive astrocytes. Color image is available online at www.liebertpub.com/neu
Calcitonin gene-related peptide enzyme-linked immunosorbent assay and immunohistochemistry
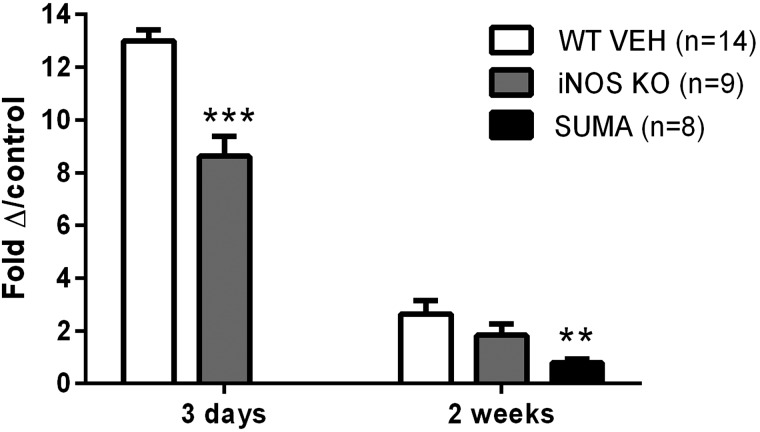
CCI injury results in increased CGRP levels in the TNC (2.5-fold) over control levels at 2 weeks postinjury with an exaggerated increase on postoperative day 3 (12.8-fold increase; Fig. 9; ANOVA, p < 0.0001; F = 57.06). Knockout of iNOS significantly reduced CGRP levels at 3 days, but not 2 weeks postinjury (ANOVA, p < 0.0001; F = 44.65). A significant reduction in CGRP levels was found in iNOS knockouts at 3 days postinjury, compared to CCI-injured WT mice (p < 0.01). Despite this significant reduction at 3 days, CGRP was still substantially elevated, compared to control, by 8.3-fold. Sumatriptan was included as a positive control because it has been shown to reduce CGRP levels39 and resulted in a significant reduction in CGRP levels at 2 weeks (ANOVA, p < 0.01; F = 6.32). Treatment with sumatriptan significantly reduced CGRP levels, compared to vehicle controls (p < 0.01). CGRP levels for naïve WT mice were not statistically different from naïve iNOS knockout mice (39.7 ± 10.3 and 40.2 ± 3.9; p > 0.05).
FIG. 9.
Enzyme-linked immunosorbent assay quantification of calcitonin gene-related peptide (CGRP) levels in the trigeminal nucleus caudalis of controlled cortical impact mice. CGRP level at 3 days postinjury in wild-type (n = 6) and iNOS KO (n = 4) mice and 2 weeks in wild-type mice treated with vehicle (n = 8), iNOS KO mice (n = 5), and wild-type mice treated with sumatriptan (n = 8) ***p < 0.001 and **p < 0.01. Data are reported as means ± standard error of the mean. iNOS, inducible nitric oxide synthase; SUMA, sumatriptan; WT VEH, wild-type treated with vehicle.
Percent CGRP immunoreactivity in the TNC for CCI mice was significantly increased on the right (ipsilateral) and left sides, compared to incision controls (p < 0.01; Table 1). Percent CGRP immunoreactivity was not significantly different between the right and left sides (p = 0.2). IHC analysis of CGRP in the TNC revealed increases for CCI, compared to control, but no side differences were found. However, the technique in quantifying the changes in the levels of neuropeptide in the TNC has limitations in terms of detection sensitivity, particularly for small changes in a neuropeptide. The percentage of CGRP positive ganglia cells was increased on the right and left sides of CCI mice compared to controls (p < 0.01 and p < 0.001, respectively).
Table 1.
Percent CGRP Immunoreactivity in the TNC and Percentage of Positive Trigeminal Ganglia Cells Post-CCI: An Analysis of Side Differences
| Group | Side | % CGRP IR in TNC Mean ± SD | % CGRP+ Ganglia cells Mean ± SD |
|---|---|---|---|
| Control | Right | 0.87 ± 0.7 | 12.6 ± 1.7 |
| Control | Left | 0.67 ± 0.7 | 14.95 ± 3.2 |
| CCI | Right | 9.1 ± 3.4* | 48.63 ± 18.9† |
| CCI | Left | 5.8 ± 1.3* | 50.9 ± 8.3‡ |
Data are reported as means ± standard error of the mean.
CCI, controlled cortical impact; CGRP, calcitonin gene-related peptide; IR, immunoreactive; SD, standard deviation; TNC, trigeminal nucleus caudalis.
p < 0.01; †p < 0.01; ‡p < 0.001.
CGRP IHC analysis was performed to determine whether meningeal inflammation was present after CCI injury (Fig. 10). Significant CGRP immunoreactivity was found in areas of the pial layers in the CCI-injured brains examined, whereas there was negligible immunoreactivity in control tissues. During dissection of the brain, the dura is typically removed; however, in certain samples, this layer can be found. In a few samples, where both the pia and dura were present, there was a substantial amount of CGRP found, indicating neurogenic inflammation postinjury. Microglia/macrophage-specific marker Iba-1 immunoreactivity was also found in the pial layers postinjury in addition to CGRP. Microglial/macrophage cell morphology was visualized using Iba-1 compared to the punctate, granular appearance of CGRP in the meningeal layers. CGRP released from the peripheral afferent terminal of the trigeminal ganglia in the meningeal layers is indicative of neurogenic inflammation, whereas infiltrating macrophage cells or activated migrating microglia in the meninges is a general inflammatory response to injury.
FIG. 10.

Calcitonin gene-related peptide (CGRP) immunoreactivity in the meningeal layers post-CCI (controlled cortical injury). CGRP expression is minimal in the control brain tissue (A, C, and E), whereas substantial CGRP immunoreactive product is visualized in the pial layers in the CCI-injured brain (B, D, and F). Inset of (B) shows CGRP immunoreactivity in both pial meningeal layer (arrowhead) and dural layer (arrow). CGRP immunoeactivity appears as a punctate neuropeptide in the meningeal layers (arrows; D and F). Ionized calcium-binding protein 1 (Iba-1) shows macrophage/microglial–specific cellular morphologies in the meningeal layers after injury (G and H). Color image is available online at www.liebertpub.com/neu
Discussion
Focal injury to the sensory cortex increases transcription and protein synthesis of iNOS, as well as levels of CGRP in the ascending trigeminal pain pathway; these changes are accompanied by trigeminal allodynia and photosensitivity 2 weeks postinjury. Drugs known to inhibit the actions of CGRP, MK8825, and sumatriptan significantly reduced iNOS gene and protein levels in the trigeminal ganglia and caudal trigeminal nuclei. Genetic knockout of iNOS attenuated CGRP levels at 3 days, but not 2 weeks, postinjury. Behaviorally, treatment with MK8825 and sumatriptan 2 weeks postinjury returned trigeminal allodynia to baseline levels, whereas allodynia in the iNOS knockout group was not different from the WT group with injury. MK8825 treatment and iNOS knockout abolished injury-induced photosensitivity behavior, whereas sumatriptan-treated mice continued to demonstrate an aversion to bright light.
Trauma-induced pain signaling molecules and their modulation
Inflammation is an early promoter of pain that can be persistent post-TBI48; however, the role in the pathogenesis of PTH is not well defined.49 Inflammation-induced sensitization of meningeal nociceptors was evidenced by increases in CGRP and iNOS at the level of the meninges, trigeminal ganglia, and/or TNC. Previously, our laboratory found the neurogenic inflammatory mediator and nociceptive neuropetide, substance P, perivascularly in the injured cortex and in the caudal brainstem.8,36 Proinflammatory mediators within the injured cortex may activate the meningeal nociceptors and, over time, render them sensitized especially because the blood–brain barrier is compromised in our model.35,50,51 In addition to directly inflamed meningeal and cortical tissues, periosteal inflammation also contributes to activation of the trigeminal ganglia neurons after closed-head injury.16,52
The initial release of inflammatory mediators, such as cytokines, prostaglandins, NO, bradykinins, and histamines, are hypothesized to sensitize trigeminal pain neurons leading to development of chronic headache post-TBI.8,9,14–16,53 Localized release of inflammatory molecules may lower the threshold for activation of meningeal nociceptors and, as a result, sensitize them to activation by a lesser degree of vessel dilatation.13,53 Proinflammatory cytokines interleukin 1 beta (IL-1β), and tumor necrosis factor alpha (TNF-α) have been shown to stimulate prostaglandins postinjury.54–57 Prostaglandin E2 stimulates neuronal excitability, triggers the trigeminal ganglia neurons to release CGRP, and, in turn, signals pain.58,59 Neurons, microglia, and astrocytes not only release proinflammatory molecules, but also play a role in handling or reacting with pronociceptive mediators (e,g., glutamate, CGRP, and substance P) that contribute to neuronal sensitization and chronic pain.60–63
Cortical injury increases iNOS gene and protein synthesis in the ascending trigeminal pain pathway. Pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) stimulate iNOS gene transcription in many cell types, such as endothelial cells, neurons, microglia, and astrocytes, post-TBI.64 Previously, we reported an influx of iNOS-labeled macrophage or microglia in the proximity of the injured cortex post-CCI,51 although a neuronal source has been identified as well.65 The acute and long-lasting excessive quantities of NO release post-TBI are generated from iNOS and far outweigh the increases in eNOS and nNOS.66–68 In vivo and in culture, iNOS was shown to augment the release of CGRP from trigeminal afferents.69,70 However, considering the evidence for nNOS in migraine, as well as inflammatory pain studies,26,71 it is reasonable to expect nNOS involvement in the pain pathway post-TBI. This study does not exclude nNOS as a contributor to CGRP increases or headache behavior postinjury, in which investigation is warranted.
Injury-triggered increases in CGRP were found at 3 levels of the trigeminal pain pathway in the 1) meningeal layers, 2) trigeminal ganglia neurons, and 3) TNC. In the ascending pain pathway, remote from the site of cortical injury, CGRP is expressed and released from the peripheral terminals of the trigeminal ganglia, neuronal cell bodies, and central terminals in the brainstem.47,72 CGRP in the trigeminal ganglia neurons and perivascular nerve fibers surrounding the meningeal and cerebral blood vessels acts as a potent vasodilator facilitating cerebral perfusion, but also plays an important role in nociceptive neurotransmission. Studies using sensitive tracers identified the trigeminal nerve and upper cervical ganglia as the afferent pain pathway for the meningeal and cerebral vessels, in which CGRP and NO are important signaling molecules.73,74 The trigeminal nerve also transmits nociceptive, tactile, temperature afferent input from the skin of the face and sinus mucous membranes.75
Blocking the actions of CGRP using an antagonist and sumatriptan reduces iNOS and alleviates both allodynia and photosensitivitiy. Deleting iNOS reduces CGRP and photosensitivity, but not allodynia, which is explained by the levels of CGRP being well above control levels. We believe these results to be evidence of synergism (a coordinated action of two or more agents, or physiological processes, such that the combined effect is greater than the sum of each independently) between iNOS and CGRP. Reciprocal interactions between CGRP and NO/NOS have been previously proposed.22,23,76 Postinjury, release of CGRP and NO by iNOS is proposed to enter a feed-forward cycle between individual ganglia cells promoting reciprocity among individual ganglia cells,22 in turn leading to peptide, enzyme, or transmitter overproduction and transport to the afferent terminal in the TNC.26 It should be noted that MK8825 and sumatriptan block CGRP through two different mechanisms. MK8825 binds to the CGRP receptors, RAMP1 and CLR, in the trigeminal ganglia to inhibit binding.77 MK8825 blocks NO-induced neuronal activation in the caudal brainstem, where the first synapse is formed by the central terminal of the trigeminal ganglia.77 Sumatriptan is a triptan-class medication and inhibits the release of CGRP from the central afferents of the trigeminal ganglia in addition to being a 5HT1B/1D receptor agonist with a role in descending pain modulation.18,39
Sensitization of trigeminal pain neurons and headache behavior
Injury-induced increases in pain-signaling molecules CGRP and iNOS in the trigeminal pain pathway are associated with headache-like behaviors, trigeminal allodynia, and photosensitivity. Previously increased CGRP also correlated with trigeminal allodynia for 1 month after cortical injury in mice.8 Mechanical allodynia (cutaneous hypersensitivity to a mechanical stimulus that is otherwise innocuous under normal conditions) is a painful response in humans, common in patients with migraine, and has been reported in patients with PTH.5–7 Allodynia in the trigeminal (periorbital) zone after cortical injury in mice and rats indicates that trigeminal pain neurons have become sensitized postinjury.6,8,9 The phenomenon known as peripheral sensitization of neurons in the trigeminal ganglia originates at the level of the afferent terminal innervating the meningeal vasculature (i.e., the meningeal nociceptor) and involves activation of the trigeminovascular system during a migraine attack.14 Central sensitization of the second-order neuron in the brainstem nuclei (i.e., the trigeminal nucleus caudalis) and thalamus is involved in chronic migraine. Inflammatory stimulation of the dura results in sensitization of the second-order neurons that is associated with allodynia at the referred pain area7,78; dural infammation also induces headache in humans.14 It should be noted, however, that peripheral input is not required in the pathophysiology of migraine; rather, it is believed that thalamic sensitization or cortical spreading depression may underlie migraine pathophysiology.7,79 This may also be the case for PTH. Persistent inflammation in the posterior thalamic nuclei has been shown post-CCI previously by our laboratory.44 Inflammation, hyperactivation, or circuit disruptions within the brain's central nociceptive pathways cannot be ruled out as a generator of PTH.7,48,78,80–82
Interactions between CGRP- and iNOS/NO-signaling pathways may converge in the thalamus eliciting photosensitivity. Neurons in the posterior thalamus respond dually to input from dural nociceptors (trigeminal ganglion cells) and photoreceptors (retinal ganglion cells)24,83; this pathway is proposed to explain photosensitivity during primary and, perhaps, PTH. Cortical contusion injury induced an aversion to bright light, but not to ambient light conditions, a behavior indicative of headache.28 A lack of group differences in the time spent in the light under ambient conditions, combined with a group effect for the bright light condition, indicate that light-avoidance behavior is a sensitivity to bright light and not anxiety. However, a finding of anxiety, induced only by bright light conditions, cannot be ruled out entirely. In addition, testing for stimulated signs of pain in an isolated pain system (allodynia and light stimuli), we also assessed spontaneous signs of pain, such as an arched back, squinting, vocalization, or inactivity. Interestingly, injury resulted in reduced exploratory rearing behavior during ambient and bright light conditions, indicating that injured mice may show signs of spontaneous pain; however, additional assessments, such as longer observations, including overnight monitoring, would be helpful to verify spontaneous pain behavior.
Treatments with sumatripan and MK8825 alleviated post-traumatic trigeminal allodynia, results that were paralleled by reductions in iNOS gene and protein expression. These findings indicate that CGRP and iNOS play a role in mediating trigeminal allodynia. These data echo the finding of CGRP antagonists and/or sumatriptan attenuated facial allodynia triggered by inflammation or NO in models of headache.28,82 Trigeminal allodynia was not different in iNOS knockout and WT injured groups at 2 weeks, which may be explained by CGRP levels being well above control levels. In contrast, nNOS and iNOS inhibitors attenuated mechanical hypersensitivity after nerve injury.30 Perhaps a pharmacological approach that utilizes NOS inhibitors or nNOS knockouts would have a greater impact on CGRP and allodynia post-TBI.
CGRP antagonism with MK8825 and iNOS knockout in the present study returned photosensitivity and exploratory behavior in bright light conditions to baseline levels postinjury. Interestingly, photosensitivity was attenuated by iNOS in the absence of CGRP reduction at 2 weeks, leading to the proposal that the iNOS/NO-signaling pathway contributes to photosensitivity in a CGRP-independent manner. In a transgenic CGRP-sensitive mouse model exhibiting photosensitivity, light aversion was blocked by a CGRP antagonist, olcegepant.42,85,86 Sumatriptan did not attenuate photosensitivity after cortical injury. In a study by Kaiser and colleagues, a triptan drug, rizatriptan, which has mechanisms of action similar to sumatriptan, was assessed for reducing photophobia in a CGRP receptor transgenic model of migraine.86 Rizatriptan reduced CGRP-induced light aversion and improved exploratory behavior in the migraine model.86 The conflicting findings between the present study and those by Kaiser and colleagues may be explained by differences in the levels of CGRP levels or CGRP receptor expression between the animal models.46,86 A lack of treatment effect and reduced exploratory behaviors in the sumatriptan group may be secondary to known side effects of sumatriptan in humans, such as paresthesia, drowsiness, tiredness, weakness, dizziness, upset stomach, and muscle cramps. Overall, CGRP antagonism showed better efficacy for attenuating headache behaviors, trigeminal allodynia, and photosensitivity, although MK8825 and sumatriptan had similar effects on iNOS transcription and protein synthesis.
Conclusions and Future Directions
In conclusion, CGRP and iNOS play an important role in the trigeminal pain pathway, contributing to allodynia and photosensitivity post-TBI, although their pathways may be independent of each other for photosensitivity. At the level of the trigeminal ganglia, iNOS is expressed by neuronal and non-neuronal cell types. Blockade of CGRP has therapeutic potential in the management of PTH in which there may be a delayed therapeutic window. Understanding the interplay between CGRP and iNOS in post-TBI headache not only clarifies central and peripheral trigeminal pain pathways involved in its pathogenesis, but also may contribute to future development of migraine therapeutics with lower side-effect profiles.
Acknowledgments
This work was supported, in part, by a research grant from the Department of Defense (W81XWH-12-1-0326; to M.B.E.) and the Investigator Initiated Studies Program of Merck Sharp & Dohme Corp. The opinions expressed in this article are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp. Thank you to Drs. Michael Oshinsky and Stephen Silberstein, and the Thomas Jefferson University Headache Center for their continued feedback on this ongoing research. The authors also thank Christine Macolino for surgical contributions.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lucas S., Hoffman J.M., Bell K.R. and Dikmen S. (2014). A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 34, 93–102 [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society. (2013). The International Classification of Headache Disorders, 3rd ed. (beta version). Cephalalgia 33, 629–808 [DOI] [PubMed] [Google Scholar]
- 3.Theeler B., Lucas S., Riechers R.G., 2nd, and Ruff R.L. (2013). Post-traumatic headaches in civilians and military personnel: a comparative, clinical review. Headache 53, 881–900 [DOI] [PubMed] [Google Scholar]
- 4.Theeler B.J., Flynn F.G., and Erickson J.C. (2010). Headaches after concussion in US soldiers returning from Iraq or Afghanistan. Headache 50, 1262–1272 [DOI] [PubMed] [Google Scholar]
- 5.Ofek H., and Defrin R. (2007). The characteristics of chronic central pain after traumatic brain injury. Pain 131, 330–340 [DOI] [PubMed] [Google Scholar]
- 6.Burstein R., Yarnitsky D., Goor-Aryeh I., Ransil B.J., and Bajwa Z.H. (2000). An association between migraine and cutaneous allodynia. Ann. Neurol. 47, 614–624 [PubMed] [Google Scholar]
- 7.Burstein R., Jakubowski M., Garcia-Nicas E., Kainz V., Bajwa Z., Hargreaves R., Becerra L., and Borsook D. (2010). Thalamic sensitization transforms localized pain into widespread allodynia. Ann. Neurol. 68, 81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott M.B., Oshinsky M.L., Amenta P.S., Awe O.O., and Jallo J.I. (2012). Nociceptive neuropeptide increases and periorbital allodynia in a model of traumatic brain injury. Headache 52, 966–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macolino C.M., Daiutolo B.V., Alberston B.K., and Elliott M.B. (2014). Mechanical alloydnia induced by traumatic brain injury is independent of restraint stress. J. Neurosci. Methods 226, 139–146 [DOI] [PubMed] [Google Scholar]
- 10.Recober A., Kuburas A., Zhang Z., Wemmie J.A., Anderson M.G., and Russo A.F. (2009). Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J. Neurosci. 29, 8798–8804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dussor G., Yan J., Xie J.Y., Ossipov M.H., Dodick D.W., and Porreca F. (2014). Targeting TRP channels for novel migraine therapeutics. ACS Chem. Neurosci. 5, 1085–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goadsby P.J., Charbit A.R., Andreou A.P., Akerman S., and Holland P.R. (2009). Neurobiology of migraine. Neuroscience 161, 327–341 [DOI] [PubMed] [Google Scholar]
- 13.Levy D., and Burstein R. (2011). The vascular theory of migraine: leave it or love it? Ann Neurol. 69, 600–601 [DOI] [PubMed] [Google Scholar]
- 14.Bernstein C., and Burstein R. (2012). Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J. Clin. Neurol. 8, 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benromano T., Defrin R., Ahn A.H., Zhao J., Pick C.G., and Levy D. (2015). Mild closed head injury promotes a selective trigeminal hypernociception: Implications for the acute emergence of post-traumatic headache. Eur. J. Pain 19, 621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feliciano D.P., Sahbaie P., Shi X., Klukinov M., Clark J.D., and Yeomans D.C. (2014). Nociceptive sensitization and BDNF up-regulation in a rat model of traumatic brain injury. Neurosci. Lett. 583, 55–59 [DOI] [PubMed] [Google Scholar]
- 17.Goadsby P.J., Edvinsson L., and Ekman R. (1990). Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 28, 183–187 [DOI] [PubMed] [Google Scholar]
- 18.Recober A., and Goadsby P.J. (2010). Calcitonin gene-related peptide: a molecular link between obesity and migraine? Drug News Perspect. 23, 112–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edvinsson L., Mulder H., Goadsby P.J., and Uddman R. (1998). Calcitonin gene-related peptide and nitric oxide in the trigeminal ganglion: cerebral vasodilatation from trigeminal nerve stimulation involves mainly calcitonin gene-related peptide. J. Auton. Nerv. Syst. 70, 15–22 [DOI] [PubMed] [Google Scholar]
- 20.Olesen J. (2008). The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol. Ther. 120, 157–171 [DOI] [PubMed] [Google Scholar]
- 21.Recober A., and Russo A.F. (2009). Calcitonin gene-related peptide: an update on the biology. Curr. Opin. Neurol. 22, 241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eberhardt M., Dux M., Namer B., Miljkovic J., Cordasic N., Will C., Kichko T.I., de la Roche J., Fischer M., Suarez S.A., Bikiel D., Dorsch K., Leffler A., Babes A., Lampert A., Lennerz J.K., Jacobi J., Marti M.A., Doctorovich F., Hogestatt E.D., Zygmunt P.M., Ivanovic-Burmazovic I., Messlinger K., Reeh P., and Filipovic M.R. (2014). H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat. Commun. 5, 4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann J., and Goadsby P.J. (2012). New agents for acute treatment of migraine: CGRP receptor antagonists, iNOS inhibitors. Curr. Treat. Options Neurol. 14, 50–59 [DOI] [PubMed] [Google Scholar]
- 24.Russo A.F. (2015). Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu. Rev. Pharmacol. Toxicol. 55, 533–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olesen J. (2010). Nitric oxide-related drug targets in headache. Neurotherapeutics 7, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akerman S., Williamson D.J., Kaube H., and Goadsby P.J. (2002). Nitric oxide synthase inhibitors can antagonize neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. Br. J. Pharmacol. 137, 62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshinsky M.L., and Gomonchareonsiri S. (2007). Episodic dural stimulation in awake rats: a model for recurrent headache. Headache 47, 1026–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bates E.A., Nikai T., Brennan K.C., Fu Y.H., Charles A.C., Basbaum A.I., Ptacek L.J., and Ahn A.H. (2010). Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia 30, 170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Alba J., Clayton N.M., Collins S.D., Colthup P., Chessell I., and Knowles R.G. (2006). GW274150, a novel and highly selective inhibitor of the inducible isoform of nitric oxide synthase (iNOS), shows analgesic effects in rat models of inflammatory and neuropathic pain. Pain 120, 170–181 [DOI] [PubMed] [Google Scholar]
- 30.Tanabe M., Nagatani Y., Saitoh K., Takasu K., and Ono H. (2009). Pharmacological assessments of nitric oxide synthase isoforms and downstream diversity of NO signaling in the maintenance of thermal and mechanical hypersensitivity after peripheral nerve injury in mice. Neuropharmacology 56, 702–708 [DOI] [PubMed] [Google Scholar]
- 31.MacMicking J.D., Nathan C., Hom G., Chartrain N., Fletcher D.S., Trumbauer M., Stevens K., Xie Q.W., Sokol K., Hutchinson N., Mudget J.S., and Chen H. (1995). Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81, 641–650 [DOI] [PubMed] [Google Scholar]
- 32.Cole J.T., Yarnell A., Kean W.S., Gold E., Lewis B., Ren M., McMullen D.C., Jacobowitz D.M., Pollard H.B., O‘Neill J.T., Grunberg N.E., Dalgard C.L., Frank J.A. and Watson W.D. (2011). Craniotomy: true sham for traumatic brain injury, or a sham of a sham? J. Neurotrauma 28, 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flexman A.M., Ng J.L., and Gelb A.W. (2010). Acute and chronic pain following craniotomy. Curr. Opin. Anaesthesiol. 23, 551–557 [DOI] [PubMed] [Google Scholar]
- 34.Elliott M.B., Tuma R.F., Amenta P.S., Barbe M.F., and Jallo J.I. (2011). Acute effects of a selective cannabinoid-2 receptor agonist on neuroinflammation in a murine model of traumatic brain injury. J. Neurotrauma 28, 973–981 [DOI] [PubMed] [Google Scholar]
- 35.Amenta P.S., Jallo J.I., Tuma R.F., and Elliott M.B. (2012). A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J. Neurosci. Res. 90, 2293–2305 [DOI] [PubMed] [Google Scholar]
- 36.Elliott M.B., Tuma R.F., Amenta P.S., Barbe M.F., and Jallo J.I. (2011). Acute effects of a selective cannabinoid-2 receptor agonist on neuroinflammation in a model of traumatic brain injury. J. Neurotrauma 28, 973–981 [DOI] [PubMed] [Google Scholar]
- 37.Bell I.M., Stump C.A., Gallicchio S.N., Staas D.D., Zartman C.B., Moore E.L., Sain N., Urban M., Bruno J.G., Calamari A., Kemmerer A.L., Mosser S.D., Fandozzi C., White R.B., Zrada M.M., Selnick H.G., Graham S.L., Vacca J.P., Kane S.A., and Salvatore C.A. (2012). MK-8825: a potent and selective CGRP receptor antagonist with good oral activity in rats. Bioorg. Med. Chem. Lett. 22, 3941–3945 [DOI] [PubMed] [Google Scholar]
- 38.Shivanand K., Raju S., Nizamuddin S. and Jayakar B. (2011). In vivo bioavailability studies of sumatriptan succinate buccal tablets. Daru 19, 224–230 [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X., Fang Y., Liang J., Yin Z., Miao J., and Luo N. (2010). Selective inhibition of 5-HT7 receptor reduces CGRP release in an experimental model for migraine. Headache 50, 579–587 [DOI] [PubMed] [Google Scholar]
- 40.Dobson C.F., Tohyama Y., Diksic M., and Hamel E. (2004). Effects of acute or chronic administration of anti-migraine drugs sumatriptan and zolmitriptan on serotonin synthesis in the rat brain. Cephalalgia 24, 2–11 [DOI] [PubMed] [Google Scholar]
- 41.Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., and Yaksh T.L. (1994). Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 [DOI] [PubMed] [Google Scholar]
- 42.Russo A.F., Kuburas A., Kaiser E.A., Raddant A.C. and Recober A. (2009). A potential preclinical migraine model: CGRP-sensitized mice. Mol. Cell Pharmacol. 1, 264–270 [PMC free article] [PubMed] [Google Scholar]
- 43.Mouton P.R. (ed). (2002). Principles and Practices of Unbiased Stereology. The Johns Hopkins University Press: Baltimore, MD [Google Scholar]
- 44.Hazra A., Macolino C., Elliott M.B., and Chin J. (2014). Delayed thalamic astrocytosis and disrupted sleep-wake patterns in a preclinical model of traumatic brain injury. J. Neurosci. Res. 92, 1434–1445 [DOI] [PubMed] [Google Scholar]
- 45.Bogdan C. (2015). Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 36, 161–178 [DOI] [PubMed] [Google Scholar]
- 46.Seiler K., Nusser J.I., Lennerz J.K., Neuhuber W.L., and Messlinger K. (2013). Changes in calcitonin gene-related peptide (CGRP) receptor component and nitric oxide receptor (sGC) immunoreactivity in rat trigeminal ganglion following glyceroltrinitrate pretreatment. J. Headache Pain 14, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lennerz J.K., Ruhle V., Ceppa E.P., Neuhuber W.L., Bunnett N.W., Grady E.F., and Messlinger K. (2008). Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J. Comp. Neurol. 507, 1277–1299 [DOI] [PubMed] [Google Scholar]
- 48.Onyszchuk G., LeVine S.M., Brooks W.M., and Berman N.E. (2009). Post-acute pathological changes in the thalamus and internal capsule in aged mice following controlled cortical impact injury: a magnetic resonance imaging, iron histochemical, and glial immunohistochemical study. Neurosci. Lett. 452, 204–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mayer C.L., Huber B.R., and Peskind E. (2013). Traumatic brain injury, neuroinflammation, and post-traumatic headaches. Headache 53, 1523–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woolf C.J., and Salter M.W. (2000). Neuronal plasticity: increasing the gain in pain. Science 288, 1765–1769 [DOI] [PubMed] [Google Scholar]
- 51.Amenta P.S., Jallo J.I., Tuma R.F., Hooper D.C., and Elliott M.B. (2014). Cannabinoid receptor type-2 stimulation, blockade, and deletion alters the vascular inflammatory responses to traumatic brain injury. J. Neuroinflamm. 11, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benromano T., Defrin R., Ahn A.H., Zhao J., Pick C.G., and Levy D. (2015). Mild closed head injury promotes a selective trigeminal hypernociception: Implications for the acute emergence of post-traumatic headache. Eur. J. Pain. 19, 621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy D., Kainz V., Burstein R., and Strassman A.M. (2012). Mast cell degranulation distinctly activates trigemino-cervical and lumbosacral pain pathways and elicits widespread tactile pain hypersensitivity. Brain Behav. Immun. 26, 311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antonova M., Wienecke T., Olesen J., and Ashina M. (2012). Prostaglandin E(2) induces immediate migraine-like attack in migraine patients without aura. Cephalalgia 32, 822–833 [DOI] [PubMed] [Google Scholar]
- 55.Kawabata A. (2011). Prostaglandin E2 and pain—an update. Biol. Pharm. Bull. 34, 1170–1173 [DOI] [PubMed] [Google Scholar]
- 56.Petraglia A.L., Maroon J.C., and Bailes J.E. (2012). From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery 70, 1520–1533; discussion, 1533. [DOI] [PubMed] [Google Scholar]
- 57.Hughes F.J., Buttery L.D., Hukkanen M.V., O'Donnell A., Maclouf J., and Polak J.M. (1999). Cytokine-induced prostaglandin E2 synthesis and cyclooxygenase-2 activity are regulated both by a nitric oxide-dependent and -independent mechanism in rat osteoblasts in vitro. J. Biol. Chem. 274, 1776–1782 [DOI] [PubMed] [Google Scholar]
- 58.Bartfai T. (2001). Immunology. Telling the brain about pain. Nature 410, 425, 427 [DOI] [PubMed] [Google Scholar]
- 59.Neeb L., Hellen P., Boehnke C., Hoffmann J., Schuh-Hofer S., Dirnagl U., and Reuter U. (2011). IL-1beta stimulates COX-2 dependent PGE(2) synthesis and CGRP release in rat trigeminal ganglia cells. PLoS One 6, e17360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeLeo J.A., and Yezierski R.P. (2001). The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 90, 1–6 [DOI] [PubMed] [Google Scholar]
- 61.Scholz J., and Woolf C.J. (2007). The neuropathic pain triad: neurons, immune cells and glia. Nat. Neurosci. 10, 1361–1368 [DOI] [PubMed] [Google Scholar]
- 62.Watkins L.R., Milligan E.D., and Maier S.F. (2001). Glial activation: a driving force for pathological pain. Trends Neurosci. 24, 450–455 [DOI] [PubMed] [Google Scholar]
- 63.Wieseler-Frank J., Maier S.F., and Watkins L.R. (2004). Glial activation and pathological pain. Neurochem. Int. 45, 389–395 [DOI] [PubMed] [Google Scholar]
- 64.Kroncke K.D., Fehsel K., and Kolb-Bachofen V. (1998). Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 113, 147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bechade C., Colasse S., Diana M.A., Rouault M., and Bessis A. (2014). NOS2 expression is restricted to neurons in the healthy brain but is triggered in microglia upon inflammation. Glia 62, 956–963 [DOI] [PubMed] [Google Scholar]
- 66.Cherian L., Hlatky R., and Robertson C.S. (2004). Nitric oxide in traumatic brain injury. Brain Pathol. 14, 195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gahm C., Holmin S., Rudehill S., and Mathiesen T. (2005). Neuronal degeneration and iNOS expression in experimental brain contusion following treatment with colchicine, dexamethasone, tirilazad mesylate and nimodipine. Acta Neurochir. (Wien) 147, 1071–1084; discussion, 1084 [DOI] [PubMed] [Google Scholar]
- 68.Wada K., Chatzipanteli K., Kraydieh S., Busto R., and Dietrich W.D. (1998). Inducible nitric oxide synthase expression after traumatic brain injury and neuroprotection with aminoguanidine treatment in rats. Neurosurgery 43, 1427–1436 [DOI] [PubMed] [Google Scholar]
- 69.Bellamy J., Bowen E.J., Russo A.F., and Durham P.L. (2006). Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur. J. Neurosci. 23, 2057–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dieterle A., Fischer M.J., Link A.S., Neuhuber W.L., and Messlinger K. (2011). Increase in CGRP- and nNOS-immunoreactive neurons in the rat trigeminal ganglion after infusion of an NO donor. Cephalalgia 31, 31–42 [DOI] [PubMed] [Google Scholar]
- 71.Maihofner C., Euchenhofer C., Tegeder I., Beck K.F., Pfeilschifter J., and Geisslinger G. (2000). Regulation and immunhistochemical localization of nitric oxide synthases and soluble guanylyl cyclase in mouse spinal cord following nociceptive stimulation. Neurosci. Lett. 290, 71–75 [DOI] [PubMed] [Google Scholar]
- 72.Fischer M.J. (2010). Calcitonin gene-related peptide receptor antagonists for migraine. Expert Opin. Investig. Drugs 19, 815–823 [DOI] [PubMed] [Google Scholar]
- 73.May A., and Goadsby P.J. (1999). The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J. Cereb. Blood Flow Metab. 19, 115–127 [DOI] [PubMed] [Google Scholar]
- 74.Liu Y., Broman J., Edvinsson L. (2008). Central projections of the sensory innervation of the rat middle meningeal artery. Brain Res. 1208, 103–110 [DOI] [PubMed] [Google Scholar]
- 75.Burstein R., Zhang X., Levy D., Aoki K.R., and Brin M.F. (2014). Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: therapeutic implications for migraine and other pains. Cephalalgia 34, 853–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J., Vause C.V., and Durham P.L. (2008). Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res. 1196, 22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feistel S., Albrecht S., and Messlinger K. (2013). The calcitonin gene-related peptide receptor antagonist MK-8825 decreases spinal trigeminal activity during nitroglycerin infusion. J. Headache Pain 14, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burstein R., Yamamura H., Malick A., and Strassman A.M. (1998). Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J. Neurophysiol. 79, 964–982 [DOI] [PubMed] [Google Scholar]
- 79.Goadsby P.J., and Akerman S. (2012). The trigeminovascular system does not require a peripheral sensory input to be activated—migraine is a central disorder. Focus on ‘Effect of cortical spreading depression on basal and evoked traffic in the trigeminovascular sensory system’. Cephalalgia 32, 3–5 [DOI] [PubMed] [Google Scholar]
- 80.Hall E.D., Bryant Y.D., Cho W., and Sullivan P.G. (2008). Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J. Neurotrauma 25, 235–247 [DOI] [PubMed] [Google Scholar]
- 81.Hall K.D., and Lifshitz J. (2010). Diffuse traumatic brain injury initially attenuates and later expands activation of the rat somatosensory whisker circuit concomitant with neuroplastic responses. Brain Res. 1323, 161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Edelmayer R.M., Vanderah T.W., Majuta L., Zhang E.T., Fioravanti B., De Felice M., Chichorro J.G., Ossipov M.H., King T., Lai J., Kori S.H., Nelsen A.C., Cannon K.E., Heinricher M.M., and Porreca F. (2009). Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann. Neurol. 65, 184–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noseda R., Kainz V., Borsook D., and Burstein R. (2014). Neurochemical pathways that converge on thalamic trigeminovascular neurons: potential substrate for modulation of migraine by sleep, food intake, stress and anxiety. PLoS One 9, e103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bourin M., and Hascoet M. (2003). The mouse light/dark box test. Eur. J. Pharmacol. 463, 55–65 [DOI] [PubMed] [Google Scholar]
- 85.Thompson S., Recober A., Vogel T.W., Kuburas A., Owens J.A., Sheffield V.C., Russo A.F., and Stone E.M. (2010). Light aversion in mice depends on nonimage-forming irradiance detection. Behav. Neurosci. 124, 821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaiser E.A., Kuburas A., Recober A., and Russo A.F. (2012). Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J. Neurosci. 32, 15439–15449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lenaerts M.E. (2008). Post-traumatic headache: from classification challenges to biological underpinnings. Cephalalgia 28 Suppl. 1, 12–15 [DOI] [PubMed] [Google Scholar]
- 88.Phares T.W., Kean R.B., Mikheeva T., and Hooper D.C. (2006). Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J. Immunol. 176, 7666–7675 [DOI] [PubMed] [Google Scholar]