Abstract
The incidence of breast cancer brain metastasis (BCBM) is increasing due in part to improved management of systemic disease and prolonged survival. Despite this growing population of patients, there exists little consensus for the treatment of HER2-positive BCBM. Lapatinib, the only brain permeable targeted agent for HER2-positive cancer, has demonstrated limited intracranial response rates and little improvement in progression free survival (PFS) for HER-2 positive patients. Size constraints are believed to prevent larger monoclonal antibodies, such as pertuzumab and trastuzumab, from crossing the blood brain barrier (BBB). However, emerging evidence reveals that the BBB is perturbed in the setting of metastases, allowing for improved penetrance of these larger targeted agents. The disrupted BBB may allow for passage of ado-trastuzumab emtansine (TDM1), though little clinical information about its activity in BCBM patients is currently known.
Keywords: Breast Cancer, Brain Metastasis, T-DM1, Kadcyla, Adotrastuzumab, emtansine
Introduction
Brain metastases are an increasingly common problem among patients with HER2-positive advanced breast cancer [1]. Historically, studies illustrate the incidence of intracranial recurrence is approximately 30% among those with advanced HER2-positive breast cancer [2]. The current standard of care for patients with Her2-positive breast cancer brain metastases is radiation with or without neurosurgical resection [3]. Whole brain radiation therapy is generally recommended for those with multiple brain metastases, while focused stereotactic radiotherapy is recommended for those with 4 or fewer brain metastases. Neurosurgery is reserved for those with a solitary brain metastasis, when the diagnosis is unclear, or when an intracranial lesion is exerting significant mass effect on the neighboring normal brain.
The landscape of systemic therapies to treat advanced HER2-positive breast cancer is evolving rapidly. Presently, a taxane, pertuzumab, and trastuzumab combination regimen is preferred first line treatment, while the antibody conjugate trastuzumab-emtansine (TDM1) is preferred second line therapy [4]. In third line and beyond, lapatinib and/or trastuzumab-containing regimens and/or clinical trials are recommended [5]. This algorithm in the setting of progressive HER2-positive breast cancer brain metastases is less clear. The majority of efficacy data has been with the brain permeable small molecule inhibitor of HER1/HER2, lapatinib, alone or in combination with other cytotoxics. However, intracranial, response rates are generally less than 20% and progression free survival less (PFS) is less than 6 months in the refractory setting [6–8].
Larger monoclonal antibodies, such as pertuzumab and TDM1, have generally been thought not to cross the blood brain barrier due to sheer size constraints. However, emerging evidence reveals that the blood-brain barrier is perturbed in the setting of metastases, often termed the “blood-tumor” barrier [9]. Moreover, radiolabeled studies of pertuzumab and TDM1 have shown accumulation of both molecules in HER2-positive breast cancer brain metastases [10]. Herein, we report intracranial response to TDM1 among four patients treated at the University of North Carolina at Chapel Hill with refractory HER2-positive brain metastases. Each case is briefly summarized in Table 1.
Table 1.
Case Descriptions
| Patient # | Date of 1° Dx | Stage at 1° Dx | Tx for 1° BC | Mets Dx Date | Mets Dx Bx Site | Date of BM Dx | Rads for BM | Systemic Tx for BM |
|---|---|---|---|---|---|---|---|---|
| 1 | Nov 2011 | Stage IV | – | – | Liver | June 2013 | SRS 1: June 2013 SRS 2: October 2014 |
capecitabine / lapatinib / PI3K inhibitor (7/2013 – 2/2014) vinorelbine / trastuzumab/everolimus (10/2014 – 4/2015) |
| 2 | Oct 2008 | Stage IA | Mastectomy (no syst Tx) | Oct 2011 | Liver | April 2013 | WBRT (4/2013) SRS (2/2014) |
TDM1 (5/2015 – present) nab-paclitaxel / herceptin / lapatinib (6/13 – 2/14) vinorelbine / trastuzumab/everolimus (3/14 – 5/14) TDM1 (5/14 – present) TDM-1 (9/14 – present |
| 3 | Nov 2003 | Stage IIB | AC × 4 (12/03 – 3/04) Docetaxel (5/04 – 8/04) |
Sept 2009 | Bone | Aug 2014 | WBRT (8/2014) | *TDM-1 d/c’ed briefly 5/15 during radiation to hip; resumed 6/15 capecitabine / lapatinib / PI3K inhibitor (11/13 – 4/14) vinorelbine / everolimus / trastuzumab (5/14 9/14) paclitaxel / trastuzumab / pertuzumab (9/14–12/14) trastuzumab / pertuzumab / aromatase inhbitor (3/15 – 5/15) TDM-1 (5/15 – present) |
| 4 | June 2010 | Stage IIB | Docetaxel / trastuzumab × 4 (10/06 – 11/06) Trastuzumab (12/06 – 11/07) Tamoxifen (2007 – 2012) |
Sept 2013 | Brain | Sept 2013 | SRS (10/13) WBRT (2/15) |
Patient 1
50-year-old female presented in November 2011 with a de novo Stage IV invasive ductal carcinoma of the breast with multiple hepatic and lung metastases. Biopsy of the primary breast tumor was negative for estrogen and progesterone receptors (ER/PR), but HER2 overexpressed (3+ by immunohistochemistry [IHC]). She initially received 2 cycles of dose-dense Adriamycin and Cyclophosphamide (AC) followed by dose-dense paclitaxel concurrent with trastuzumab; paclitaxel was discontinued due to anaphylactic reaction. Treatment was transitioned to docetaxel/trastuzumab and 2 cycles were completed before continuing on singleagent trastuzumab. Following response to therapy, she underwent bilateral mastectomies in August of 2012.
In the summer of 2013, the patient presented with significant headaches that led to neuroimaging and the identification of several brain metastases throughout the cerebellum and cerebral hemispheres. Three intracranial lesions were treated with stereotactic radiosurgery (SRS) (20Gy, 18Gy, and 25 Gy respectively). She then transitioned to capecitabine, lapatinib, and an investigational phosphotidyl-inosital 3 kinase (PI3K) inhibitor. After 9 cycles, she experienced intracranial disease progression and was transitioned to capecitabine/trastuzumab.
In July of 2014, an enlarging and symptomatic intracranial lesion in the frontal lobe was surgically resected; pathology revealed radiation necrosis. SRS was subsequently performed on 3 progressive intracranial lesions in October 2014. A restaging brain magnetic resonance imaging (MRI) showed progression in 2 intracranial lesions, prompting initiation of vinorelbine/everolimus/trastuzumab on a clinical trial which was discontinued after 5 cycles again due to intracranial disease progression. T-DM1 was initiated and after 4 cycles, a brain MRI illustrated a measurable reduction in the size of several intra-cranial lesions (Figure 1, Patient 1). The largest lesion, a 22 mm enhancing lesion in the corpus callosum, decreased to 14 mm. A 22 mm lesion in the left cerebellar hemisphere decreased to 17 mm. The patient’s neurologic status was stable and steroids were no longer required to maintain symptom control.
Figure 1.
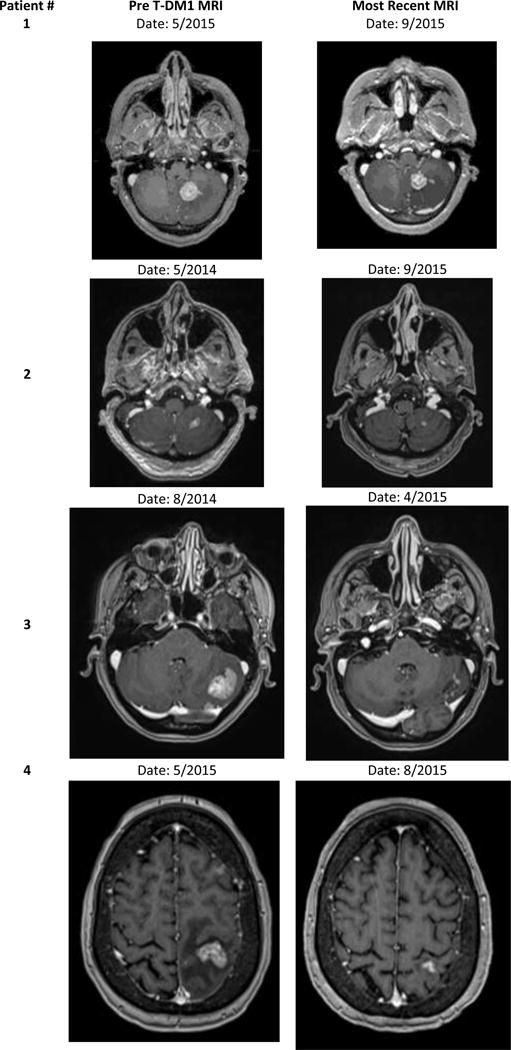
Representative images of intracranial response to TDM1 among four patients treated at the University of North Carolina at Chapel Hill
Patient 2
51-year-old female initially diagnosed with ductal carcinoma in-situ (DICS) via core needle biopsy following an abnormal screening mammogram in November of 2008. The patient underwent lumpectomy with sentinel lymph node biopsy which revealed 2cm of DCIS with associated microinvasion and lymph node micro-metastasis. Due to positive surgical margins, she proceeded to completion mastectomy.
In July 2011, she presented with left upper quadrant abdominal pain with nausea and poor appetite. A computed tomography (CT) of the abdomen and pelvis showed extensive masses throughout the liver which were biopsy-proven adenocarcinoma from breast primary, ER positive, PR negative, HER2 positive (3+ by IHC). She was treated with nab-paclitaxel and trastuzumab from November of 2011 until August of 2012 at which point nab-paclitaxel was discontinued; she continued on trastuzumab alone. Letrozole was added to trastuzumab in October 2012.
In April 2013 headaches prompted a brain MRI; multiple brain metastases throughout both the cerebellum and left cerebral hemispheres were discovered. She received whole-brain radiation therapy (WBRT) to a total dose of 35 Gy in April 2013. Systemic therapy was restarted with nab-paclitaxel, trastuzumab, and lapatinib in June 2013 through January 2014 when intracranial disease progression prompted SRS therapy to a single cerebellar lesion at a total dose of 25 Gy. Then patient then transitioned to vinorelbine, everolimus, trastuzumab on a clinical trial in March 2014 which was discontinued due to intracranial progression in May 2014. She initiated TDM1 and has remained clinically stable on treatment for over 16 months with measurable reduction in the size of numerous intracranial lesions as per brain MRI September 2015 (Figure 1, Patient 2).
Patient 3
47-year-old female diagnosed in November 2003 with a Stage IIIA invasive ductal carcinoma after self-palpating a mass in her left breast. She was treated with a left mastectomy and sentinel lymph node biopsy. IHC staining of the breast tumor revealed ER positivity, negative PR, and HER2 positivity (3+). Following mastectomy, the patient completed 4 cycles of AC followed by 4 cycles of docetaxel. Chest well radiation was followed by Tamoxifen therapy until 2009.
In September 2009, the patient was diagnosed with biopsy-proven bone metastases. Treatment with trastuzumab and zolendronic acid was initiated in October 2009 and paclitaxel was added in January 2009. This combination therapy was continued until progression in April 2011. The patient then transitioned to a clinical trial with vinorelbine, trastuzumab, and an investigational vaccine agent. Following 2 cycles, treatment was discontinued as a result of disease progression. Treatment with lapatinib and trastuzumab was then initiated and continued through January 2013; therapy was discontinued due to progressive disease.
Letrozole/trastuzumab with zolendronic acid stabilized was continued until disease progression in August 2014 at which time pulmonary nodules and 2 new brain metastases were found on routine restaging. The patient received 14 fractions of WBRT at 250 cGy per fraction for a total dose of 35 Gy. Systemic therapy was transitioned to T-DM1 following WBRT in September 2014. The patient remained stable and progression free with reduction in her 2 brain lesions and no new lesions while on TDM1 for over 12 months (Figure 1, Patient 3).
Patient 4
49-year-old female was diagnosed in October of 2006 with a T2N1 invasive ductal carcinoma of the left breast, ER and PR positive, and HER2 amplified by fluorescence in situ hybridization (FISH). She received 4 cycles of neoadjuvant docetaxel/trastuzumab with a pathologic complete response. Five years of endocrine therapy with tamoxifen was completed in September of 2012.
In the fall of 2013, the patient experienced severe nausea, vomiting, and dizziness. Brain MRI revealed multiple intracranial lesions with a large cystic right cerebellar lesion. An emergency craniotomy was performed to manage the dominant lesion while five remaining lesions were treated via SRS (21 Gy to each lesion).
In October of 2013, she began capecitabine, lapatinib, and an investigational PI3K inhibitor. This combination therapy was discontinued in April 2014 due to intracranial progression and new hepatic lesions. The patient was transitioned in October 2014 to vinorelbine, everolimus, trastuzumab on a clinical trial followed by paclitaxel, trastuzumab, pertuzumab due to intracranial progressive disease.
In February 2015, the patient received WBRT at a dose 35 Gy followed by one additional cycle of trastuzumab/pertuzumab with letrozole. Due to progressive liver disease, she was transitioned to T-DM1 in May of 2015, and after only 3 months on treatment, marked reduction in both intracranial and hepatic lesions was noted. The largest intracranial lesion in the left posterior frontal lobe decreased from 17 mm in size to 10 mm (Figure 1, Patient 4)
Discussion
While HER2-targeted therapies, namely the brain penetrant small molecule inhibitor lapatinib, have demonstrated efficacy in the treatment of brain metastasis derived from breast cancer, response rates are relatively low, 6% as a single agent and 20% in combination with capecitabine, and rarely durable (median PFS ~5.5 months) [6, 7]. Systemic treatment options for patients with advanced HER2 positive breast cancer and progressive brain metastases remain limited. In this report, we present 4 patients with treatment-refractory brain metastases treated at the University of North Carolina at Chapel Hill who benefited both clinically and radiographically from systemic therapy T-DM1.
Our experience is concordant with recently published preclinical data demonstrating the efficacy of T-DM1 in the CNS metastatic microenvironment [11], as well as other early reports illustrating benefit of T-DM1 in the treatment of patients with progressive HER2-positive brain metastases [12–14]. In preclinical models, T-DM1 demonstrated the ability to overcome resistance to trastuzumab therapy in HER2-driven or PI3K-driven breast cancer brain lesions, significantly delaying metastatic growth of these tumors. In an early clinical case series of 10 patients with progressive HER2-positive brain metastases treated at a single institution, response was observed in three patients while four patients had stable disease for at least six months. The median progression free survival in that study was 5 months, with a median overall survival of 8.5 months [14].
In this brief case series, we present four clinical cases of heavily pre-treated patients with treatment refractory Her2-positive brain metastases who derived clinical benefit and radiographic response to TDM1, one of which was maintained on therapy for 16 months. At the time of this writing, all patients remain alive and on-treatment with measureable reductions in intracranial tumor size. All 4 patients had 30% or greater reduction in size + at least 5 mm absolute reduction. Intracranial response was evaluated using modified response evaluation criteria in solid tumors criteria (RECIST 1.1).
Collectively, this data adds to a growing body of evidence illustrating clinical benefit of T-DM1 in the HER2-positive breast cancer brain metastases population which warrants continued study in early phase clinical trials appropriately powered to determine benefit and effect on quality of life for this patient population.
Acknowledgments
Financial Support:
Research reported in this publication was supported by the National Cancer Institute of the NIH under award number K23CA157728 (to C.K. Anders) and NCI Breast SPORE program (P50-CA58223)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Puhalla S, et al. Unsanctifying the sanctuary: challenges and opportunities with brain metastases. Neuro Oncol. 2015;17(5):639–51. doi: 10.1093/neuonc/nov023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendell JC, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–7. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 3.Ewend MG, et al. Guidelines for the initial management of metastatic brain tumors: role of surgery, radiosurgery, and radiation therapy. J Natl Compr Canc Netw. 2008;6(5):505–13. doi: 10.6004/jnccn.2008.0038. quiz 514. [DOI] [PubMed] [Google Scholar]
- 4.Swain SM, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–34. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma S. Advances in treating HER2-positive breast cancer: an interview with Sunil Verma. BMC Med. 2014;12:129. doi: 10.1186/s12916-014-0129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson EM, et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013;22(4):525–31. doi: 10.1016/j.breast.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaz-Luis I, et al. Impact of hormone receptor status on patterns of recurrence and clinical outcomes among patients with human epidermal growth factor-2-positive breast cancer in the National Comprehensive Cancer Network: a prospective cohort study. Breast Cancer Res. 2012;14(5):R129. doi: 10.1186/bcr3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachelot T, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 9.Lockman PR, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–78. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ningaraj NS, et al. Targeted brain tumor treatment-current perspectives. Drug Target Insights. 2007;2:197–207. [PMC free article] [PubMed] [Google Scholar]
- 11.Askoxylakis V, et al. Preclinical Efficacy of Ado-trastuzumab Emtansine in the Brain Microenvironment. J Natl Cancer Inst. 2016;108(2) doi: 10.1093/jnci/djv313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krop IE, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113–9. doi: 10.1093/annonc/mdu486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalsi R, et al. Brain metastasis and response to ado-trastuzumab emtansine: a case report and literature review. Clin Breast Cancer. 2015;15(2):e163–6. doi: 10.1016/j.clbc.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Bartsch R, et al. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin Exp Metastasis. 2015;32(7):729–37. doi: 10.1007/s10585-015-9740-3. [DOI] [PubMed] [Google Scholar]


